Abstract
The advent of serum prostate-specific antigen (PSA) as a biomarker has enabled early detection of prostate cancer and, hence, improved clinical outcome. However, a low PSA is not a guarantee of disease-free status, and an elevated PSA is frequently associated with a negative biopsy. Therefore, our goal is to identify molecular markers that can detect prostate cancer with greater specificity in body fluids such as urine or blood. We used the RT-PCR differential display method to first identify mRNA transcripts differentially expressed in tumor vs. patient-matched nontumor prostate tissue. This analysis led to the identification of 44 mRNA transcripts that were expressed differentially in some but not all tumor specimens examined. To identify mRNA transcripts that are differentially expressed in most tumor specimens, we turned to differential display of pooled tissue samples, a technique we name averaged differential expression (ADE). We performed differential display of mRNA from patient-matched nontumor vs. tumor tissue, each pooled from 10 patients with various Gleason scores. Differentially expressed mRNA transcripts identified by ADE were fewer in number, but were expressed in a greater percentage of tumors (>75%) than those identified by differential display of mRNA from individual patient samples. Differential expression of these mRNA transcripts was also detected by RT-PCR in mRNA isolated from urine and blood samples of prostate cancer patients. Our findings demonstrate the principle that specific cDNA probes of frequently differentially expressed mRNA transcripts identified by ADE can be used for the detection of prostate cancer in urine and blood samples.
Keywords: body fluids, differential display
Early detection is important for effective treatment and management of cancer. Prostate cancer is the most commonly diagnosed nonskin malignancy and the second leading cause of cancer death in American men. For two decades, serum prostate-specific antigen (PSA) has been widely used as a marker for prostate cancer detection. However, elevated serum PSA lacks the specificity required to distinguish prostate cancer from other prostatic disorders, such as benign prostatic hyperplasia (BPH) and prostatitis (1, 2). Furthermore, PSA also lacks the sensitivity to detect a large fraction of early-stage tumors, because >15% of men with a normal serum PSA level have biopsy-proven prostate cancer (3). Histological confirmation of prostate cancer requires multiple biopsies of the prostate using procedures that are too invasive to repeat at regular intervals. Therefore, a screening test for prostate cancer detection requires a noninvasive approach that is specific and sensitive.
With these objectives, we used differential display (DD) (4, 5) to identify mRNA transcripts that are expressed differentially in tumor compared with matched nontumor prostate tissues from patients who underwent radical prostatectomy. DD analysis has been widely used to identify genes that are differentially expressed in cells and tissue samples (4, 6, 7). This sensitive technique requires small amounts of starting RNA, and offers a great potential for rapid identification of overexpressed and down-regulated messages and low-abundance mRNAs that are involved in many of the regulatory processes of the cell (8). DD analysis of individual tumors provided information on a number of genes, but the differential expression of several of these genes could be verified by RT-PCR in <20% of tumors. We then investigated the use of DD to compare pooled tumors vs. their pooled nontumor contralateral prostate specimens; we reasoned that this approach would more likely reveal genes differentially expressed in the majority of samples. DD of pooled tumors is referred to as averaged differential expression (ADE). ADE, as expected, identified fewer genes than did DD of individual tumors. But their expression was confirmed in >75% of the tumors under study. Furthermore, gene changes identified by ADE were readily detectable in urine and blood of patients with advanced prostate cancer. Thus, ADE offers an effective strategy for the identification of genes whose expression is altered in a wide population of patients with a heterogeneous cancer such as that of the prostate. The relative levels of overexpressed and down-regulated genes identified in body fluids may provide a viable option for reliable and, possibly, early detection of prostate cancer.
Results
Identification of Genes Differentially Expressed Between Tumor and Nontumor Prostate Tissue from Radical Prostatectomy Patients.
In an effort to identify biomarkers for prostate cancer detection, we performed DD on tumor and matched nontumor prostate tissues from prostatectomy patients to investigate differences in expression of numerous genes (5). DD was performed on tissues from seven patients representing Gleason grades 3 + 3 (three patients), 3 + 4 (one patient), 4 + 4 (two patients), and 5 + 4 (one patient), by using 24 different anchor and arbitrary primer sets for cDNA amplification. Using this approach, we identified 286 differentially expressed cDNA bands (191 overexpressed and 95 down-regulated). Of these 286 bands, 44 (37 overexpressed and 7 down-regulated) have been extracted from the gels and sequenced to date. The accession number and gene identity of each of these sequences is presented in supporting information (SI) Table 1. Of these 44 sequenced mRNAs, only 13 matched mRNA sequences in GenBank; the rest are expressed sequence tags that have not been reported previously. Thus, by applying DD to tumor and patient-matched nontumor prostate tissue, we were able to identify a number of mRNA transcripts not reported previously.
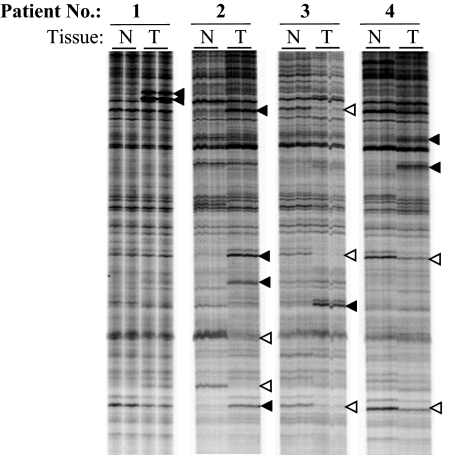
A representative DD of RNA amplified from tumor vs. patient-matched nontumor prostate tissue from four different patients using the same anchor and arbitrary primer set (H-T11C and H-AP17) is presented in Fig. 1. DD performed on different days with the same tissue samples using the same anchor and arbitrary primer pairs yielded essentially the same profile (data not shown). Notably, as expected, most of the bands were of similar intensity in matched tumor and nontumor RNA. On the other hand, however, bands differentially expressed in one tumor–nontumor pair were not necessarily differentially expressed in other tumor–nontumor pairs. For example, even tumors with the same Gleason grade differed (compare differentially expressed cDNA bands identified by arrowheads in patients 1 vs. 2, both with Gleason grades 3 + 3, and patients 3 vs. 4, both with Gleason grades 4 + 4).
Fig. 1.
DD analysis of RNA from tumor vs. patient-matched nontumor prostate tissue: RNA was isolated from prostate tumor (T) and matched nontumor (N) prostate tissue from individual patients and reverse-transcribed with anchor primer H-T11C. The resultant cDNA was amplified with primer H-T11C and arbitrary primer H-AP19 as described in Materials and Methods. The PCRs for each sample were run in duplicate. The amplified products were separated on an extended format 6% polyacrylamide gel. Differentially expressed mRNA transcripts in individual patients are indicated by arrowheads; filled arrowheads indicate overexpressed mRNA transcripts, and open arrowheads indicate down-regulated mRNA transcripts in tumor, as compared with nontumor, prostate tissue from individual patients. Tumors of patients 1 and 2 were of Gleason grade 3 + 3, and those in patients 3 and 4 were of Gleason grade 4 + 4.
Of the 44 transcripts listed in SI Table 1, most were differentially expressed in only one of seven tumors and were not studied further by RT-PCR to evaluate changes in a cross-section of patients. However, a few were differentially expressed in multiple tumor–nontumor pairs, and these were analyzed further by RT-PCR with gene-specific primers, by using RNA isolated from another set of tumor–nontumor pairs (SI Fig. 6). For example, TRPM8 was found by DD to be overexpressed in three of seven tumors, and RT-PCR confirmed overexpression (>1.5-fold) in another five of six tumors (SI Fig. 6). By comparison, in these same tumors, ADAMTS9 was down-regulated (to <0.5) in two of six tumors and RP11-571N1 was up-regulated (>1.5-fold) in one of six tumors, frequencies comparable to those found by DD. Thus, DD data correlate with RT-PCR data. However, although DD is sensitive enough to detect low-abundance transcript differences in individual patient samples, transcripts differentially expressed in the majority of tumor–nontumor pairs would need to be identified to develop reliable diagnostic biomarkers.
Identification of mRNA Transcripts That Can Detect Prostate Cancer in a Majority of Patients by Using ADE.
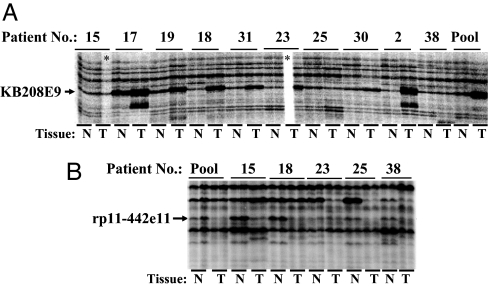
To more efficiently increase the odds of identifying transcript differences common to a majority of tumor–nontumor pairs, DD was carried out by using RNA pooled from multiple patients (pooled tumor RNA vs. pooled nontumor RNA). We use the term ADE to refer to DD of RNA pooled from multiple patients. ADE analysis of RNA pooled from 10 different patient specimens (tumor vs. nontumor) led to the identification of an mRNA transcript that was overexpressed in the pooled tumor RNA as well as in 7 of the 10 individual tumor RNAs that comprised the pool (Fig. 2A). The sequence of this mRNA transcript showed 100% identity to a 285-nucleotide sequence (accession no. EH613345) in KB208E9 (GenBank accession no. AP000345). Based on another ADE analysis of RNA pooled from five patient specimens (tumor vs. nontumor), we identified the down-regulation of an mRNA transcript in pooled, as well as in three of the five individual, tumor RNAs (Fig. 2B). The sequence of this mRNA transcript showed 100% identity to a 343-nucleotide sequence (accession no. EH613353) in rp11-442e11 (accession no. AC007707.14). These two were the only differentially expressed transcripts that were identified by ADE with the one primer pair used.
Fig. 2.
Averaged differential expression (ADE) of RNA pooled from multiple patients. RNA was isolated from tumor and patient-matched nontumor prostate tissues. DD was performed on individual tumor–nontumor pairs or on pooled tumor vs. pooled nontumor, by using anchor primer H-T11C and arbitrary primer H-AP17. Two DD profiles of pooled RNA revealed one band higher in tumor in 7 of 10 individual tumor–nontumor pairs and another band lower in three of five tumor–nontumor pairs, respectively. These bands were identified as KB208E9 (A) and rp11-442e11 (B), based on their excision, cloning, sequencing, and BLAST analysis. The Gleason grades of the tumors used in this study were 3 + 3 (patients 15, 17, and 19), 3 + 4 (patients 18 and 31), 3 + 5 (patient 23), 4 + 3 (patients 25 and 30), and 4 + 4 (patients 2 and 38). N, nontumor tissue; T, tumor tissue.
RT-PCR Validation of Differential Expression of KB208E9 and rp11-442e11 in Prostate Tissue from Cancer Patients.
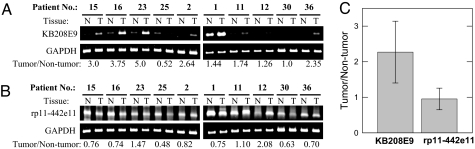
To confirm differential expression of genes identified by ADE, we used RT-PCR with gene-specific primers to measure KB208E9 and rp11-442e11 transcript levels in tumor vs. nontumor pairs from 19 patients. Representative RT-PCR results from tissues (tumor vs. nontumor) of 10 of the 19 patients is presented in Fig. 3 A and B. KB2088E9 was overexpressed in 13, and rp11-442e11 was down-regulated in 12 of these 19 patients. The mean tumor–nontumor ratio of the KB208E9 transcript, normalized to GAPDH, in 19 patients was 1.96 ± 0.263, and the mean tumor–nontumor ratio of rp11-442e11 was 0.89 ± 0.09 (P = 0.01) (Fig. 3C). Because both transcripts were analyzed in each tumor vs. nontumor pair, we calculated the ratio of these transcripts; the mean ratio of KB208E9/rp11-442e11 was 2.13 ± 0.27 (n = 19). These data suggest that the ratio of KB208E9 to rp11-442e11 may be of diagnostic value.
Fig. 3.
RT-PCR analysis of genes identified by ADE. RT-PCR using gene-specific primers was carried out to analyze the levels of KB208E9 (A) and rp11-442e11 (B) mRNA in tumors and matched nontumor prostate tissue. GAPDH was included as a housekeeping gene. KB208E9 and GAPDH were amplified by using 25 cycles; rp11-442e11, present at lower levels, was amplified by using 30 cycles. The number of PCR cycles used for each of these transcripts was determined to be in a linear range for semiquantitative analysis. KB208E9 (A) and rp11-442e11 (B) were quantitated by densitometry, normalized to GAPDH, and expressed as a ratio in tumor vs. nontumor (numbers below). (A and B) Data from 10 tumor–nontumor pairs. (C) Summary of data from these 10 patients plus an additional 9 patients; the mean tumor to nontumor ratio of KB208E9 and rp11-442e11 was 1.96 ± 0.263 and 0.89 ± 0.09 (n = 19), respectively.
Detection of KB208E9 and rp11-442e11 in Blood and Urine of Prostate Cancer Patients.
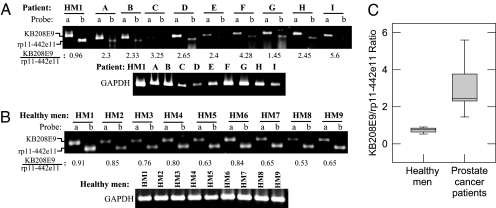
We then sought to investigate whether mRNA transcripts identified by ADE could be detected in body fluids. We obtained blood and urine samples from nine patients (SI Table 2) undergoing treatment for disseminated prostate cancer. Blood and urine specimens from nine healthy men were used as controls. RNA was prepared from blood and urine and analyzed for KB208E9, rp11-442e11 and GAPDH transcript levels by RT-PCR using gene-specific primers. As shown in Fig. 4, KB208E9 (lanes labeled probe a) and rp11-442e11 (lanes labeled probe b) transcript levels were substantially higher in the urine of patients (Fig. 4A) than of healthy men (Fig. 4B). Most noticeably, the ratio of KB208E9 to rp11-442e11 in urine was 6-fold higher in prostate cancer patients (4.04 ± 1.67; n = 9) than in healthy men (0.66 ± 0.12; n = 9) (Fig. 4C).
Fig. 4.
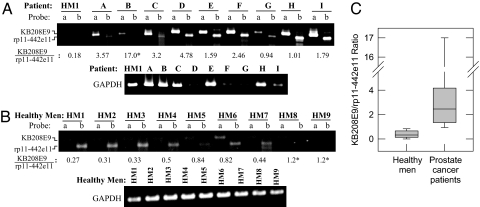
RT-PCR analysis of KB208E9 and rp11-442e11 mRNA in urine of prostate cancer patients. RNA was isolated from individual urine specimens, and RT-PCR was performed with sequence-specific primers for KB208E9, rp11-442e11, and GAPDH. PCRs were performed for 30 cycles. Numbers below represent the ratio of KB208E9 to rp11-442e11, based on densitometry. GAPDH is shown as an indicator of RNA in each sample. (A) The level of KB208E9 (probe a) and rp11-442e11 (probe b) in the urine RNA of a healthy man (HM1) and nine prostate cancer patients (A–I). (B) The level of KB208E9 (probe a) and rp11-442e11 (probe b) in urine RNA of nine healthy men (HM1-HM9). (C) The mean ratio of KB208E9 to rp11-442e11 in healthy men (0.66 ± 0.12; n = 9) vs. prostate cancer patients (4.04 ± 1.67; n = 9). ∗, Approximate value; a more reliable value could not be obtained because of low rp11-442e11 levels in the sample.
KB208E9 and rp11-442e11 were also detected in the blood of these subjects (Fig. 5). The ratio of KB208E9 to rp11-442e11 was 2.97 ± 0.42 (n = 9) in the prostate cancer patients (Fig. 5A) vs. 0.74 ± 0.42 (n = 9) in the blood of the healthy men (Fig. 5 B and C). Thus, the ratio of KB208E9 to rp11-442e11 in both urine and blood was 4- to 6-fold higher in prostate cancer patients than in healthy men (Figs. 4C and 5C).
Fig. 5.
RT-PCR analysis of KB208E9 and rp11-442e11 mRNA in blood of prostate cancer patients. RNA was isolated from individual blood specimens, and RT-PCR was performed with sequence-specific primers for KB208E9, rp11-442e11, and GAPDH. PCRs were performed for 30 cycles. Numbers below represent the ratio of KB208E9 to rp11-442e11, based on densitometry. GAPDH is shown as an indicator of RNA per sample. (A) The level of KB208E9 (probe a) and rp11-442e11 (probe b) in blood RNA of one healthy man (HM1) and nine prostate cancer patients (A–I). (B) The level of KB208E9 (probe a) and rp11-442e11 (probe b) in blood RNA of nine healthy men (HM1–HM9). (C) The mean ratio of KB208E9 to rp11-442e11 in healthy men (0.74 ± 0.04; n = 9) and prostate cancer patients (2.97 ± 0.42; n = 9).
We found no difference in the level of expression of PSA mRNA between tumor vs. nontumor tissue specimens from prostate cancer patients (data not shown). It is reported that quantitative RT-PCR showed no difference in PSA mRNA levels between blood samples from patients with localized prostate cancer and healthy men (9). We also observed no significant difference in PSA mRNA levels between blood samples of patients undergoing treatment for disseminated prostate cancer and healthy men (data not shown). Furthermore, as shown in SI Table 2, there were also some prostate cancer patients (patients D and E) on androgen-deprivation therapy (ADT) and/or radiation therapy who had serum PSA levels <0.2 ng/ml yet had detectable levels of KB208E9 in their urine and blood.
Discussion
Differential Display.
Recent advances in several gene-detection technologies have led to the discovery of differences in gene expression between normal and cancer cells. Most of these technologies rely on examining mRNA transcripts that are present in publicly available databases such as GenBank, EMBL, Swiss-Prot, etc., and are limited in their ability to identify novel mRNA transcripts that are unique to the cancer cell. DD (4, 5) has a potential to identify mRNA transcripts that not only match sequences in databases but also those that are novel and whose expression is altered in a majority of prostate cancers. By using this technique we identified mRNA transcripts that are expressed differentially in many individual tumors as compared with matched nontumor prostate tissues from patients who underwent radical prostatectomy. This analysis led to the identification of 44 differentially expressed mRNA transcripts of which 31 were previously unidentified (SI Table 1). Thus, the majority of the DD mRNA transcripts identified in this study do not correspond to transcripts previously deposited in GenBank. The few DD mRNA transcripts that matched GenBank transcripts are reported to be altered in a variety of cancer types.
Noteworthy among the mRNA transcripts that matched sequences in GenBank were TRPM8 and ADAMTS9. TRPM8 was overexpressed, and ADAMTS9 was down-regulated in tumors from >70% of the prostate cancer patients examined (SI Fig. 6). TRPM8 is a member of the transient receptor potential (TRP) family of Ca2+-channel proteins that is reported to be androgen-regulated and required for the survival of prostate cancer cells (10) and overexpressed in several cancers including prostate, breast, colorectal and lung (11). ADAMTS9 belongs to a subgroup of “a distinctive and metalloproteinase with thrombospondin motifs” (ADAMTS) family of enzymes capable of cleaving versican (chondroitin sulfate proteoglycan-2). Increased expression of versican is associated with the local spread of tumor cells, potentially through destabilization of focal adhesion (12). Down-regulation of ADAMTS9 therefore can result in the accumulation of versican in the stromal compartment of the prostate (13). Our observation that ADAMTS9 is down-regulated in prostate tumor tissue is consistent with such a possibility.
The expression profile of most of the genes identified in this study varied from patient to patient (Fig. 1), in part because of the heterogeneous nature of the disease, and in part because of admixture of tumor cells with nontumor cells. The differential expression of some of these genes could be verified by RT-PCR in <20% of tumors. Thus, genes identified by DD of an individual tumor provide information on the expression profile of that individual, but it is not helpful in defining a profile common to all prostate cancer patients.
ADE.
Our study shows that a profile common to most prostate cancer patients can be obtained by performing DD on pooled RNAs from multiple patients' tumor and matched nontumor prostate tissues. Differentially expressed mRNA transcripts identified by ADE were expressed in a greater percentage of tumors (>70%) than those identified by DD of mRNA from individual patient samples and were fewer in number.
With one primer combination, we identified two genes, KB208E9 and rp11-442e11, which were differentially expressed in > 70% of the prostate cancer tumors. KB208E9 was elevated in tumor tissues of most patients who underwent radical prostatectomy irrespective of whether they presented with Gleason grade 3, 4, or 5 disease (Fig. 2). A differentially expressed cDNA sequence of 285 nucleotides showed 100% homology to a portion of genomic sequence (clone KB208E9, GenBank accession no. AP000346.1, at Chr22q11.2) that contains no known genes or expressed sequence tags. The sequence also had 97% identity with a 277-bp region of human endogenous retrovirus K (HERV-K) mRNA (GenBank accession no. U39937), implicated in certain cancers (14), and a recent study has shown the presence of HERV-K mRNA in human breast cancer cell lines (15). Another cDNA sequence of 343 nucleotides showed 100% homology to a portion of genomic sequence (clone rp11-442e11, GenBank accession no. 007707.14, at Chr 11q23.3) that corresponds to intron 4 of the RefSeq gene KIAA0999 (http://genome.ucsc.edu). Thus, it appears that prostate cancer expresses decreased levels of an alternate splice variant of KIAA0999 that has not been identified previously. The functions of these transcripts require further investigation.
Thus, whereas DD in general allows the detection of novel and low-abundance mRNA transcripts with altered expression in individual patients, ADE identifies uncommon mRNA transcripts whose expression is altered in most of the patients.
Detection of Prostate Cancer.
For two decades, early detection of prostate cancer and, hence, improved clinical outcome, can be attributed to the advent of PSA in serum as a biomarker. However, a low PSA is not a guarantee of disease-free status, and elevated serum PSA lacks the specificity required to distinguish prostate cancer from other prostatic disorders. We observed no difference in the level of expression of PSA mRNA between tumor vs. nontumor tissue specimens from prostate cancer patients (data not shown).
Circulating epithelial cells in cancer patients permit detection of DNA- (16), protein- (17), and RNA- (18) based prostate cancer markers. It is evident from biochemical recurrence in nearly 25% of patients who have undergone radical prostatectomy for organ-confined prostate cancer (19) that tumor cells can escape from the primary site into the circulation during very early stages of the disease. Prostate epithelial cells indeed have been found in the blood of patients diagnosed with prostate cancer (2, 20–22). It is also evident that, at an early stage, localized primary tumors may harbor cells with metastatic potential and exhibit a gene-expression signature matching that observed in metastatic colonies (23, 24). Some genes that are increased in prostate cancer tissue (25, 26) are also found to be elevated in patient urine (27). Thus, cancer cells that enter the circulation even during early stages of tumor growth might display characteristics of cancer that is either likely to metastasize or remain indolent. Therefore we have focused on identifying molecular markers that are sensitive and specific enough to detect prostate cancer in easily obtainable body fluids such as blood and urine.
The gene expression changes identified by ADE were readily detectable by RT-PCR of mRNA isolated from urine and blood of patients undergoing treatment for disseminated prostate cancer. KB208E9 and rp11–442e1 were present at different levels in urine and blood of prostate cancer patients relative to healthy men, and the ratio of KB208E9 to rp11-442e11 was 4- to 6-fold higher in prostate cancer patients (Figs. 4 and 5). An increase in KB208E9 levels was observed in all patients irrespective of whether the disease was in remission (patients undergoing ADT and/or radiation therapy for biochemical recurrence after radical prostatectomy) or hormone-refractory (metastatic patients undergoing chemotherapy). Thus increased KB208E9 or increased ratio of KB208E9/rp11-442e11 characterizes patients with localized and advanced disease. Thus, these studies establish proof of principle that frequently differentially expressed mRNA transcripts identified by using ADE can be used for the detection of prostate cancer in body fluids such as urine and blood.
Early detection is very important for effective treatment and management of cancer. It remains to be determined whether KB208E9 shows a similar increase in urine or blood of patients at the time of initial presentation with elevated PSA, which would offer an additional screening tool for the detection of prostate cancer. The KB208E9/rp11-442e11 ratios of prostate cancer patients compared with healthy men show little or no overlap (Figs. 4 and 5), suggesting that there is considerable potential for this measurement in screening for prostate cancer. Optimism, however, must be tempered by the small sample size (nine prostate cancer patients and nine healthy men) and by the fact that patients with disseminated prostate cancer may not accurately represent patients at (or before) the time of diagnosis, when utility as a screening tool would be relevant.
Clinical Applications.
Autopsy data from American men indicates that there is an ≈49% lifetime risk of developing prostate cancer. However, the risk of having clinically detected prostate cancer in the same population is <18% (28), suggesting that the development and progression of prostate cancer are different in different men. Prostate cancer is a heterogeneous disease (29) whose development and progression involve changes in expression of a number of genes that determine oncogenic transformation, survival, and invasiveness of prostate cancer cells. Reliable detection and prediction of outcome of the disease, therefore, require identification of changes in expression of not just one or two genes but a number of genes that influence disease development and progression.
A profile of changes in expression of multiple genes can provide a “signature” or a “barcode” that is capable of not only discriminating prostate cancer from other prostatic disorders, but also providing insight into the responsiveness of the disease to currently available treatment strategies and final outcome. In addition, such a profile might provide information about tumor subtype characteristics, such as androgen dependence, drug sensitivity or resistance, metastatic potential, etc. To this end, we envision that a microarray containing selected cDNA probes for mRNA transcripts identified in this study, along with probes for some of the disease progression-associated genes such as hepsin (30), GSTP1 (31), α-methylacyl-CoA racemase (AMACR) (32), and CAMKK2 (33–37) that have been identified by using expression arrays, will help not only in the detection of prostate cancer but also provide a signature for good and poor prognosis of the disease (38).
Materials and Methods
Tissue Specimens.
Prostate tumors were obtained from radical prostatectomy specimens. None of the patients included in this study had received hormonal therapy, chemotherapy, or radiation therapy. This protocol was reviewed and approved by the Institutional Review Board of Henry Ford Health System. Cancerous tissues were graded by a pathologist according to the Gleason scoring system. Nontumor prostate tissue was obtained from the contralateral lobe of the same specimen. Cancer and matched nontumor tissues were stored frozen at −80°C within 1 hour of surgical excision.
Blood and Urine Specimens.
Peripheral blood and urine samples were obtained from prostate cancer patients undergoing chemotherapy at Henry Ford Hospital. Blood was collected in PAXgene blood RNA tubes for RNA stabilization (Qiagen, Valencia, CA). As the procedure requires, these tubes were stored at room temperature for at least 2 h before RNA isolation was performed. Urine was collected in an equal volume of lysis buffer containing 5.64 M guanidinium thiocyanate, 0.5% sarcosyl, 50 mM sodium acetate (pH 6.5), and 1 mM β-mercaptoethanol, and the pH was adjusted to 7.0 with 1.5 M Hepes (pH 8.0); these samples were frozen at −80°C until extraction of RNA was performed. This procedure allows recovery of total RNA (both intra- and extracellular) in urine. All patients provided written informed consent, and protocols were approved by the Institutional Review Board of Henry Ford Hospital.
RNA Isolation.
Total RNA was extracted from frozen prostate tissue specimens with RNeasy Mini kit (Qiagen) according to the manufacturer's protocols. For isolation of total cellular RNA from blood, the PAXgene Blood RNA kit was used (Qiagen). Isolation of RNA from urine was carried out by using the protocol of Menke and Warnecke (39). DNA was removed by performing on-column DNase digestion with RNase-free DNase (Qiagen). The integrity and size distribution of RNA was monitored by agarose gel electrophoresis.
RT-PCR DD.
DD was performed by using the RNAimage kit (GenHunter, Nashville, TN) as described by Liang and Pardee (5). RNAs isolated from tumor and matched nontumor prostate tissues obtained from the same surgical specimen were compared by DD. RT-PCR for DD of individual surgical specimens was performed by using 24 different primer-pair combinations involving three anchor primers (H-T11C, H-T11G, and H-T11A) and eight arbitrary primers (H-AP17–H-AP24) from GenHunter. RT-PCR for DD of pooled surgical specimens from multiple patients (ADE) was performed by using anchor primer H-T11C and arbitrary primer H-AP17. Reverse transcription of 200 ng of individual or pooled RNA was performed with Sensiscript RT (Qiagen, Santa Clarita, CA). Reactions containing 2 μl of 10× RT buffer, 2 μl of 5 mM dNTP (final concentration 500 μM), 2 μl of 10 μM anchor primer (final concentration 1 μM), 2 μl of RT, 1 μl of RNase inhibitor (10 units/μl), and 10 μl of RNase-free water were incubated at 37°C for 30 min and then at 93°C for 5 min. Ten percent of the RT reaction was used for subsequent PCR, in duplicate. The PCR contained 200 nM of each anchor primer and arbitrary primer (e.g., H-T11C and H-AP19, or H-T11C and H-AP17), 10 mM Tris-Cl (pH 8.4), 50 mM KCl, 1.5 mM MgCl2, 5 mM DTT, 2 μM dNTP mix, 20 Ci/mmol (1 Ci = 37 GBq) [α-33P]dATP and 2 units Taq Polymerase (Qiagen) in a total volume of 20 μl. The cycling parameters were 94°C for 15 sec, 40°C for 2 min, and 72°C for 30 sec, followed by 72°C for 5 min. Forty PCR cycles were performed for amplification of RNA from both tumor and patient-matched nontumor tissues. PCR products were subjected to denaturing 6% PAGE on an extended format using programmable Genomyx LR gel electrophoresis apparatus (Beckman Coulter, Columbia, MD). cDNA bands that were either more abundant or less abundant in tumor than in nontumor RNA were excised, reamplified by using the same primers used for DD, and sequenced directly or after cloning into pGEM-T vector (Invitrogen, Carlsbad, CA), as described (5). Clones were screened for the insert and then sequenced. Sequences of differentially expressed mRNA transcripts were then searched for homology to known gene sequences in GenBank by using the BLAST algorithm (40).
RT-PCR Analysis of Differentially Expressed Genes.
To confirm differential expression of genes identified by DD, we performed semiquantitative RT-PCR using primers we designed based on the sequence of the DD cDNA fragments. These primer sequences were 5′-gattttcaccaatgaccgccg (forward) and 5′-ccccagcagcattgatgtcg (reverse) for TRPM8, 5′-caggggaaacagacgatgacaact (forward) and 5′-tgcggtaacccaagccacact (reverse) for ADAMTS9, 5′-gagccaaaagttcttctacactgc (forward) and 5′-agattccagatggttctgccta (reverse) for RP11-571N1, 5′-tgcctcagggaatgcttaat (forward) and 5′-cctctacctgcattcccaag (reverse) for KB208E9, 5′-ggtgtttttcagcaggctct (forward) and 5′-aaaatggtgggtttgaggtg (reverse) for rp11-442e11, and 5′-gagatccctccaaaatcaagtg (forward) and 5′-ccttccacgataccaaagttgt (reverse) for GAPDH. cMaster RTplus PCR system (Brinkman Instruments, Westbury, NY) was used to reverse transcribe and amplify total RNA from tissue, blood, or urine. RNA was reverse transcribed by using oligo(dT) primer and cMaster reverse transcriptase according to the manufacturer's protocol. The enzyme was inactivated for 5 min at 85°C, and cDNA was stored at −80°C until use. Amplification of cDNA was carried out by using primers described above for each gene. Different PCR cycle numbers were tested for each gene to ensure that the assay was in the linear range of amplification. The housekeeping gene GAPDH was amplified from each sample to normalize the level of each test gene. PCR products were run on a 2% agarose gel. Quantitation was carried out by digital analysis of band intensity in the gel with an Eagle Eye II Still Video system, using the EagleSight software (version 3.2; Stratagene, La Jolla, CA).
Supplementary Material
Acknowledgments
This work was supported by a generous gift from the Vattikuti Foundation, and by National Institutes of Health Grant R01-DK57864 and Department of Defense (DOD) Grant W81XWH-05-1-0071 (to G.P.-V.R.), and DOD Grant 17-02-1-0692 (to A.B.P.).
Abbreviations
- ADE
averaged differential expression
- DD
mRNA RT-PCR differential display
- PSA
prostate-specific antigen.
Footnotes
The authors declare no conflict of interest.
Data deposition: The sequences of all mRNA transcripts identified by DD and ADE in this paper have been deposited in the GenBank database (accession nos. EH613309–EH613353).
This article contains supporting information online at www.pnas.org/cgi/content/full/0610504104/DC1.
References
- 1.Mazzucchelli R, Colanzi P, Pomante R, Muzzonigro G, Montironi R. Adv Clin Pathol. 2000;4:111–120. [PubMed] [Google Scholar]
- 2.Schamhart DH, Maiazza R, Kurth KH. Int J Oncol. 2005;26:565–577. [PubMed] [Google Scholar]
- 3.Thompson IM, Pauler DK, Goodman PJ, Tangen CM, Lucia MS, Parnes HL, Minasian LM, Ford LG, Lippman SM, Crawford ED, et al. N Engl J Med. 2004;350:2239–2246. doi: 10.1056/NEJMoa031918. [DOI] [PubMed] [Google Scholar]
- 4.Martin KJ, Graner E, Li Y, Price LM, Kritzman BM, Fournier MV, Rhei E, Pardee AB. Proc Natl Acad Sci USA. 2001;98:2646–2651. doi: 10.1073/pnas.041622398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liang P, Pardee AB. Mol Biotechnol. 1998;10:261–267. doi: 10.1007/BF02740847. [DOI] [PubMed] [Google Scholar]
- 6.Kim MY, Park E, Park JH, Park DH, Moon WS, Cho BH, Shin HS, Kim DG. Oncogene. 2001;20:4568–4575. doi: 10.1038/sj.onc.1204626. [DOI] [PubMed] [Google Scholar]
- 7.Chakrabarti R, Robles LD, Gibson J, Muroski M. Cancer Genet Cytogenet. 2002;139:115–125. doi: 10.1016/s0165-4608(02)00641-6. [DOI] [PubMed] [Google Scholar]
- 8.Liang P, Pardee AB. Nat Rev Cancer. 2003;3:869–876. doi: 10.1038/nrc1214. [DOI] [PubMed] [Google Scholar]
- 9.Patel K, Whelan PJ, Prescott S, Brownhill SC, Johnston CF, Selby PJ, Burchill SA. Clin Cancer Res. 2004;10:7511–7519. doi: 10.1158/1078-0432.CCR-04-0166. [DOI] [PubMed] [Google Scholar]
- 10.Zhang L, Barritt GJ. Cancer Res. 2004;64:8365–8373. doi: 10.1158/0008-5472.CAN-04-2146. [DOI] [PubMed] [Google Scholar]
- 11.Tsavaler L, Shapero MH, Morkowski S, Laus R. Cancer Res. 2001;61:3760–3769. [PubMed] [Google Scholar]
- 12.Sakko AJ, Ricciardelli C, Mayne K, Suwiwat S, LeBaron RG, Marshall VR, Tilley WD, Horsfall DJ. Cancer Res. 2003;63:4786–4791. [PubMed] [Google Scholar]
- 13.Cross NA, Chandrasekharan S, Jokonya N, Fowles A, Hamdy FC, Buttle DJ, Eaton CL. Prostate. 2005;63:269–275. doi: 10.1002/pros.20182. [DOI] [PubMed] [Google Scholar]
- 14.Boller K, Konig H, Sauter M, Mueller-Lantzsch N, Lower R, Lower J, Kurth R. Virology. 1993;196:349–353. doi: 10.1006/viro.1993.1487. [DOI] [PubMed] [Google Scholar]
- 15.Ejthadi HD, Martin JH, Junying J, Roden DA, Lahiri M, Warren P, Murray PG, Nelson PN. Arch Virol. 2005;150:177–184. doi: 10.1007/s00705-004-0378-8. [DOI] [PubMed] [Google Scholar]
- 16.Goessl C, Krause H, Muller M, Heicappell R, Schrader M, Sachsinger J, Miller K. Cancer Res. 2000;60:5941–5945. [PubMed] [Google Scholar]
- 17.Paul B, Dhir R, Landsittel D, Hitchens MR, Getzenberg RH. Cancer Res. 2005;65:4097–4100. doi: 10.1158/0008-5472.CAN-04-4523. [DOI] [PubMed] [Google Scholar]
- 18.Tombal B, Van Cangh PJ, Loric S, Gala JL. Prostate. 2003;56:163–170. doi: 10.1002/pros.10237. [DOI] [PubMed] [Google Scholar]
- 19.Zimmerman RA, Culkin DJ. Clin Prostate Cancer. 2003;2:160–166. doi: 10.3816/cgc.2003.n.024. [DOI] [PubMed] [Google Scholar]
- 20.Wang ZP, Eisenberger MA, Carducci MA, Partin AW, Scher HI, Ts'o PO. Cancer. 2000;88:2787–2795. doi: 10.1002/1097-0142(20000615)88:12<2787::aid-cncr18>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 21.Ts'o PO, Pannek J, Wang ZP, Lesko SA, Bova GS, Partin AW. Urology. 1997;49:881–885. doi: 10.1016/s0090-4295(97)00157-x. [DOI] [PubMed] [Google Scholar]
- 22.Fehm T, Sagalowsky A, Clifford E, Beitsch P, Saboorian H, Euhus D, Meng S, Morrison L, Tucker T, Lane N, et al. Clin Cancer Res. 2002;8:2073–2084. [PubMed] [Google Scholar]
- 23.Liotta LA, Kohn EC. Nat Genet. 2003;33:10–11. doi: 10.1038/ng0103-10. [DOI] [PubMed] [Google Scholar]
- 24.Ramaswamy S, Ross KN, Lander ES, Golub TR. Nat Genet. 2003;33:49–54. doi: 10.1038/ng1060. [DOI] [PubMed] [Google Scholar]
- 25.Chatterjee SK, Zetter BR. Future Oncol. 2005;1:37–50. doi: 10.1517/14796694.1.1.37. [DOI] [PubMed] [Google Scholar]
- 26.Tricoli JV, Schoenfeldt M, Conley BA. Clin Cancer Res. 2004;10:3943–3953. doi: 10.1158/1078-0432.CCR-03-0200. [DOI] [PubMed] [Google Scholar]
- 27.Hutchinson LM, Chang EL, Becker CM, Ushiyama N, Behonick D, Shih MC, DeWolf WC, Gaston SM, Zetter BR. Clin Biochem. 2005;38:558–571. doi: 10.1016/j.clinbiochem.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 28.Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, Feuer EJ, Thun MJ. CA Cancer J Clin. 2005;55:10–30. doi: 10.3322/canjclin.55.1.10. [DOI] [PubMed] [Google Scholar]
- 29.Scher HI, Heller G. Urology. 2000;55:323–327. doi: 10.1016/s0090-4295(99)00471-9. [DOI] [PubMed] [Google Scholar]
- 30.Magee JA, Araki T, Patil S, Ehrig T, True L, Humphrey PA, Catalona WJ, Watson MA, Milbrandt J. Cancer Res. 2001;61:5692–5696. [PubMed] [Google Scholar]
- 31.Jeronimo C, Usadel H, Henrique R, Oliveira J, Lopes C, Nelson WG, Sidransky D. J Natl Cancer Inst. 2001;93:1747–1752. doi: 10.1093/jnci/93.22.1747. [DOI] [PubMed] [Google Scholar]
- 32.Dhanasekaran SM, Barrette TR, Ghosh D, Shah R, Varambally S, Kurachi K, Pienta KJ, Rubin MA, Chinnaiyan AM. Nature. 2001;412:822–826. doi: 10.1038/35090585. [DOI] [PubMed] [Google Scholar]
- 33.Chaib H, Cockrell EK, Rubin MA, Macoska JA. Neoplasia. 2001;3:43–52. doi: 10.1038/sj.neo.7900126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bubendorf L, Kolmer M, Kononen J, Koivisto P, Mousses S, Chen Y, Mahlamaki E, Schraml P, Moch H, Willi N, et al. J Natl Cancer Inst. 1999;91:1758–1764. doi: 10.1093/jnci/91.20.1758. [DOI] [PubMed] [Google Scholar]
- 35.Singh D, Febbo PG, Ross K, Jackson DG, Manola J, Ladd C, Tamayo P, Renshaw AA, D'Amico AV, Richie JP, et al. Cancer Cell. 2002;1:203–209. doi: 10.1016/s1535-6108(02)00030-2. [DOI] [PubMed] [Google Scholar]
- 36.Lapointe J, Li C, Higgins JP, van de Rijn M, Bair E, Montgomery K, Ferrari M, Egevad L, Rayford W, Bergerheim U, et al. Proc Natl Acad Sci USA. 2004;101:811–816. doi: 10.1073/pnas.0304146101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.True L, Coleman I, Hawley S, Huang CY, Gifford D, Coleman R, Beer TM, Gelmann E, Datta M, Mostaghel E, et al. Proc Natl Acad Sci USA. 2006;103:10991–10996. doi: 10.1073/pnas.0603678103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weinberg RA. The Biology of Cancer. New York: Garland Science; 2007. pp. 587–654. [Google Scholar]
- 39.Menke TB, Warnecke JM. Ann N Y Acad Sci. 2004;1022:185–189. doi: 10.1196/annals.1318.028. [DOI] [PubMed] [Google Scholar]
- 40.Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.