Abstract
There is considerable evidence from animal studies that the mesolimbic and mesocortical dopamine systems are sensitive to circulating gonadal steroid hormones. Less is known about the influence of estrogen and progesterone on the human reward system. To investigate this directly, we used functional MRI and an event-related monetary reward paradigm to study women with a repeated-measures, counterbalanced design across the menstrual cycle. Here we show that during the midfollicular phase (days 4–8 after onset of menses) women anticipating uncertain rewards activated the orbitofrontal cortex and amygdala more than during the luteal phase (6–10 days after luteinizing hormone surge). At the time of reward delivery, women in the follicular phase activated the midbrain, striatum, and left fronto-polar cortex more than during the luteal phase. These data demonstrate augmented reactivity of the reward system in women during the midfollicular phase when estrogen is unopposed by progesterone. Moreover, investigation of between-sex differences revealed that men activated ventral putamen more than women during anticipation of uncertain rewards, whereas women more strongly activated the anterior medial prefrontal cortex at the time of reward delivery. Correlation between brain activity and gonadal steroid levels also revealed that the amygdalo-hippocampal complex was positively correlated with estradiol level, regardless of menstrual cycle phase. Together, our findings provide evidence of neurofunctional modulation of the reward system by gonadal steroid hormones in humans and establish a neurobiological foundation for understanding their impact on vulnerability to drug abuse, neuropsychiatric diseases with differential expression across males and females, and hormonally mediated mood disorders.
Behavioral, biochemical, and physiological data in animals demonstrate that the gonadal steroid hormones estrogen and progesterone affect behavior and modulate neuronal activity (1–4). These hormones not only influence ovulation and reproductive behavior but also affect cognitive functions, affective state, vulnerability to drugs of abuse, and pain sensitivity. Although ovarian steroids have widespread neurophysiological effects, including on the dopaminergic system, and although estrogen and progesterone receptors are densely present along midbrain dopaminergic neurons and other components of the reward system (such as the amygdala and striatum), little is known about the influences of estrogen and progesterone on the dopamine-dependent reward system in women.
Substantial preclinical data, including behavioral and neurochemical differences between sexes, across the estrous cycle, and in postovariectomy hormone replacement (5, 6), attest to neuroregulatory effects of both estrogen and progesterone on the dopaminergic system (7, 8), not only on the tuberoinfundibular dopaminergic system involved in control of the anterior pituitary and important for ovulation and reproductive behavior, but also on the mesolimbic and mesocortical dopaminergic systems relevant for cognitive activities, affective state, and reward processing. For example, estrogen serves as a neuroprotectant in female, but not male, mice during methamphetamine-induced neurotoxicity of the nigrostriatal dopaminergic system (9). The facts that female rats show the highest rates of cocaine self-administration shortly after estradiol peaks and that estradiol administration to ovariectomized females enhances the acquisition of cocaine self-administration (10, 11) also offer evidence for estrogenic modulation of the rodent reward system. Conversely, systemic administration of dopamine agonists and antagonists modulates conditioned and spontaneous behavior according to sex, estrous cycle, ovariectomy, and estrogen administration (12, 13). These effects could be due, at least in part, to dopamine activation of a number of steroid receptors, including progesterone receptors, by a ligand-independent mechanism (14–16). Together, these data show complex interactions between ovarian hormones and the reward dopaminergic system in rodents.
A better understanding of these neural influences in humans would have crucial implications for sex-related differences and menstrual cycle effects on prevalence, course, and treatment response characteristics of neuropsychiatric disorders, as well as on vulnerability to drug abuse, in which dysfunction of the dopaminergic reward system plays an important role. For example, such information could elucidate the mechanism by which women experience greater subjective response to both cocaine (17) and amphetamine (18) in the follicular phase of the menstrual cycle as compared with the luteal phase, and by which women with schizophrenia have later disease onset and less severe course of illness than men (19). These clinical observations provide evidence that neurosteroids modulate the dopaminergic system in women, but they leave open the question of gonadal steroid hormone modulation of human reward neural circuitry.
Previous functional MRI (fMRI) and lesion studies distinguish specific functions for different components of the highly interconnected brain reward system, providing clues for likely neural structures on which gonadal steroid may have an effect. fMRI studies have proposed that the amygdala and the ventral striatum respond to predictors of reward (rather than to the reward itself), whereas the medial part of the prefrontal cortex is, conversely, more activated at the time of reward delivery (20, 21). Lesion studies confirm the crucial importance of the basolateral complex of the amygdala, the orbitofrontal cortex, and the ventral striatum for coding stimulus reward value and expectancies of reinforcers (21–24). Indeed, rats and monkeys with neurotoxic lesions of the amygdala or the orbitofrontal cortex fail to show devaluation effects when the incentive value of a food is reduced by satiation (25, 26). A recent fMRI study using a Pavlovian conditioning paradigm also indicates that predictive representations in the amygdala and orbitofrontal cortex are linked to specific reward value, because these brain regions are activated during pairing of two visual conditioned stimuli with two food odors but respond less to devaluated cues than to nondevaluated cues for food items after participants consumed one food to satiation (24). Importantly, the brain network coding reward value receives direct inputs from dopaminergic neurons, which send signals related to reward uncertainty and to error prediction (the latter representing the discrepancy between the probability with which reward is predicted and the actual outcome). These signals vary differentially with reward probabilities and may help to create an updated representation of reward value (27).
To investigate directly the neurophysiological effects of gonadal steroid hormones on the human reward system, we studied women in a counterbalanced, repeated-measures design during the midfollicular and luteal phases of the menstrual cycle using event-related fMRI and a monetary reward task that distinguishes neural concomitants of anticipating uncertain rewards from those of reward outcome (28). We also assessed plasma estradiol and progesterone levels as correlates of reward-related activation and tested a group of men with the same task, allowing us to identify components of reward neural circuitry that are sensitive to levels of circulating gonadal steroids and to investigate between-sex differences in reward activation. If specific components of the reward system depend on gonadal steroids, we would expect to observe a modulation of fMRI activity in brain regions such as the amygdala, orbitofrontal cortex, and ventral striatum by menstrual cycle phases and by levels of gonadal steroids as well as neurofunctional differences between women and men that are consistent with menstrual cycle findings and correlations with gonadal steroid levels.
Results
Hormone Measures and Clinical Ratings.
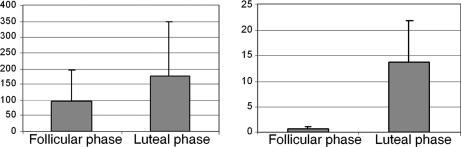
Plasma estradiol and progesterone levels were both significantly higher during the luteal than follicular phase (paired t tests, P < 0.01 and P < 0.0005, respectively) (Fig. 1). Symptom rating scores (Beck Depression Inventory and Premenstrual Tension Syndrome rating scale) did not differ across menstrual cycle phases and confirmed the absence of clinically relevant symptoms during each study phase. Similarly, daily self-ratings of the severity of several mood and behavioral symptoms recorded for at least 2 months confirmed the absence of significant menstrual cycle-related symptoms in all women studied.
Fig. 1.
Serum steroid hormone levels. Shown are mean estradiol (Left; in pg/ml) and progesterone (Right; in ng/ml) levels during the follicular and luteal phases. Estradiol and progesterone levels were both higher during the luteal phase than during the follicular phase.
fMRI Results.
Comparison between menstrual cycle phases.
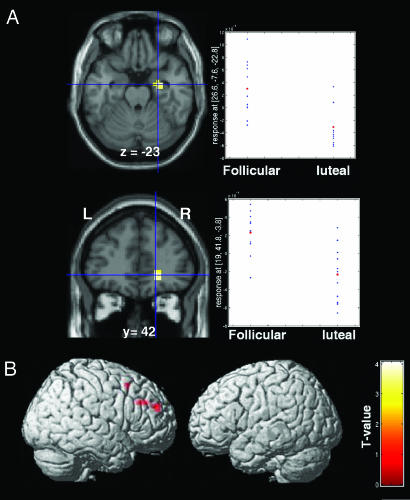
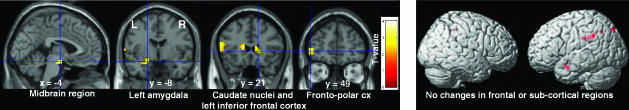
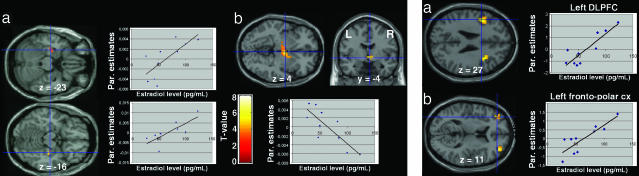
Because the first goal of this study was to assess menstrual cycle influences on reward processing, the main comparison of interest was between the follicular and luteal phases. The order of the scans was counterbalanced across women. As predicted, we found that the reward system, especially the amygdala, orbitofrontal cortex, striatum, and midbrain, was modulated by menstrual cycle phase. Indeed, during anticipation of uncertain rewards, the right amygdala and the right superior orbitofrontal cortex, in the depth of the medial orbital sulcus, between the medial aspect of the anterior prefrontal cortex (BA 10) and the middle frontal gyrus (BA 11), were activated more during the follicular than luteal phase, whereas the right dorsolateral prefrontal and anterior cingulate cortices were activated more during the luteal phase compared with the follicular phase [Fig. 2; see also supporting information (SI) Table 1]. At the time of reward delivery, increased BOLD response was observed in the midbrain, left amygdala, heads of the caudate nuclei, left inferior frontal gyrus, and left fronto-polar cortex in the follicular vs luteal phases of the cycle (Fig. 3 and SI Table 2). Conversely, activations of the left intraparietal region and left inferior temporal cortex were greater during the luteal than the follicular phase. No changes were observed in frontal or subcortical regions.
Fig. 2.
Cross-menstrual cycle phase differences in BOLD response during anticipation of uncertain rewards. (A) Statistical maps overlaid onto structural MRI showing BOLD fMRI responses greater in follicular phase than in luteal phase in the right amygdala (Upper) and orbitofrontal cortex (Lower). To the right of each map is shown distributions of BOLD signal response for each woman. (B) Greater right dorsolateral prefrontal cortex activity in the luteal phase compared with the follicular phase.
Fig. 3.
Cross-menstrual cycle phase differences in BOLD response at the time of reward outcome. (Left) Greater BOLD response during follicular phase than during luteal phase in midbrain, left amygdala, heads of the caudate nuclei, left inferior frontal gyrus, and left fronto-polar cortex. (Right) Greater BOLD response during luteal phase than during follicular phase was observed in the left intraparietal region and the inferior-temporal cortex.
Between-sex comparisons.
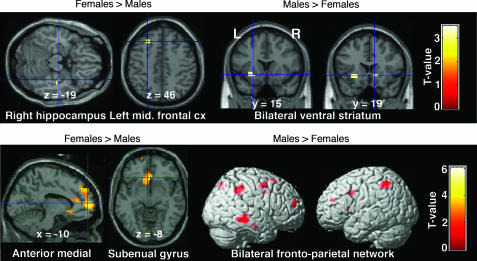
We compared men (tested once) and women on their first day of testing (to equalize task training and exposure in both groups) to investigate sex differences regardless of menstrual cycle effect (Fig. 4; SI Table 3). During anticipation of uncertain rewards, the right hippocampus and left middle frontal gyrus were more robustly activated in women (Fig. 4 Upper). In contrast, men activated the ventral putamen bilaterally more than women. At the time of reward delivery, women more strongly activated a large anterior medial prefrontal cortex/rostral anterior cingulate cortex region, the subgenual gyrus (Fig. 4 Lower), and a small left amygdala region (not shown in Fig. 4). The hypothalamus was also more activated in women at a lower threshold of P < 0.01 (uncorrected, random effects) whereas men showed more activation in a bilateral prefronto-parietal network, the supplementary motor area, and the right inferior temporal region.
Fig. 4.
Between-sex differences in brain activity. (Upper) During anticipation of uncertain rewards. Statistical maps showing greater right hippocampal and left middle frontal gyrus activity in women than in men (Left) whereas men showed greater activation (Right) in bilateral ventral striatum. (Lower) At the time of reward delivery. Women showed more activation in anterior medial prefrontal cortex and subgenual gyrus compared with men (Left), who, in turn, showed more activation in a bilateral fronto-parietal network, the right inferior temporal cortex, and the supplementary motor area (Right).
Separate analyses of men compared with first-day scans of women in their luteal phase and to first-day scans of women in their follicular phase corroborated results from the first-day scans of all women in the study taken together.
Correlation with gonadal steroid levels in women.
To pinpoint specific effects of circulating gonadal steroids on brain regions differentially activated across the menstrual cycle or displaying sex differences, we investigated the relationship between brain activation and plasma progesterone/estradiol levels. During the follicular phase only estradiol levels were considered because plasma progesterone levels were at the lower limits of the assay sensitivity/detectability. A number of correlations were noted in regions either identified in our cross-menstrual cycle analysis or reported in prior studies to be modulated by ovarian steroids. In the follicular phase, during anticipation of uncertain rewards, positive correlation with estradiol was found in the amygdalo-hippocampal complex bilaterally, whereas negative correlations with estradiol levels were demonstrated in a network including the hypothalamus (peak of activity falling in the paraventricular hypothalamic nuclei and extending into neighboring regions), bilateral fronto-polar cortex, thalamus, dorsolateral prefrontal cortices, and anterior cingulate cortex (SI Table 4 and Fig. 5 Left). At time of reward delivery, positive correlation with estradiol levels was found in the bilateral fronto-polar and dorsolateral prefrontal cortices (Fig. 5 Right). These correlations show specific actions of estrogens on brain activation because progesterone level is minimal in the follicular phase.
Fig. 5.
Statistical maps of regression analyses between brain activity and estradiol level during the follicular phase of the menstrual cycle. (Left) During anticipation of uncertain rewards. Estradiol level correlated positively with activity of the bilateral amygdalo-hippocampal complex (a) and negatively with activity of the hypothalamus (b). (Right) At the time of rewarded outcome relative to no reward. Estradiol level correlated positively with activity of the bilateral dorsolateral (a) and fronto-polar (b) cortices.
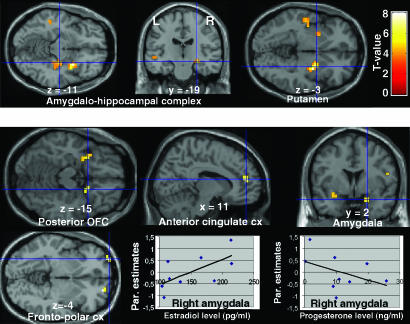
During the luteal phase we performed correlation analyses with both estradiol and progesterone levels (SI Table 5). First, during anticipation of uncertain rewards, no significant correlation (positive or negative) was found between brain activation and estrogen levels. However, right amygdalo-hippocampal complex and bilateral putamen activity correlated positively with progesterone (Fig. 6 Upper). Thus, unlike the follicular phase, when anticipation of uncertain rewards provoked estradiol-linked activation in the amygdalo-hippocampal complex, in the luteal phase this activation was driven by progesterone level.
Fig. 6.
Regression analysis between brain activity and estradiol/progesterone levels during the luteal phase of the menstrual cycle. (Upper) During anticipation of uncertain rewards, progesterone level correlated positively with activity of the bilateral amygdalo-hippocampal complex and putamen. (Lower) Interactions between the effects of estradiol and progesterone levels on brain activity in the posterior orbitofrontal, anterior cingulate, and fronto-polar cortices, and amygdala at the time of the rewarded outcome. The graphs show such interactions in the right amygdala.
Second, at time of reward delivery in the luteal phase, estradiol level correlated positively with activity of a network that included the posterior orbitofrontal cortex and the amygdala and correlated negatively with the response of the anterior cingulate and fronto-polar cortices. Conversely, progesterone level correlated positively with cingulate and fronto-polar cortex activity, but negatively with activity in posterior orbitofrontal cortex and amygdala. Thus, at time of reward delivery, estrogen and progesterone levels act in opposite fashion on the cingulate, fronto-polar, posterior orbitofrontal and amygdala. This was confirmed by a formal interaction analysis between the effects of estrogen and progesterone levels on brain activation (Fig. 6 Lower), revealing brain regions in which correlations with estradiol were opposite in direction and significantly different from those with progesterone.
Discussion
These data show that ovarian steroids modulate reward-evoked neural activity in humans which has not been reported previously. Our results establish that components of a highly interconnected network of brain areas including orbitofrontal cortex, amygdala, striatum, and midbrain are modulated by the menstrual cycle during distinct stages of reward processing (anticipation vs. reward outcome) (Figs. 2 and 3). The results also indicate that some of these findings can be related to levels of circulating gonadal steroids and that certain between-sex differences in reward processing can be better understood within this context.
First, regarding the effect of menstrual cycle phase during anticipation of uncertain rewards, women in the follicular phase, compared with the luteal phase, showed more robust activation in the orbitofrontal cortex and the amygdala (Fig. 2), two regions that are anatomically and functionally connected and part of a network involved in both autonomic control and emotion. Our findings are particularly interesting in light of evidence from rodents, humans, and nonhuman primates that (i) the basolateral complex of the amygdala and the orbitofrontal cortex are crucial in coding stimulus reward value and expectancies of reinforcers (21–24) and that (ii) in animals, selective lesions of the basolateral amygdala or the orbitofrontal cortex impair the acquisition of drug seeking and increase the choice of small, immediate rewards over larger, delayed rewards, supporting the role of these structures in delaying reinforcement (29, 30). Because addictive drugs and gambling share common mechanisms for reinforcing properties (27), our results suggest that the orbitofrontal cortex and amygdala may be crucial sites of hormonal modulation that could underlie women's greater subjective responses to addictive drugs during the follicular phase (11, 17, 18). Damage to these regions may also result in disturbed social and emotional behavior and abnormalities in strategic decision-making. The central amygdala is reciprocally connected with hypothalamic nuclei and modulates pituitary hormone secretion and reproductive functions. The amygdala and hypothalamus also project to the brainstem, sending inputs to the limbic system for the control of the autonomic nervous system. In addition to its connections to the amygdala, the orbitofrontal cortex may also influence arousal, autonomic, and endocrine functions through its connections with the hypothalamus and ventral tegmental area (31). Although it appears that the amygdala-orbital cortex findings are in the right hemisphere for reward uncertainty and are on the left during reward delivery, several of these apparent lateralizations disappear with a more liberal statistical threshold, suggesting a need for further study of potential lateralization effects.
Second, at the time of reward delivery, women in the follicular phase, relative to luteal phase, activated the heads of the caudate nuclei, midbrain region, left amygdala, and left fronto-polar cortex (Fig. 3). In contrast, no changes in subcortical and frontal brain regions were observed during luteal phase at the time of reward outcome. Thus, the reward system was more responsive during the follicular phase, both at the time of the rewarded outcome and during reward anticipation. These findings are interesting in light of the facts that ovariectomy decreases, whereas administration of estradiol potentiates, release of dopamine in rat striatum (32) and that dopaminergic innervation to the striatum may have a specific role in drug-seeking behavior (33, 34).
Sex differences are due to a combination of genetic and hormonal events that begin early during development. Recent fMRI findings highlight a role for brain structures such as the amygdala in mediating sex differences in emotionally related behavior. For example, men show stronger response to visual sexual stimuli and women retain stronger and more vivid memories for emotional events. Normal women demonstrate greater responses to aversive arousing stimuli in amygdala and orbitofrontal cortex compared with men (35) but show less activation of the amygdala and hypothalamus with visual sexually arousing stimuli (36). Our study let us investigate whether brain activation in the domain of monetary reward varies with sex differences and with gonadal steroid levels. We found sex differences in subcortical regions (amygdalo-hippocampal complex and ventral striatum) that were related to changes in gonadal steroid levels. Indeed, the activity in the right amygdalo-hippocampal complex, which was more robust in the midfollicular phase when estradiol is unopposed by progesterone (Fig. 2), was also more robustly engaged in women than men (Fig. 4 Upper) and was positively correlated with hormone levels during both menstrual cycle phase (Figs. 5 Left and 6 Lower).
These results extend to the reward domain recent fMRI reports of a modulation of the amygdala and orbitofrontal cortex by the hormonal cycle both in premenopausal and younger women during passive viewing of negative stimuli (pictures or words) (37, 38). Although these recent studies did not compare sex differences in brain activity and did not assess directly hormonal levels, and therefore could not specify whether the observed changes correlated with estrogen and/or progesterone, they elegantly demonstrate that generally arousing stimuli may modulate similar brain networks across menstrual cycle phases. Our results add to the understanding of sex differences in emotion-related behavior and show that the response of the amygdalo-hippocampal complex to different types of arousing stimuli depends on both sex differences and the actions of gonadal steroids. These sex differences in the amygdala's function may be related to structural and developmental sex differences, such as the higher concentration of sex hormone receptors and larger size of the amygdala in men (39), as well as to circulating estrogen and progesterone levels.
Previous neuroimaging studies of reward that grouped men and women together proposed different functions for the ventral striatum and the anterior medial prefrontal cortex, linking the former to reward anticipation and the latter to the time of reward outcome (20, 21). Extending these reports to anticipation of rewards with maximal uncertainty, we recently found robust ventral striatum activation in a large group of subjects that included both men and women (scanned without monitoring their menstrual cycle) (28). Our current data suggest that these findings may, in part, be driven by sex-specific differences, with men showing higher ventral striatal activity and women exhibiting higher anterior medial prefrontal cortex activity (Fig. 4). Thus, reward studies must consider both sex differences and gonadal steroid actions at the time of testing. Our findings extend to humans' previous observations in animals that the actions of estrogen and progesterone on midbrain dopaminergic projections to the striatum are sexually dimorphic and involve both prodopaminergic and antidopaminergic effects (5, 6, 32).
Finally, BOLD response in a number of brain areas correlated with gonadal steroid levels. Apart from the previously discussed amygdalo-hippocampal complex, the hypothalamus and lateral prefrontal cortex displayed interesting patterns of correlation with hormonal levels. First, hypothalamic activity negatively correlated with estrogen levels during anticipation of rewards with maximal uncertainty in the follicular phase (Fig. 5 Left). The hypothalamus is regulated by both estrogen and progesterone and receives direct dopaminergic inputs via projections from tuberohypophysial dopamine neurons as well as indirect inputs from reward centers (e.g., amygdala). Conversely, hypothalamic neurons modulate reward-seeking through direct projections to midbrain dopaminergic neurons (40). Second, at the time of reward outcome, the dorsolateral prefrontal and the fronto-polar cortex responses correlated positively with estrogen levels during the follicular phase (Fig. 5 Right). These results are consistent with the view that prefrontal cortex is a prime mediator of estrogen's effects on cognition, as revealed by an early PET study in young women under pharmacological ovarian suppression (41), by comparison between menopausal women with and without hormone replacement therapy (42, 43), and by the fact that ovarian steroids are potent regulators of dopaminergic innervation to the prefrontal cortex. Ovariectomy reduces, and subsequent estrogen and progesterone replacement restores, the density of axons immunoreactive for tyrosine hydroxylase in monkey dorsolateral prefrontal cortex (44). Estradiol treatment, which is associated with changes in dorsolateral prefrontal cortex structural plasticity, also reverses age-related impairment in prefrontal cognitive function in ovariectomized monkeys (45).
We believe that the changes in brain activity we observed are mediated by sex steroids because activity in reward-related brain regions was both modulated by menstrual cycle phase and correlated with gonadal steroid hormone levels. However, the observed BOLD changes are not simply explained by the correlations. Indeed, the direction of many of these relationships, at first glance, appears to be opposite to that which might be predicted by the average hormone levels during each menstrual cycle phase along with the corresponding across-phase changes in BOLD signal. For example, both estrogen and progesterone levels were higher in the luteal phase; however, the BOLD signal in many regions was not only higher during the follicular phase, but also positively correlated with estrogen level. Thus, simple correlations between BOLD signal and a single hormone level during a specific menstrual cycle phase do not adequately explain the manifold neuroregulatory interactions between estrogen and progesterone that presumably led to the differences we observed across the menstrual cycle. Preclinical data (11) demonstrate that estrogen and progesterone interact to affect neuronal signaling and that the effects of estrogen differ in the presence of progesterone (i.e., in the luteal phase), consistent with the present results.
Our findings provide compelling evidence for a neurofunctional effect of the menstrual cycle on the human reward system, and they bring important new sources of information to bear on previous findings in rodents demonstrating interactions between dopamine and female gonadal steroid hormones (12, 13). It is interesting to note that, from an evolutionary perspective, the increased availability, receptivity, and desire that may occur during the ovulatory period has been thought to facilitate procreation. Thus, increased activity of specific components of the reward system during the follicular phase may modulate basic behavioral functions of reward, such as approach behavior during reward anticipation as well as consummatory and hedonic behavior at the time of reward delivery. This may elucidate the neurobiological substrates of menstrual cycle influence on different types of motivated behavior (e.g., sexual preference and drug abuse), as evidenced by women's stronger attraction to testosterone-dependent traits (masculine voice and virile face shape) during the most fertile phase of the menstrual cycle (late-follicular) (46). Moreover, in female rats it has been suggested that gonadal hormone modulation of dopamine activity in the striatum may have evolved to facilitate reproductive success by enhancing the pacing of sexual behavior (47). The present work offers a step toward extending understanding of the complex interactions between the reward system and gonadal steroid hormones to humans.
Materials and Methods
Subjects.
Subjects provided written informed consent as approved by the National Institute of Mental Health Institutional Review Board. Thirteen right-handed healthy women (mean age = 29, range = 6) were scanned twice during presentation of images of “slot machines.” Each woman was tested once during the midfollicular phase (days 4–8 after the onset of menses) and once during the luteal phase (days 6–10 after the luteinizing hormone surge as determined by urinary luteinizing hormone assay). Two women initially enrolled were excluded; one woman had an anovulatory menstrual cycle (progesterone levels <0.3 ng/ml during the mid-luteal phase), and the other reported the presence of depressive symptoms in her luteal daily ratings. One woman with an estradiol level of 428 pg/m (more than four times the mean of the group) was excluded from the steroid level correlational analysis before the correlations were performed to avoid spurious results. This subject was included in the between-sex and between-menstrual cycle analyses because she had a normal ovulatory menstrual cycle, was scanned within the time window of our study protocol, and had an estradiol level within the physiological range for the follicular phase. Moreover, excluding her from these analyses did not significantly change the results. The order of the scans was counterbalanced across women (5 of the 11 women included were first scanned in their follicular phase). The inclusion criteria were as follows: no hormonal medication within the last 6 months, regular menstrual cycle duration for the women (average cycle length = 29 days), no CNS-active medication or illicit drugs, and no regular consumption of nicotine or alcohol. Thirteen right-handed men matched with the women for age (mean = 27, range = 5) and level of education were scanned (once) following the same procedure. No subject had a history of gambling, and all were free of past and present neurologic and psychiatric diseases as determined by a normal medical history, physical examination, laboratory tests, and structured psychiatric diagnostic interview. Subjects were paid for participating and earned extra money for performing the task described below. Subjects were told that they would earn a percentage of each of the $10 and $20 bills presented on the screen but were not told the exact percentage.
Clinical Assessments.
Study participants were selected to be free of clinically significant menstrually related symptoms to avoid potential confounding effects of concurrent mood changes on the fMRI data. Their Beck Depression Inventory scores ranged from 0 to 4 and may not typify the general population. The ratings administered to the women include (i) daily symptom self-ratings for at least 2 months consisting of a visual analogue scale and a six-point Likert-type scale, both rating the severity of several mood and behavioral symptoms and used to confirm the presence or absence of menstrual cycle-related symptoms; and (ii) on the day of the procedure the Beck Depression Inventory and the Premenstrual Tension Syndrome rating scale (both self and objective versions).
Hormone Assessment.
A venous blood sample was drawn before each MRI investigation to determine estradiol and progesterone. Parameters were measured by commercially available immunometric assays with an automated chemiluminescent immunoassay system. Progesterone level was not obtained from one woman.
Experimental Paradigm.
We used an event-related monetary reward task (see SI Methods and ref. 28) during presentation of “slot machines” that varied reward probability and magnitude. Briefly, experimental trials were divided into two phases: anticipation, in which spinners rotated and stopped successively, and an outcome phase presenting the pictures of $0, or $10 or $20 bills. During these two phases, a pie chart indicated the probability of winning a certain amount of money. Subjects pressed a response button at the cue and outcome.
fMRI Data Acquisition.
Imaging was conducted on a General Electric 3-Tesla scanner with a real-time functional imaging upgrade. Functional imaging involved a series of 29 contiguous 3.3-mm axial slices per volume collected over six runs, plus eight “dummy” volumes at the start of each session. These functional scans used an echo-planar single shot repetition time gradient echo T2* weighting (echo-planar imaging repetition-time sequence: real time, 2,300 ms; echo time, 23 ms; field of view, 24 cm; 64 × 64 matrix; voxel size, 3.75 × 3.75 × 3.3; flip angle, 90°). Signal dropout in orbitofrontal cortex from susceptibility artifact was reduced with local high-order z-shimming performed in the axial direction and by tilting subjects' heads 30° relative to the AC-PC line (see SI Methods and SI Fig. 7). High-resolution T1-weighted structural scans were acquired by using a magnetization prepared rapid gradient echo sequence (180 sagittal slices of 1 mm; field of view, 256 mm; number of excitations, 1; repetition time, 11.4 ms; echo time, 4.4 ms; matrix, 256 × 256; inversion time, 300 ms).
Image Analysis.
Data were analyzed by using Statistical Parametric Mapping (SPM99). Preprocessing included slice timing and motion correction, coregistration to a standard template, alignment to the first volume for each subject, and spatial normalization to the Montreal Neurological Institute T1-weighted template image. The data were then smoothed with a 10-mm FWHM Gaussian kernel.
The BOLD response to each event type was modeled as δ functions at the appearance of the slot (1 s) and at the outcome (2 s), and as a rectangular pulse during the presence of the slot machine on the screen (15 s), and was convolved with a canonical hemodynamic response function. Within-subject time series modeling accounted for the following 15 regressors: four at the time of appearance of the slot machine (one for each stimulus type), four during the delay, and seven regressors at the outcome (rewarded vs. nonrewarded × 3, plus 100% chance of no reward). In the current analyses, only the anticipatory (delay) and outcome phases were assessed. The default high-pass filter was applied to the time series. Condition-specific estimates of neural activity (β) were computed independently at each voxel for each subject by using the general linear model. We used random-effects models for menstrual cycle, sex differences, and gonadal steroid correlational analyses, and we set a threshold of P < 0.005, uncorrected. Anticipatory and outcome phases were assessed with two comparisons: (i) anticipation of rewards with maximal uncertainty [DelaySlot__B > Delay_Slot_A, i.e., (Delay: P = 0.5 $20, P = 0.5 $0) > (Delay: P = 0.25 $20, P = 0.75 $0)]; and (ii) response at the time of rewarded outcome relative to no reward delivery ($20_slot_A+$20_slot_B+$10_slot_C)/3 > $0_slot_D).
For the correlational analysis between gonadal steroids and brain regions activated during reward anticipation or at the time of reward delivery, we entered the progesterone and estradiol levels of each subject as regressors. During the follicular phase only estradiol levels were considered because progesterone levels were at the lower limits of the assay sensitivity. During the luteal phase, correlational analyses were performed with positive and negative estradiol and progesterone levels as well as with the interaction term revealing brain regions in which estradiol levels act in opposite direction and significantly differ from those with progesterone.
Supplementary Material
Acknowledgments
We thank R. Olsen and P. Koch for research assistance. This work was supported by the National Institute of Mental Health intramural research program (K.F.B.). J.-C.D. was supported in part by grants from the Fondation pour la Recherche Médicale and by a FPG Young Investigator International Reintegration Grant from the European Union.
Abbreviation
- fMRI
functional MRI.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0605569104/DC1.
References
- 1.McEwen B. Recent Prog Horm Res. 2002;57:357–384. doi: 10.1210/rp.57.1.357. [DOI] [PubMed] [Google Scholar]
- 2.McEwen BS, Alves SE. Endocr Rev. 1999;20:279–307. doi: 10.1210/edrv.20.3.0365. [DOI] [PubMed] [Google Scholar]
- 3.Pfaff DW, Vasudevan N, Kia H, Zhu Y, Chan J, Garey J, Morgan M, Ogawa S. J Steroid Biochem Mol Biol. 2000;74:365–373. doi: 10.1016/s0960-0760(00)00114-x. [DOI] [PubMed] [Google Scholar]
- 4.Pfaff D. J Endocrinol. 2005;184:447–453. doi: 10.1677/joe.1.05897. [DOI] [PubMed] [Google Scholar]
- 5.Becker JB, Cha JH. Behav Brain Res. 1989;35:117–125. doi: 10.1016/s0166-4328(89)80112-3. [DOI] [PubMed] [Google Scholar]
- 6.Becker JB, Robinson T, Lorenz K. Eur J Pharmacol. 1982;80:65–72. doi: 10.1016/0014-2999(82)90178-9. [DOI] [PubMed] [Google Scholar]
- 7.Creutz LM, Kritzer MF. J Comp Neurol. 2004;476:348–362. doi: 10.1002/cne.20229. [DOI] [PubMed] [Google Scholar]
- 8.Lynch WJ, Roth ME, Mickelberg JL, Carroll ME. Pharmacol Biochem Behav. 2001;68:641–646. doi: 10.1016/s0091-3057(01)00455-5. [DOI] [PubMed] [Google Scholar]
- 9.Dluzen D, Horstink M. Endocrine. 2003;21:67–75. doi: 10.1385/endo:21:1:67. [DOI] [PubMed] [Google Scholar]
- 10.Lynch WJ, Roth ME, Mickelberg J, Carroll M. Pharmacol Biochem Behav. 2001;68:641–646. doi: 10.1016/s0091-3057(01)00455-5. [DOI] [PubMed] [Google Scholar]
- 11.Jackson L, Robinson TE, Becker JB. Neuropsychopharmacology. 2006;31:129–138. doi: 10.1038/sj.npp.1300778. [DOI] [PubMed] [Google Scholar]
- 12.Diaz-Veliz G, Butron S, Benavides MS, Dussaubat N, Mora S. Pharmacol Biochem Behav. 2000;66:887–892. doi: 10.1016/s0091-3057(00)00283-5. [DOI] [PubMed] [Google Scholar]
- 13.Diaz-Veliz G, Benavides MS, Butron S, Dussaubat N, Mora S. Pharmacol Biochem Behav. 1999;62:21–29. doi: 10.1016/s0091-3057(98)00097-5. [DOI] [PubMed] [Google Scholar]
- 14.Mani SK, Allen JM, Clark JH, Blaustein J, O'Malley BW. Science. 1994;265:1246–1249. doi: 10.1126/science.7915049. [DOI] [PubMed] [Google Scholar]
- 15.Mani SK, Blaustein JD, O'Malley BW. Horm Behav. 1997;31:244–255. doi: 10.1006/hbeh.1997.1393. [DOI] [PubMed] [Google Scholar]
- 16.Blaustein JD. Ann NY Acad Sci. 2003;1007:238–250. doi: 10.1196/annals.1286.023. [DOI] [PubMed] [Google Scholar]
- 17.Evans SM, Haney M, Foltin R. Psychopharmacology (Berlin) 2002;159:397–406. doi: 10.1007/s00213-001-0944-7. [DOI] [PubMed] [Google Scholar]
- 18.Justice J, de Wit H. Psychopharmacology (Berlin) 1999;145:67–75. doi: 10.1007/s002130051033. [DOI] [PubMed] [Google Scholar]
- 19.Hafner H. Psychoneuroendocrinology. 2003;28(Suppl 2):17–54. doi: 10.1016/s0306-4530(02)00125-7. [DOI] [PubMed] [Google Scholar]
- 20.Knutson B, Fong G, Adams C, Varner J, Hommer D. NeuroReport. 2001;12:3683–3687. doi: 10.1097/00001756-200112040-00016. [DOI] [PubMed] [Google Scholar]
- 21.O'Doherty J. Curr Opin Neurobiol. 2004;14:769–776. doi: 10.1016/j.conb.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 22.Holland P, Gallagher M. Curr Opin Neurobiol. 2004;14:148–155. doi: 10.1016/j.conb.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 23.Schoenbaum G, Setlow B, Saddoris M, Gallagher M. Neuron. 2003;39:855–867. doi: 10.1016/s0896-6273(03)00474-4. [DOI] [PubMed] [Google Scholar]
- 24.Gottfried J, O'Doherty J, Dolan RJ. Science. 2003;301:1104–1107. doi: 10.1126/science.1087919. [DOI] [PubMed] [Google Scholar]
- 25.Blundell P, Hall G, Killcross S. J Neurosci. 2003;23:7702–7709. doi: 10.1523/JNEUROSCI.23-20-07702.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Malkova L, Gaffan D, Murray E. J Neurosci. 1997;17:6011–6020. doi: 10.1523/JNEUROSCI.17-15-06011.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fiorillo CD, Tobler P, Schultz W. Science. 2003;299:1898–1902. doi: 10.1126/science.1077349. [DOI] [PubMed] [Google Scholar]
- 28.Dreher JC, Kohn P, Berman KF. Cereb Cortex. 2006;16:561–573. doi: 10.1093/cercor/bhj004. [DOI] [PubMed] [Google Scholar]
- 29.Winstanley CA, Theobald D, Cardinal R, Robbins TW. J Neurosci. 2004;24:4718–4722. doi: 10.1523/JNEUROSCI.5606-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hutcheson DM, Everitt BJ. Ann NY Acad Sci. 2003;1003:410–411. doi: 10.1196/annals.1300.038. [DOI] [PubMed] [Google Scholar]
- 31.Price JL. Ann NY Acad Sci. 1999;877:383–396. doi: 10.1111/j.1749-6632.1999.tb09278.x. [DOI] [PubMed] [Google Scholar]
- 32.DiPaolo T. Rev Neurosci. 1994;5:27–42. [Google Scholar]
- 33.Bradberry CW. J Neurosci. 2000;20:7109–7115. doi: 10.1523/JNEUROSCI.20-18-07109.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ito R, Dalley J, Robbins TW, Everitt BJ. J Neurosci. 2002;22:6247–6253. doi: 10.1523/JNEUROSCI.22-14-06247.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bradley M, Codispoti M, Sabatinelli D, Lang P. Emotion. 2001;1:300–319. [PubMed] [Google Scholar]
- 36.Hamann S, Herman R, Nolan C, Wallen K. Nat Neurosci. 2004;7:411–416. doi: 10.1038/nn1208. [DOI] [PubMed] [Google Scholar]
- 37.Goldstein JM, Jerram M, Poldrack R, Ahern T, Kennedy D, Seidman L, Makris N. J Neurosci. 2005;25:9309–9316. doi: 10.1523/JNEUROSCI.2239-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Protopopescu X, Pan H, Altemus M, Tuescher O, Polanecsky M, McEwen B, Silbersweig D, Stern E. Proc Natl Acad Sci USA. 2005;102:16060–16065. doi: 10.1073/pnas.0502818102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Newman S. Ann NY Acad Sci. 1999;877:242–257. doi: 10.1111/j.1749-6632.1999.tb09271.x. [DOI] [PubMed] [Google Scholar]
- 40.Harris G, Wimmer M, Aston-Jones G. Nature. 2005;437:556–559. doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- 41.Berman KF, Schmidt P, Rubinow D, Danaceau M, Van Horn J, Esposito G, Ostrem J, Weinberger DR. Proc Natl Acad Sci USA. 1997;94:8836–8841. doi: 10.1073/pnas.94.16.8836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Keenan P, Ezzat W, Ginsburg K, Moore G. Psychoneuroendocrinology. 2001;26:577–590. doi: 10.1016/s0306-4530(01)00013-0. [DOI] [PubMed] [Google Scholar]
- 43.Resnick SM, Maki PM. Ann NY Acad Sci. 2001;949:203–214. doi: 10.1111/j.1749-6632.2001.tb04023.x. [DOI] [PubMed] [Google Scholar]
- 44.Kritzer M, Kohama S. J Comp Neurol. 1998;395:1–17. [PubMed] [Google Scholar]
- 45.Hao J, Rapp P, Leffler A, Leffler S, Janssen W, Lou W, McKay H, Roberts J, Wearne SL, Hof P, Morrison J. J Neurosci. 2006;26:2571–2578. doi: 10.1523/JNEUROSCI.3440-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Feinberg D, Jones B, Law Smith M, Moore F, DeBruine L, Cornwell R, Hillier S, Perrett D. Horm Behav. 2006;49:215–222. doi: 10.1016/j.yhbeh.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 47.Becker JB. Pharmacol Biochem Behav. 1999;64:803–812. doi: 10.1016/s0091-3057(99)00168-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.