Abstract
Although copper has been reported to influence numerous proteins known to be important for angiogenesis, the enhanced sensitivity of this developmental process to copper bioavailability has remained an enigma, because copper metalloproteins are prevalent and essential throughout all cells. Recent developments in x-ray optics at third-generation synchrotron sources have provided a resource for highly sensitive visualization and quantitation of metalloproteins in biological samples. Here, we report the application of x-ray fluorescence microscopy (XFM) toin vitro models of angiogenesis and neurogenesis, revealing a surprisingly dramatic spatial relocalization specific to capillary formation of 80–90% of endogenous cellular copper stores from intracellular compartments to the tips of nascent endothelial cell filopodia and across the cell membrane. Although copper chelation had no effect on process formation, an almost complete ablation of network formation was observed. XFM of highly vascularized ductal carcinomas showed copper clustering in putative neoangiogenic areas. This use of XFM for the study of a dynamic developmental process not only sheds light on the copper requirement for endothelial tube formation but highlights the value of synchrotron-based facilities in biological research.
Keywords: copper chelation, human microvascular endothelial cells, infiltrating ductal breast carcinoma
Endogenous metals, such as Cu, Fe, and Zn, are subject to complex regulation in cellular systems. They are required as cofactors or regulators of numerous proteins (1) and yet, if present in overabundance, are toxic and expose the cellular environment to adventitious redox activity (2). This delicate balance is thought to be achieved by sequestration of these metals within their target proteins, metallochaperone systems, or distinct subcellular compartments (3). Although many proteins that handle transition metals within cells have been identified (4), our knowledge as to how metal content is dynamically regulated in eukaryotic cells is still limited. To what extent does regulation of the metal ion content of individual metalloproteins, mediated by protein–protein interactions, serve as an additional level of regulation of cellular metalloprotein activity? Could such regulation result in polarization of transition metal distribution throughout a cell during a biological process? To begin to explore such questions, we examined a cellular system whose biology is acutely sensitive to modulation by metals.
Angiogenesis, the process by which nascent vasculature is formed, has long been characterized by a heightened sensitivity to copper (5, 6). Copper or copper complexes have been shown to directly stimulate angiogenesis in several model systems, including the rabbit corneal system (6) and the bovine aorta endothelial cell system (7), as well as in vivo (8). Furthermore, copper chelation has been shown to inhibit angiogenesis in numerous animal and xenograft models (9). Such findings have led to clinical trials for the treatment of solid tumors by copper chelation with some findings of efficacy in disease stabilization (10, 11), yet the molecular basis for the sensitivity of angiogenesis to copper remains elusive. Copper has been shown to influence either the bioactivity or production of a number of factors involved in the initiation of angiogenesis, including VEGF (12), basic fibroblast growth factor (bFGF) (13), and angiogenin (14). The effect of copper on these factors may arise directly, or it may occur through a more complex, indirect process, but it is difficult to rationalize how the modulation of these factors by copper could fully explain the heightened sensitivity of endothelial cells in comparison to other cell types. A window of copper deficiency appears to exist in which, although angiogenesis is impaired, other cellular processes dependant on copper show no clinical disruption (10).
Because tube morphogenic processes rely heavily on the spatial redistribution of existing components (15, 16), an experimental approach that both measures and localizes elemental content is ideally suited to provide particular insight into this phenomenon. Synchrotron-derived x-rays from third-generation sources can now be focused such that both quantitation and spatial discrimination of metals can be achieved at the submicrometer scale by using x-ray fluorescence microprobe (XFM) analysis. Most of the initial biological applications of XFM analysis to date have focused on the distribution of nonendogenous metals within the cellular space, such as the localization of TiO2-labeled oligonucleotides (17), the examination of platinum oxidation states (18), and bacterial uptake of chromium (19). However, more recent work has begun to demonstrate the utility of this technology for the quantitation and localization of endogenous metals, such as in studies on the phagosome environment in pathogen-infected macrophages (20), on the copper content and topography of fibroblasts (21), and on the relocalization of zinc during early stages of macrophage differentiation (22). We have used XFM to explore the relationship between copper and angiogenesis both in vitro and in vivo and have discovered that massive relocalization of cellular copper stores appears to be a requirement for proper angiogenic network formation.
Results and Discussion
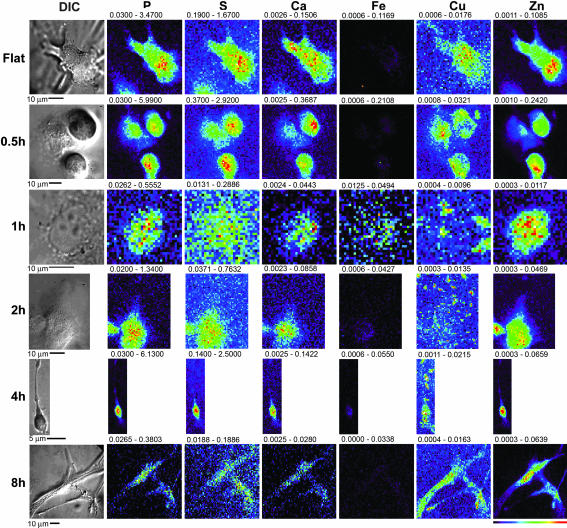
As a model system for the study of angiogenesis, we used human microvascular endothelial cells (HMVECs) which, when stimulated with angiogenic factors and plated at an appropriate density on basement membrane matrices such as Matrigel, form a complex network consisting of multicellular lumen-containing structures that mimic early angiogenesis (23, 24). These cells display a temporally synchronized differentiation from fully dispersed cells at 0 h to a loose network of connected cells by 1 h after plating, extensive cell-to-cell contact and primitive network formation by 2 h, and a mature lumen-containing network by 8 h. Examination of the subcellular distribution of multiple elements (P to Zn on the atomic chart by K-line fluorescence) over time during morphogenic network formation by HMVECs was carried out at the Advanced Photon Source by using XFM analysis. As a control for cellular growth, cells were plated on gelatin-coated surfaces, conditions which, despite stimulation with identical angiogenic factors, result in proliferation rather than tubulogenesis. XFM analysis of these control cells demonstrated elemental distributions for P, S, and Zn typical of eukaryotic cells, with S correlating to overall cell thickness and Zn most prevalent in the nucleus (Fig. 1, Flat) (22). Because of the high background content of Fe in the surrounding matrix, cellular distribution maps for this metal were uninformative for the in vitro samples. Copper was consistently centrally localized to an area at the periphery of the nucleus (Fig. 1, Flat), consistent with the copper topography observed in other cell types (data not shown) and consistent with reports of some colocalization of copper with Golgi and mitochondrial organelles (21). When cells are stimulated with VEGF and bFGF and plated onto the basement membrane surface, copper translocates outwards toward the periphery of the cell within 30- 60 min (Fig. 1, 0.5h and 1h). As the cell becomes more fully attached to the matrix and filopodia are extended, an extensive concentration of copper is translocated into the tips of the multiple extended processes (Fig. 1, 2h). As polarity is established and filopodial commitment is made, a large portion of the copper has been relocated from the cell body out along the axis of these growing processes (Fig. 1, 4h). Once formation of contacts between adjacent cells and consolidation of the network is complete, copper distributions similar to control proliferating cells appear to be reestablished (Fig. 1, 8h). Other measured elements displayed no such dramatic relocalization during tubulogenesis (Fig. 1, matching columns for P, S, Ca, and Zn). To assess the significance of these changes in cellular copper distribution, we sought to estimate the proportion of the total copper that is redistributed during tubulogenesis. Because the penetrating nature of XFM effectively renders single cells flat, we divided each cell into two morphological compartments of equal area, defining regions of interest consisting of the inner 50% and peripheral 50% of this two-dimensional projection of the cellular volume. Copper content of each region of interest was quantitated by integration of the calibrated x-ray fluorescence signal over each volume with MAPS software (25). In proliferating control cells, the total copper content was approximately evenly distributed between these two compartments; the mean ratio (± SD) between the periphery and the centrally localized cell body was measured to be 1.0 ± 0.3 for all cells in this group. As cells began to polarize and extend filopodia, this mean ratio increased to 11 ± 2 after 1 h and to 20 ± 5 after the 2 h. To achieve such high ratios, 80–90% of cellular copper must be redirected from the central cell body to the periphery during tubulogenesis.
Fig. 1.
XFM scans of HMVECs undergoing tubulogenesis. Silicon nitride windows were coated with either a thin layer of Matrigel or a layer of gelatin. HMVECs were plated on these substrates and exposed to VEGF and bFGF. The process was stopped at various times subsequent to initiation; the cells were then fixed, and both light microscope and XFM images were obtained. The scans are representative of the five cells scanned at each time point. The scale of the scans is shown below each image, and a color table is shown to the bottom right. The maximum and minimum threshold values in micrograms per squared centimeter are given above each frame. Scans were obtained by using 10.0-keV incident energy with dwell times of 1 sec per pixel and 1-μm steps through the sample.
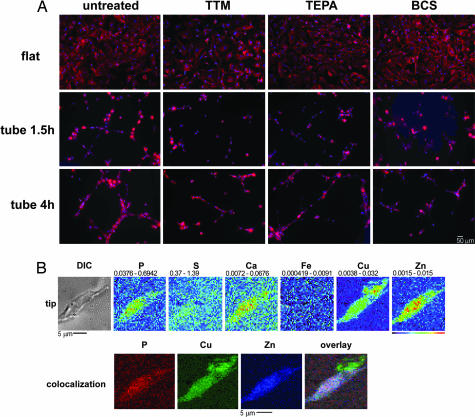
Although the overall process of angiogenesis has been shown to be sensitive to copper chelation, it has not been reported whether filopodial extension, process extension, or cell-to-cell contact is the particular aspect of tubulogenesis that is most sensitive. To resolve this issue, HMVEC tubulogenesis was initiated and the cells were exposed to three copper chelators: ammonium tetrathiomolybdate (TTM), tetraethylpentamine (TEPA), or bathocuproinedisulfonic acid (BCS). These chelators were chosen for their copper specificity and reported low toxicity in a number of cell types (10, 26, 27). Their low toxicity in HMVECs was confirmed by a trypan blue exclusion cell viability assay, which found no statistically significant decrease in viability from the use of any of the three chelators at the concentrations used in our experimental conditions. Morphologically, early aspects of HMVEC tubulogenesis are not inhibited by copper chelation (Fig. 2A Middle), nor is attachment and spreading on gelatin-coated surfaces in control cells significantly impaired (Fig. 2A Top). Cells attached to their substrate, flattened, and then generated filopodia. However, in cells untreated by copper chelators (Fig. 2A Left), filopodia that did not meet another cell were retracted and networks formed only between cells that contacted one another. In contrast, in cells chelated either by TTM, TEPA, or BCS, unsuccessfully networked filopodia were not retracted and still appeared to be generated in a seemingly random number of orientations (Fig. 2A Right). Although some intercell contact was established, the generation of a mature network is significantly impaired. This correlates to the time period when copper has been mobilized to the leading edge of filopodia. Overlays of x-ray fluorescence images and optical images derived from cells at these time points suggested that some of this extended copper might be present exterior to the cell. We therefore focused on areas at the extreme tips of growing filopodia and performed XFM scans at a spatial resolution of 400 nm (vertical) × 600 nm (horizontal). These higher-resolution scans revealed that copper at these filopodial tips is present external to the signal derived from phosphorous and zinc fluorescence (Fig. 2B, overlay) and to any cellular structure evident in the optical image (Fig. 2B). This finding suggests that a portion of the copper that is relocalized toward the tips of branching endothelial cell filopodia is translocated across the cell membrane into the extracellular space, which may explain the ability of charged, largely membrane-impermeant copper chelators to ablate network formation. Although the precise target of the mobilized cellular copper is unclear, the preservation of this extracellular copper through chemical fixation suggests that it is tightly sequestered within a protein or other macromolecule.
Fig. 2.
Role of copper in HMVEC filopodia extension. (A) HMVECs were plated in either gelatin- or Matrigel-coated dishes, stimulated with bFGF and VEGF, and untreated or exposed to 100 μM TEPA, 100 μM TTM, or 100 μM BCS. The cells were incubated for either 1.5 or 4 h, fixed and permeabilized, and stained with Hoescht 33342 and Alexa Fluor 660–phalloidin. Cells were then imaged, and the Hoescht signal and phalloidin signal were pseudocolored blue and red, respectively. (B) Areas at the tips of HMVEC filopodia extensions were scanned by XFM at high resolution. (Upper) The optical image and metal maps. (Lower) False-color images of P, Zn, and Cu and the overlay of these images.
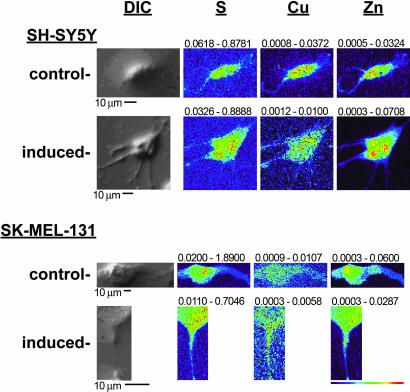
The in vivo specificity of copper chelation in inhibiting angiogenesis suggests that relocalization of copper may be specific to angiogenesis and not a general characteristic of cellular filopodia or process extension. Many proteins, such as semaphorins (28), ephrins (29), and netrin (30), have been shown to drive both endothelial and neuronal cell directional migration, yet only angiogenesis is known to be sensitive to copper chelation. We hypothesized therefore that there would not be a copper translocation during neural cell morphogenesis, as shown here during endothelial cell morphogenesis. We examined metal content and distribution over time in SH-SY5Y neuroblastoma cells, which when exposed to cytokines on basement membrane surfaces undergo maturative and morphological differentiation, including the extension of numerous neuritic processes (31). The results (Fig. 3) demonstrate that, although there is some degree of redistribution of copper along these newly formed neurites, no large-scale redistributions analogous to those seen in HMVECs were found. The mean ratio (± SD) of integrated copper between the periphery and the centrally localized cell body was 0.7 ± 0.1 in both cases. To extend this observation, we examined distributions of copper as neurally derived SK-MEL-131 cells were induced to extend cellular processes by treatment with topoisomerase-II and the tyrosine kinase inhibitor genistein (32). Here too, there was no statistically significant translocation of copper like that seen in the endothelial cells (Fig. 3). The average results for all three cells mapped under each condition gave mean ratios of copper between inner and outer regions of interest for untreated and treated cells at 0.8 ± 0.1 and 0.9 ± 0.1, respectively. Taken together, these data suggest that, although many cell types share some of the same molecular machinery for process extension, extensive copper translocation and secretion is uniquely associated with endothelial cell branching morphogenesis.
Fig. 3.
XFM scans of cells undergoing neural process extension. SH-SY5Y cells were applied to either gelatin- or Matrigel-coated silicon nitride windows and exposed to bFGF and IGF for 3 d. SK-MEL-131 cells were plated directly on silicon nitride windows and either untreated or exposed to 45 μM genistein for 2 d. The cells were fixed and washed, optical images were acquired, and the cells were scanned by XFM analysis. Representative output is shown, with the scale bar and color table shown below the scans. The maximum and minimum threshold values in micrograms per squared centimeter are given above each frame.
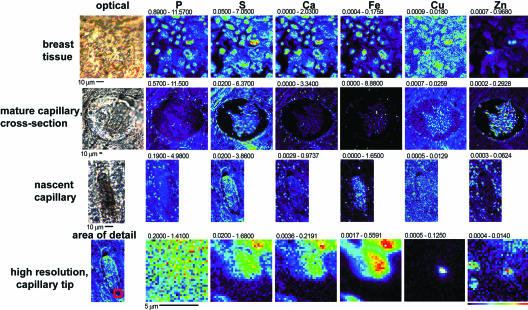
Although in vitro HMVEC tubulogenesis is a standard model of early tumor-derived angiogenesis, translation of these findings to a more physiologically intact system would deepen their significance in explaining the sensitivity of angiogenesis to copper. To extend our findings to an in vivo setting, we sought to image metal content in a tumor system physiologically dependent on angiogenesis and in samples characterized as having a high microvascular density. We therefore examined five 5-μm-thick sections of high-grade Estrogen Receptor/Progesterone Receptor-negative VEGF-positive infiltrating ductal breast carcinomas with a range of HER-2 expression (negative to highly positive) and high microvascular density (6.3–9.5 on the Chalkey index). Areas of high vascularization in each tumor section were identified by using rapid XFM scans to locate regions of high iron content, a result of residual hemoglobin-dense, iron-rich blood. Thirty-six longer-dwell-time (1–2 sec), higher-resolution (1 μm) scans of these vascularized sections, as well as 10 areas of nonvascularized breast tissue (Fig. 4), were carried out. Of the 36 scans of vascularized regions, 10 appeared representative of lymphatic space invasion, 15 were axial cuts of mature vessels, and 10 were parallel cuts of mature vessels. Nonvascularized breast tissue displayed elemental content roughly uniformly distributed according to cell thickness, resulting in images that appear similar to those obtained for other eukaryotic cells, with individual cells clearly visible in P, S, Ca, Fe, Cu, and Zn maps (22) (Fig. 4, breast tissue). A representative scan of a mature, larger (200-μm diameter) cross-sectional capillary illustrates several items of note. Iron content was significantly higher than surrounding tissue, as expected for residual heme-containing blood, with a maximal concentration of 8.88 μg/cm2 compared with 0.176 μg/cm2 in the adjacent breast tissue. Zinc content appeared to be higher in the residual blood material as well, whereas phosphorous content was higher in the surrounding tissue as a probable consequence of enucleated red blood cells lacking phosphorous-dense DNA. Sulfur, calcium, and copper content appeared to be equally distributed in the two tissue types. The localization of copper to the center of endothelial cells in these mature capillaries is consistent with our data from HMVECs, in which copper was redistributed only in cells actively forming new processes. In contrast, copper and, to a lesser extent, zinc consistently demonstrated distinct distributions in all 10 of the scans, which were classified as nascent lymphatic space invasions (Fig. 4, nascent capillary) whereas phosphorous, sulfur, calcium, and iron distributions remained similar to those seen in mature capillaries. Zinc appeared to be concentrated external to the lymphatic space in these putatively neoangiogenic areas. It is possible that additional signaling events involving zinc may be occurring in vivo or that this zinc could be coming from stromal cells or pericytes that are not physically represented in the HMVEC model system. Most dramatically, copper was consistently present in particularly high concentrations in areas of endothelial cells where branching morphogenesis may be active and displayed a punctuate pattern at the leading edge of endothelial cells. Higher resolution (0.25-μm step sizes) scans of an 8- × 8-μm area surrounding one of these representative punctate copper signals (in Fig. 4, high resolution, capillary tip) revealed that copper and, to some degree, zinc appeared to be present either at the periphery or transported across the leading edge of endothelial cells involved in angiogenesis. These results suggest that copper may be transported to the extracellular space in areas in which nascent capillaries are forming similar to the findings in the in vitro HMVEC system. Such a phenomenon could certainly explain the inherent in vivo sensitivity of angiogenesis to copper chelation.
Fig. 4.
XFM scans of human breast-infiltrating ductal carcinoma tumor tissues. Areas of breast and endothelial cells were identified in paraffin-embedded tissue, and XFM scans (1–2 sec per pixel) of such areas were performed. For each representative scan, an optical image is shown to the left. Maps of each metal show areas of lowest content to highest content scaled to a rainbow color scale shown to the bottom right. The size scale of each scan is shown by the scale bar under each scan, and the minimal and maximal content displayed in micrograms per squared centimeter is listed above each image. The region of the nascent capillary on the left from which the high-resolution scans to the right are derived is shown as a red square.
Conclusions
Although all cells possess cupric metalloproteins, the data presented here imply that the particular sensitivity of angiogenesis to bioavailable copper may arise from the mobilization of this metal to the extracellular space by endothelial cells during this process. The mode of action of copper chelators has often been suggested to involve reducing the amount of bioavailable copper in circulating plasma proteins (9). However, our findings here suggest that at least some of the copper that may be acting in a regulatory capacity is instead arising from intracellular stores. Chelation may only work by competition against protein targets of this endothelial-cell-derived copper, and as such this competition may be locally only partially effective. It is possible that a number of regulatory angiogenic proteins, such as VEGF or angiogenin, known to be critical for angiogenesis and copper-dependent, or other uncharacterized proteins are being activated by growth factor-stimulated endothelial-cell-excreted copper. It has been noted that simply adding exogenous copper to a cell system can initiate angiogenesis (6); translocation of intracellular copper to the extracellular space as demonstrated here may be serving the same purpose. Alternatively, peptides resulting from the proteolytic degradation of extracellular matrix proteins such as SPARC/osteonectin (33) may be activated by excreted copper and behave as proangiogenics in an autocrine manner (34).
The concept that endogenous metal content is subject to dynamic regulation has been established for some metal ions, such as calcium. Yet a similar role for transition metals, such as copper and zinc, metal ions which are much more tightly sequestered by the cell, has far less experimental support. This may partially be a result of an historical lag in the ability to sensitively measure and spatially localize these ligand- or chaperone-bound transition metals relative to calcium, for which sensitive, selective, and optically visible probes have been available for many years. The redistribution data presented here suggest that transition metals like copper may be subject to dynamic spatial regulation and, furthermore, that this regulation may play a role in developmental processes. XFM analysis, which provides high elemental sensitivity for medium to high Z elements irrespective of coordination or redox status, as well as quantitative information, speciation, and spatial mapping, represents a powerful evolution in the tools used to study endogenous metal usage.
Materials and Methods
Cell Specimens.
Sections (5 μm thick) of formalin-fixed paraffin-embedded infiltrating ductal breast carcinomas were obtained from the University of Chicago tumor tissue archive. For x-ray imaging, the sections were mounted intact on silicon nitride windows (area, 2 × 2 mm; thickness, 1,000 nm) manufactured by Silson (Blisworth, U.K.) and attached by brief heating to 55°C. Primary HMVECs from neonatal foreskin epidermis were obtained from Cascade Biologics (Portland, OR) and grown in medium 131 supplemented with microvascular growth supplement (Cascade Biologics) on tissue culture plates coated with gelatin. For the induction of tubulogenesis, a 1:1 mixture of supplemented medium 131 and Matrigel (Sigma, St. Louis, MO) was created at 4°C and layered onto either 100-mesh, carbon/formvar-coated gold-finder grids (Electron Microscopy Sciences, Fort Washington, PA) or silicon nitride windows. HMVECs were collected and plated at 2.25 × 104 per squared centimeter of culture surface area and supplemented with 50 ng/ml VEGF (Alpha Diagnostics International, San Antonio, TX). Tubulogenesis was allowed to proceed, and the process was stopped at various time points subsequent to initiation by washing cells in PBS followed by fixation in 4% paraformaldehyde in PBS for 10 min. Residual PBS was removed by several washes in 20 mM Pipes, pH 7.2/200 mM sucrose followed by air drying. This method of cell fixation and preparation for x-ray fluorescence to minimize disruption of metal ion topology has been previously described (22) and does not significantly alter typical cellular copper content relative to fixation by plunge freezing. SH-SY5Y cells (America Type Culture Collection, Manassas, VA) were plated onto grids or windows either untreated or coated with a thin layer of Matrigel at 2.07 × 104 cells per squared centimeter in RPMI medium 1640 (Invitrogen, Carlsbad, CA) supplemented with 10% FBS (American Type Culture Collection) and penicillin/streptavidin (Sigma), and neurite formation was induced with 3 nM bFGF (Promega, Madison, WI) and 5 nM IGF-1 (Chemicon International, Temecula, CA). SK-MEL-131 cells (Alan Houghton, Memorial Sloan-Kettering Cancer Center, New York, NY) were plated on silicon nitride windows in growth medium, and differentiation was induced with 45 μM genistein (Sigma). Cells were incubated for 3 d and then fixed and washed as described above. Optical images of the cells were captured on a DMXRE microscope (Leica Microsystems, Wetzlar, Germany) by using Nomarski differential interference contrast microscopy and ×40 and ×100 air objectives.
For the chelation studies, HMVECs were collected and either not treated or treated with 100 μM TTM (GFS Chemicals, Columbus, OH) or 100 μM TEPA (Sigma), or 100 μM BCS (Sigma) simultaneous to plating on either Matrigel or gelatin-coated wells. Cells were simultaneously fixed and permeabilized by incubation in 0.5% dodecyltrimethylammonium chloride/1% paraformaldehyde in PBS for 5 min, followed by 1% paraformaldehyde in PBS for 20 min. Cells were then blocked in 1% BSA and stained with 1 μg/ml Hoescht 33342 and 165 nM Alexa Fluor 660–phalloidin (both from Molecular Probes, Eugene, OR). Cells were then imaged by using a AxioImager.Z1 microscope imaging workstation (Carl Zeiss, Thornwood, NY) equipped with a 300-W scrambled Xenon source (Sutter Instrument Co., Novato, CA). Images were captured with a Sensicam QE camera (Cooke Co., Auburn Hills, MI) and processed with Slidebook software (Intelligent Imaging Innovations, Denver, CO).
X-Ray Imaging.
Specimens were imaged with the scanning x-ray microprobe at beamline 2-ID-E at the Advanced Photon Source (Argonne, IL). Undulator-generated x-rays of 10-keV incident energy were monochromatized with a single bounce Si 〈111〉 monochromator and focused to a measured spot size of 0.3 × 0.5 μm using Fresnel zone plate optics (X-radia, Concord, CA). Cells were raster-scanned in steps of 1.0 μm, and fluorescence spectra were collected for 1- to 2-sec dwell times by using a three-element UltraLE Ge-detector (Canberra, Meridien, CT). Quantitation and image-processing was performed with MAPS software (25), and standardization to convert the fluorescence signal to a two-dimensional concentration in micrograms per squared centimeter was achieved by fitting spectra against the signal derived from thin-film standards NBS-1832 and NBS-1833 (National Bureau of Standards, Gaithersburg, MD).
Acknowledgments
This study, including use of the Advanced Photon Source, was supported by U.S. Department of Energy, Office of Science Contract DE-AC-02-06CH11357. O.I.O. is a Doris Duke Distinguished Clinical Scientist.
Abbreviations
- bFGF
basic fibroblast growth factor
- XFM
x-ray fluorescence microscopy
- HMVEC
human microvascular endothelial cell
- TTM
ammonium tetrathiomolybdate
- TEPA
tetraethylpentamine
- BCS
bathocuproinedisulfonic acid.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
References
- 1.Lippard SJ, Berg JM. Principles of Bioinorganic Chemistry. Mill Valley, CA: Univ Sci Books; 1994. [Google Scholar]
- 2.Valko M, Morris H, Cronin MT. Curr Med Chem. 2005;12:1161–1208. doi: 10.2174/0929867053764635. [DOI] [PubMed] [Google Scholar]
- 3.O'Halloran TV, Culotta VC. J Biol Chem. 2000;275:25057–25060. doi: 10.1074/jbc.R000006200. [DOI] [PubMed] [Google Scholar]
- 4.Finney LA, O'Halloran TV. Science. 2003;300:931–936. doi: 10.1126/science.1085049. [DOI] [PubMed] [Google Scholar]
- 5.Ziche M, Jones J, Gullino PM. J Natl Cancer Inst. 1982;69:475–482. [PubMed] [Google Scholar]
- 6.Raju KS, Alessandri G, Ziche M, Gullino PM. J Natl Cancer Inst. 1982;69:1183–1188. [PubMed] [Google Scholar]
- 7.McAuslan BR, Reilly WG, Hannan GN, Gole GA. Microvasc Res. 1983;26:323–338. doi: 10.1016/0026-2862(83)90080-8. [DOI] [PubMed] [Google Scholar]
- 8.Alessandri G, Raju K, Gullino PM. Microcirc Endothelium Lymphatics. 1984;1:329–346. [PubMed] [Google Scholar]
- 9.Goodman VL, Brewer GJ, Merajver SD. Endocr Relat Cancer. 2004;11:255–263. doi: 10.1677/erc.0.0110255. [DOI] [PubMed] [Google Scholar]
- 10.Brewer GJ, Dick RD, Grover DK, LeClaire V, Tseng M, Wicha M, Pienta K, Redman BG, Jahan T, Sondak VK, et al. Clin Cancer Res. 2000;6:1–10. [PubMed] [Google Scholar]
- 11.Redman BG, Esper P, Pan Q, Dunn RL, Hussain HK, Chenevert T, Brewer GJ, Merajver SD. Clin Cancer Res. 2003;9:1666–1672. [PubMed] [Google Scholar]
- 12.Sen CK, Khanna S, Venojarvi M, Trikha P, Ellison EC, Hunt TK, Roy S. Am J Physiol. 2002;282:H1821–H1827. doi: 10.1152/ajpheart.01015.2001. [DOI] [PubMed] [Google Scholar]
- 13.Watanabe T, Seno M, Sasada R, Igarashi K. Mol Endocrinol. 1990;4:869–879. doi: 10.1210/mend-4-6-869. [DOI] [PubMed] [Google Scholar]
- 14.Soncin F, Guitton JD, Cartwright T, Badet J. Biochem Biophys Res Commun. 1997;236:604–610. doi: 10.1006/bbrc.1997.7018. [DOI] [PubMed] [Google Scholar]
- 15.Nelson WJ. Trends Cell Biol. 2003;13:615–621. doi: 10.1016/j.tcb.2003.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glesne DA, Zhang W, Mandava S, Ursos L, Buell ME, Makowski L, Rodi DJ. Cancer Res. 2006;66:4030–4040. doi: 10.1158/0008-5472.CAN-05-3294. [DOI] [PubMed] [Google Scholar]
- 17.Paunesku T, Rajh T, Wiederrecht G, Maser J, Vogt S, Stojicevic N, Protic M, Lai B, Oryhon J, Thurnauer M, et al. Nat Mater. 2003;2:343–346. doi: 10.1038/nmat875. [DOI] [PubMed] [Google Scholar]
- 18.Hall MD, Dillon CT, Zhang M, Beale P, Cai Z, Lai B, Stampfl AP, Hambley TW. J Biol Inorg Chem. 2003;8:726–732. doi: 10.1007/s00775-003-0471-6. [DOI] [PubMed] [Google Scholar]
- 19.Kemner KM, Kelly SD, Lai B, Maser J, O'Loughlin EJ, Sholto-Douglas D, Cai Z, Schneegurt MA, Kulpa CF, Jr, Nealson KH. Science. 2004;306:686–687. doi: 10.1126/science.1103524. [DOI] [PubMed] [Google Scholar]
- 20.Wagner D, Maser J, Lai B, Cai Z, Barry CE, III, Honer Zu Bentrup K, Russell DG, Bermudez LE. J Immunol. 2005;174:1491–1500. doi: 10.4049/jimmunol.174.3.1491. [DOI] [PubMed] [Google Scholar]
- 21.Yang L, McRae R, Henary MM, Patel R, Lai B, Vogt S, Fahrni CJ. Proc Natl Acad Sci USA. 2005;102:11179–11184. doi: 10.1073/pnas.0406547102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Glesne D, Vogt S, Maser J, Legnini D, Huberman E. J Struct Biol. 2006;155:2–11. doi: 10.1016/j.jsb.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 23.Kleinman HK, McGarvey ML, Hassell JR, Star VL, Cannon FB, Laurie GW, Martin GR. Biochemistry. 1986;25:312–318. doi: 10.1021/bi00350a005. [DOI] [PubMed] [Google Scholar]
- 24.Lawley TJ, Kubota Y. J Invest Dermatol. 1989;93:59S–61S. doi: 10.1111/1523-1747.ep12581070. [DOI] [PubMed] [Google Scholar]
- 25.Vogt S. J Phys IV. 2003;104:635–638. [Google Scholar]
- 26.Narayanan VS, Fitch CA, Levenson CW. J Nutr. 2001;131:1427–1432. doi: 10.1093/jn/131.5.1427. [DOI] [PubMed] [Google Scholar]
- 27.Mitsumoto A, Kim KR, Oshima G, Nakagawa Y. Biol Pharm Bull. 2001;24:336–341. doi: 10.1248/bpb.24.336. [DOI] [PubMed] [Google Scholar]
- 28.Palmer A, Klein R. Genes Dev. 2003;17:1429–1450. doi: 10.1101/gad.1093703. [DOI] [PubMed] [Google Scholar]
- 29.Lu X, Le Noble F, Yuan L, Jiang Q, De Lafarge B, Sugiyama D, Breant C, Claes F, De Smet F, Thomas JL, et al. Nature. 2004;432:179–186. doi: 10.1038/nature03080. [DOI] [PubMed] [Google Scholar]
- 30.Gu C, Yoshida Y, Livet J, Reimert DV, Mann F, Merte J, Henderson CE, Jessell TM, Kolodkin AL, Ginty DD. Science. 2005;307:265–268. doi: 10.1126/science.1105416. [DOI] [PubMed] [Google Scholar]
- 31.Glass TL, Raabe TD, Garcia DM, Koke JR. Brain Res. 2002;934:43–48. doi: 10.1016/s0006-8993(02)02317-x. [DOI] [PubMed] [Google Scholar]
- 32.Kiguchi K, Constantinou AI, Huberman E. Cancer Commun. 1990;2:271–277. doi: 10.3727/095535490820874218. [DOI] [PubMed] [Google Scholar]
- 33.Lane TF, Iruela-Arispe ML, Johnson RS, Sage EH. J Cell Biol. 1994;125:929–943. doi: 10.1083/jcb.125.4.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sage EH, Reed M, Funk SE, Truong T, Steadele M, Puolakkainen P, Maurice DH, Bassuk JA. J Biol Chem. 2003;278:37849–37857. doi: 10.1074/jbc.M302946200. [DOI] [PubMed] [Google Scholar]