Abstract
Retrograde axonal transport plays an important role in the maintenance of neuronal functions, but the mechanism is poorly defined partly because the constituents of the retrograde transport system and their interactions have yet to be elucidated. Of special interest is how dynein/dynactin motor proteins interact with membrane cargoes. Here, we report that an endosomal vesicle protein, termed retrolinkin, functions as a receptor tethering vesicles to dynein/dynactin through BPAG1n4. Retrolinkin, a membrane protein highly enriched in neuronal endosomes, binds directly to BPAG1n4. Deletion of retrolinkin membrane-association domains disrupts retrograde vesicular transport, recapitulating the BPAG1 null phenotype. We propose that retrolinkin acts with BPAG1n4 to specifically regulate retrograde axonal transport. Our work lays the foundation for understanding fundamental issues of axonal transport and provides insights into the molecular mechanisms underlying human neurodegenerative disorders.
Keywords: BPAG1n4, cytoskeleton, neurodegeneration, vesicles, endosomes
The intracellular trafficking of vesicles and membrane organelles driven by motor proteins, the kinesins and dynein, is essential for cellular processes (1, 2). An important issue is how motor proteins are linked to membranous cargoes. Cargo specificity may vary in different types of cells or subcellular compartments. Accessory and adaptor proteins are presumed to play an important role for both anterograde and retrograde transport, but there is little data for which proteins are involved and how they interact. For example, the accessory proteins for anterograde cargoes appear to differ from those for retrograde cargoes. Although the genetic divergence in the tail domain confers cargo selectivity for kinesins (3–5), it appears that the diverse functions for cytoplasmic dynein may require a different set of associated proteins (3, 6). Recent studies have begun to identify proteins important for retrograde transport. The data suggest that βIII-spectrin, a membrane coat protein, acts through the Arp1 subunit of dynactin (7, 8) to link the dynein motor complex to membranous cargo (7, 8) and that the p50 subunit of dynactin (also known as dynamitin) binds to a Golgi-associated protein, bicaudal D (9). But whether these interactions mediate retrograde movement of a specific membranous cargo is uncertain. Indeed, it is unlikely that βIII-spectrin serves this role because it is present on a variety of different membranes (10).
The BPAG1 null mouse is characterized by marked accumulation of vesicles and severely disrupted retrograde axonal transport (11, 12). We reasoned that this mouse might provide an opportunity to define further the membrane receptor proteins and mechanisms that mediate dynein/dynactin-based transport of vesicles. The recently identified BPAG1 isoform, BPAG1n4, was shown to play an important role in retrograde transport (12). BPAG1n4 is located nearly exclusively on vesicles in sensory neurons; specifically disrupting its interaction with dynactin disrupted retrograde transport (12). Interestingly, the analysis of the amino acid sequence reveals that BPAG1n4 has no transmembrane region, suggesting that the association of BPAG1n4 with vesicles must be mediated through an unidentified membrane acceptor. In this article, we have identified a membrane protein, retrolinkin, which binds directly to BPAG1n4; in turn, BPAG1n4 associates with dynactin. Deletion of the transmembrane domains in retrolinkin results in a severely impaired retrograde axonal transport in sensory neurons, a result recapitulating the BPAG1 null neurons. The absence of dynein motor protein on retrolinkin-associated vesicles in BPAG1 null sensory axons, revealed by double immunoelectron microscopy, suggests that the anchoring of dynein/dynactin on vesicles is mediated by BPAG1n4.
Results and Discussion
To understand how BPAG1n4 associates with vesicles, we initiated a yeast two-hybrid screen (13) with the BPAG1n4 ERM2 domain (amino acids 4680–5416) [supporting information (SI) Fig. 6] as bait. This screen identified a cDNA fragment that encodes an unknown protein. The deduced sequence of the full-length cDNA was analyzed by using the OrienTM protein prediction program (14). On the basis of the predicted structure, there are two putative transmembrane regions (amino acids 12–29 and 468–488) and the residues between them are located in the cytosol (amino acids 30–467) (Fig. 1A). The predicted structure raised the possibility that this protein resides in membrane cargoes where it binds through its cytosolic residues to BPAG1n4 and that together these proteins tether membranous cargoes to dynein/dynactin destined for retrograde transport (12). On the basis of this postulation, we tentatively named the protein retrolinkin. Antibody was generated against the predicted cytoplasmic region (amino acids 31–460) of retrolinkin by using a bacterial recombinant protein (Fig. 1B, lane 1). In detergent extracts of mouse nervous tissues, antiretrolinkin recognized a protein that migrated at ≈120 kDa (Fig. 1B, lanes 2–4), the specificity of which was confirmed by competition assays with purified recombinant protein (Fig. 1B, lanes 1′–4′). The overlapping detection of the GFP-tagged truncated retrolinkin by both anti-GFP and antiretrolinkin in transfected COS7 cells provided additional verification of the antiretrolinkin specificity (Fig. 2A, lanes 2 and 5). However, the detected molecular mass of retrolinkin (120 kDa) was much larger than the calculated size (≈60 kDa). To assess whether the discrepancy was caused by posttranslational modifications typical of membrane proteins, such as glycosylation, enzymatic digestion with endoglycosidase H (endo H) was performed. After the treatment of deglycosylation, retrolinkin, indicated by immunoblot detection, was indeed reduced to 60–65 kDa (Fig. 1B, lane 6), verifying that the addition of posttranslational glycolytic linkages accounts for the protein's slower migration.
Fig. 1.
Characterizations of retrolinkin (labeled RTLN) as an integral membrane protein. (A) Diagram of mouse retrolinkin protein structure. Amino acid residue numbers denote boundaries for the putative transmembrane (TM) regions in retrolinkin. The cytosolic domain (amino acids 31–460) was used for preparing recombinant domain protein and raising polyclonal antibodies. The fragment of amino acids 173–406 (isolated from yeast two-hybrid screen), containing the site responsible for BPAG1n4's binding, was used for dominant negative assays. (B–D) Expression of retrolinkin. (B) The blot was probed first with antiretrolinkin antibody (lanes 1–4), followed by the purified recombinant domain protein competitions (lanes 1′–4′). After deglycosylation treatment with endo H, the molecular mass of retrolinkin protein was reduced (lane 6). Recom, recombinant protein; br, brain; SC/SN, spinal cord/sciatic nerves; untr, untreated lysates; tr, endo H-treated samples. (C and D) Detections of the protein expression in mouse brain at different developmental stages (C) and in multiple tissues of embryonic day 18 (D) are indicated. Postnatally, retrolinkin could be similarly detected in dorsal and ventral roots of DRGs with NF-L as a neuronal-specific loading control (D, lanes 10 and 11). (E) Endogenous retrolinkin is found primarily in endosomal membrane fractions (lane 4). Spinal cord and sciatic nerve tissue extracts (lane 1) were fractionated by differential centrifugation first into S100 (nuclear component, lane 2) and P100 (membrane fraction, lane 3). The P100 fraction was further fractionated by floatation up through an 8–35–40% sucrose gradient at 150,000 × g for 2 h. Endosomal (lane 4) and heavy (lane 5) membranes were collected from the 8–35% and 35–40% interfaces, respectively. TrkB and Rab5B were used as endosomal vesicular markers, and Lamp2 and Rab7 were used as heavy membrane markers. An aliquot from each step of the fractionations was saved, and the loading was adjusted on equal protein measures. (F) Alkali extraction of retrolinkin. The P100 fraction was treated with 0.1 M Na2CO3 (pH 11.5) for 30 min on ice and then centrifuged at 100,000 × g for 1 h. Immunoblots were analyzed with antiretrolinkin (Top), APP C terminus (Middle), and EEA1 (Bottom). S, supernatant; P, pellet. (G) The N terminus of retrolinkin resides on the cytoplasmic face of intracellular membranes. Aliquots of total membranes (P100) were treated with 1 μg/ml proteinase K for 20 min at room temperature and then centrifuged at 100,000 × g for 1 h. Immunoblotting was done with antibodies against retrolinkin (Top), APP C terminus (Middle), and N terminus (Bottom).
Fig. 2.
The cytosolic domain of retrolinkin dissociates with vesicle membrane. (A) Expression of the N-terminal region of retrolinkin (amino acids 173–406) fused to GFP (retrolinkin-cyto-GFP) in transfected COS7 cells. RTLN, endogenous retrolinkin; retrolinkin-cyto-GFP, cytosolic domain of retrolinkin epitop-tagged with GFP. The blot was probed first with antiretrolinkin (lanes 1–3) and then with anti-GFP (lanes 4–6). (B and C) Unlike full-length retrolinkin (B), the cytosolic domain of retrolinkin without membrane docking sites (retrolinkin-cyto-GFP; C) does not display vesicular structures. Arrowheads in B point to the vesicle structures, and arrowheads in C indicate diffuse distributions. (D) COS7 cells transfected with pEGFP-N2 as a control. (Scale bar: 18 μm.)
To understand the function of retrolinkin, we examined first the spatial and temporal patterns of expression. It was detected weakly as early as embryonic day 12, and the level increased persistently through later embryonic stages (Fig. 1C). It is noteworthy that retrolinkin expression parallels that for BPAG1 neuronal isoforms (15, 16). Postnatally, the protein was consistently found in the brain and spinal cord, but was absent in tissues outside of the nervous system (Fig. 1D); thus, retrolinkin appears to be a neural-specific protein and may be expressed in both motor and sensory neurons (Fig. 1D, lanes 10 and 11).
We tested the prediction that retrolinkin is a membrane-associated protein. After differential centrifugation of spinal cord and sciatic nerve tissue extracts, the P100 fraction, containing membranes (Fig. 1E, lane 3), was further fractionated by floatation in an 8–35% to 40% sucrose gradient at 150,000 × g for 2 h. Endosomal and heavy [endoplasmic reticulum (ER) and Golgi] membranes were collected from the 8%–35% and 35%–40% interfaces, respectively (17). Similar to Rab5B and TrkB (18), retrolinkin was detected primarily in the fraction containing endosomes (Fig. 1E, lane 4), whereas Lamp2 and Rab7 were enriched in the heavy membrane fraction (Fig. 1E, lane 5). To test whether retrolinkin is integral or peripherally associated with these membranes, we treated isolated vesicles with 0.2 M sodium carbonate (pH 11.5) for 30 min. Unlike early endosome antigen 1 (EEA1), which is peripherally associated with membranes (19), retrolinkin remained in the carbonate insoluble membrane fraction after repeated washes, a result consistent with that for amyloid precursor protein (APP), an integral membrane protein (20) (Fig. 1F). These results confirm the structural prediction of retrolinkin as an integral membrane-associated protein. Next, we investigated the membrane topology of retrolinkin. The P100 fraction was prepared under conditions that maintain the integrity of vesicles and subjected to proteinase K digestion for 20 min as reported (21). Proteinase K is not membrane-permeable and at limited concentrations digests only proteins accessible at the cytoplasmic face of membranes. Under these conditions, the cytosolic domains of retrolinkin and APP were much more sensitive to digestions (Fig. 1G Top and Middle) than a luminal control, the N-terminal domain of APP (Fig. 1G Lower). Taken together, the results support the view that retrolinkin is an integral membrane protein whose BPAG1n4-binding region isolated from a yeast two-hybrid screen appears to be within the cytosolic domain on the surface of vesicles.
The presence of retrolinkin in membranes was corroborated by subcellular localization studies. The specific vesicular labeling in sciatic nerves at a ultrastructural level (SI Fig. 7 A–F) or the punctated staining patterns in PC12 cells revealed by confocol microscopy (data not shown) provided strong additional evidence supporting the predicted localization on vesicles. To define the subtypes of vesicles that carry retrolinkin, we conducted colocalization studies using various well defined vesicle markers. When Flag-tagged full-length retrolinkin was transiently overexpressed in COS7 cells, which do not express retrolinkin endogenously, the protein was largely colabeled with EEA1, an endosome marker (SI Fig. 8 A–C), but not with TGN58K, a Golgi marker (SI Fig. 8 D–F). Notably, overexpression of retrolinkin in COS7 cells led to formation of enlarged endosomes or fused multivesicular bodies (SI Fig. 8C Inset), suggesting either increased fusion or decreased budding of endosomes under the conditions of retrolinkin overexpression. In double immuno-EM, membranes showed colabeling of retrolinkin with EEA1, providing additional evidence for their presence together in vivo on endosomes. In three independent assays, ≈80.6% ± 17.3% of retrolinkin-immunostained vesicles were colabeled with EEA1; conversely, ≈81.3% ± 21.6% of EEA1-immunopositive vesicles were also positive for retrolinkin (SI Fig. 7 G–I). Thus, retrolinkin exists on the surface of endosomes.
On the basis of the protein structure, two putative transmembrane regions (amino acids 12–29 and 468–488) were predicted by the OrienTM protein prediction program (14). To analyze the disposition of retrolinkin and test the prediction, we deleted both transmembrane regions. In striking contrast to the pattern of immunostaining for the full-length form (Fig. 2B), the GFP-tagged retrolinkin truncation (retrolinkin-cyto-GFP) in COS7 cells was diffusely distributed and in no case showed the vesicular or punctate pattern expected for vesicular structures (Fig. 2C). This finding confirms that the predicted transmembrane domains of retrolinkin are required for its membrane localization.
To understand what role is played by retrolinkin, we confirmed its interaction with BPAG1n4. The fragment isolated from the two-hybrid screen was retested and mapped in the yeast two-hybrid system. The cytosolic region between amino acids 173 and 406 of retrolinkin was confirmed to be responsible for association with BPAG1n4 (Fig. 3A). The direct interaction in the yeast two-hybrid system was further verified by in vitro pull-down assays. The purified recombinant His-retrolinkin cytosolic domain (Fig. 3B, lane 1), but not His-tubulin folding cofactor B (TBCB) control (Fig. 3B, lane 2), bound to immobilized BPAG1n4 isolated from brain lysates (Fig. 3B, compare lanes 3 and 4). No binding activity was found in sham control (Fig. 3B, lanes 5 and 5′). These results provide additional evidence for direct binding between the two proteins. Ultrastructurally, BPAG1n4 and retrolinkin were present together on vesicular structures in vivo within axons in the sciatic nerves. From three independent trials, ≈70% ± 11.2% retrolinkin-immunolabeled structures were colabeled with antibodies to BPAG1n4; conversely, ≈68% ± 8.7% of BPAG1n4-associated structures were also positive for retrolinkin (Fig. 3 D–F). No significant labeling was detected in negative controls when using only secondary antibodies (data not shown). Consistently, in cultured dorsal root ganglia (DRG) neurons, retrolinkin was found to colocalize with BPAG1n4 on punctate structures, but not with control proteins such as NF-L or the ER marker protein Bip (SI Fig. 9). The in vivo association was also examined by coimmunoprecipitation assays on mouse brain extracts. Retrolinkin was detected in complexes coimmunoprecipitated with anti-BPAG1n4, confirming that the two proteins are copresent in a complex (Fig. 3C, lane 2).
Fig. 3.
Retrolinkin interacts directly with BPAG1n4. (A) Yeast two-hybrid assay for BPAG1n4-retrolinkin interaction. (1) Positive control of the system. (2) Cytosolic domain of retrolinkin plus ERM2 of BPAG1n4. (3) Cytosolic domain of retrolinkin plus empty vector. (4) Cytosolic domain of retrolinkin plus p150Glued. (B) Pull-down assay for BPAG1n4-retrolinkin binding activity: BPAG1n4 protein was immobilized either on anti-BPAG1n4 (lanes 3, 4, 3′, and 4′) or sham control (lanes 5 and 5′) antibody-conjugated Protein A-Sepharose 4B. The beads were incubated with either His-retrolinkin (amino acids 31–460) (lanes 3, 5, 3′, and 5′) or His-TBCB (lanes 4 and 4′) for binding activity. Lanes 1 and 2: the total lysates of purified His-tagged proteins of His-retrolinkin (RTLN) (lane 1) and His-TBCB (lane 2). Bound, beads fraction; unbound, supernatant. The blot was immunoprobed with anti-His antibody. (C) Coimmunoprecipitation of BPAG1n4 and retrolinkin from mouse brain extracts. The anti-BPAG1n4 antibody (lane 2), but not the sham control or the beads alone (lanes 3 and 4), was able to coimmunoprecipitate retrolinkin. Lane 1: total brain lysates as detection control. (D–F) Double Immuno-EM labeling of mouse sciatic nerve sections with antiretrolinkin (15 nm) and anti-BPAG1n4 (5 nm). Arrows indicate colocalization of retrolinkin and BPAG1n4. (Scale bar: 100 nm, D–F.)
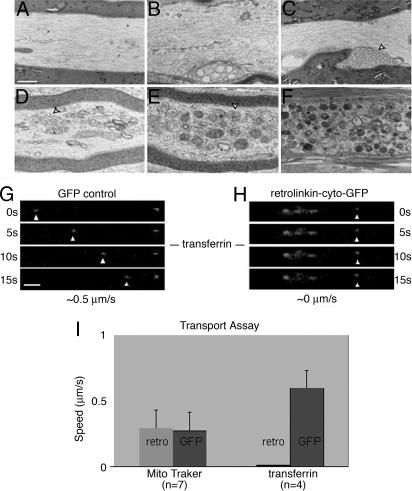
In BPAG1 null sensory axons, the progressive accumulation of vesicles and other membranous organelles is correlated with severely disrupted retrograde axonal transport (12). Before accumulating (Fig. 4F), vesicles were found to form chains or multivesicular bodies (Fig. 4 B–E), similar to those structures observed in retrolinkin-transfected cells (SI Fig. 8C Inset). One interpretation is that the absence of retrolinkin–BPAG1n4 interaction in BPAG1 null animals severs the docking of motor complexes to retrolinkin-associated membrane cargoes, resulting in a failure of retrograde axonal transport of the vesicles. Thus, disrupting retrolinkin–BPAG1n4 interaction could phenocopy disruption of BPAG1n4 function. To test this idea, we used viral transduction to overexpress the GFP-tagged cytoplasmic domain of retrolinkin (retrolinkin-Cyto-GFP) in DRG neurons. This fusion protein contains no membrane docking sites but retains BPAG1n4-binding activity. Because the truncated retrolinkin does not localize on vesicular structures in COS7 cells (Fig. 2C), the protein was expected to act in a dominant negative fashion competing with endogenous retrolinkin for BPAG1n4 binding, thus preventing BPAG1n4 (thereby dynein/dynactin motor complex) binding to retrolinkin-associated membrane cargoes. The expression of the fusion protein was monitored by green fluorescence, which was present in the cell bodies and the axons of transfected cells (SI Fig. 10 A and C). To analyze retrograde axonal transport, Texas red-conjugated transferrin (Tf-TR) transport assays were carried out 7 days posttransduction in the axons of virally transduced neurons, as described (12). Indeed, retrograde movement of Tf-TR particles was dramatically reduced in ≈90% of the transduced neurons. The vesicles barely moved (Fig. 4 H and I and SI Movie 1), a result that recapitulates that in BPAG1 null sensory neurons and WT neurons in which the interaction of BPAG1n4 with dynactin has been selectively inhibited (12). The viability of transduced cells was evaluated by using Trypan blue exclusion assays (SI Fig. 10 B, D, and F). In contrast, in GFP control neurons, Tf-TR particles were transported rapidly and consistently toward cell bodies (Fig. 4G and I and SI Movie 2). The dominant negative activity of retrolinkin-cyto-GFP demonstrates that a functional interaction between retrolinkin and BPAG1n4 is required for retrograde axonal transport of vesicles. It raises the possibility that it is through improperly anchoring BPAG1n4 (and thereby the dynein/dynactin complex) to vesicles that the dominant negative mutant of retrolinkin disrupts retrograde axonal transport. Interestingly, when mitochondrial transport was assayed with Mitotracker (22) in retrolinkin-cyto-GFP-overexpressing DRG neurons, these organelles moved bidirectionally between other stationary mitochondria, displaying no significant difference to the motions observed in GFP-expressing control neurons (Fig. 4I). The apparently unimpaired transport behavior of mitochondria in retrolinkin-cyto-GFP supports the idea that dominant negative activity of retrolinkin-cyto-GFP affects mainly the transport of vesicles.
Fig. 4.
Full-length retrolinkin function is required for retrograde axonal transport. (A–F) Vesicle fusion and accumulation caused in BPAG1 knockout mouse. EM of longitudinal sections of spinal cord dorsal column reveals fusion and accumulation of vesicles, multivesicular bodies, and other organelles in BPAG1 null (B–F), but not in WT (A). (Scale bar: 1 μm.) (G and H) BPAG1n4–retrolinkin interaction is required for retrograde transport in sensory axons. Transport assays of transferrin-labeled vesicles in cultured DRG neurons overexpressing GFP (G) or retrolinkin-cyto-GFP (H). Shown are montages of vesicle transport over time in a segment of neurite. Velocities of vesicle transport are indicated at the bottom. (Scale bar: 5 μm.) (I) Quantitative measurement of transport assays for MitoTracker-labeled mitochondria and transferrin-labeled vesicles in DRG neurons overexpressing GFP or retrolinkin-cyto-GFP.
BPAG1n4 has been shown to bind directly to p150Glued dynactin with its ERM1 domain (12). The importance of this interaction to retrograde axonal transport has been demonstrated (12). Given the findings reported herein, we propose that retrolinkin functions as a membrane receptor for BPAG1n4 to anchor the dynein/dynactin motor complex to endosomal cargoes. To examine this hypothesis, we used double immuno-EM to examine sciatic nerves by using antibodies against retrolinkin and the dynein intermediate chain (DIC). We asked whether the localization of dynein motor protein to retrolinkin positive vesicles remains consistent in the absence of BPAG1n4. Colabeling of retrolinkin and dynein was quantitatively examined in WT and BPAG1 null samples. As predicted, there was essentially no colocalization of retrolinkin and DIC on accumulated membrane structures in BPAG1 null samples (Fig. 5G–I); in total only two or three DIC-associated particles could be found in all of the retrolinkin-labeled vesicles examined (>100) in three independent trials. In contrast, ≈83.3% ± 16.7% of retrolinkin-associated structures showed colabeling with DIC in WT tissues (Fig. 5 D–F). Additional support was obtained from biochemical analysis on fractionated vesicles (23) isolated from WT or BPAG1 null mice. Although the levels of retrolinkin and vesicle markers remain comparable between WT and BPAG1 null tissues, the level of DIC in the null samples was consistently found to be significantly reduced in the endosomal fraction (Fig. 5A, compare lanes 3 and 4). When the endosomal fraction was further analyzed through a 5–10–15–20–40% sucrose gradient, the reduction of DIC became more apparent in the null samples (Fig. 5B, compare lanes 5 and 6, and 7 and 8). These results strongly support the view that it is through its associations with retrolinkin and p150Glued that BPAG1n4 mediates docking of the dynein/dynactin motor complex to vesicle cargoes for retrograde axonal transport. Given the degenerative sensory neuron phenotype that characterizes the BPAG1 null mouse (11, 15), both retrolinkin and BPAG1n4 appear to play an essential role in sensory neurons.
Fig. 5.
Dynein/dynactin is nearly absent on the surface of retrolinkin-associated vesicles in BPAG1 null axons. (A and B) Vesicle fractionation assays. (A) Spinal cord and sciatic nerve tissue extracts from WT and BPAG1 null mice (KO) were centrifuged through an 8–35–40% sucrose gradient for 30 min at 150,000 × g. The interface pellets were collected and analyzed sequentially with antiretrolinkin (RTLN) and anti-Rab5B, anti-DIC, anti-LAMP-2, and anti-Rab7 (compare lanes 3 with 4). Endo, endosomal fraction; heavy, heavy membrane fraction. (B) After this prescreening, the endosomal fractions of WT and KO samples were further analyzed through a 5–10–15–20–40% sucrose gradient and immunoprobed with anti-p150Glued, anti-DIC, and Rab5B, confirming that the level of dynein/dynactin motor proteins in the KO samples were significantly decreased in endosomal fractions (compare lanes 5 and 6, and 7 and 8). F1, interface of 5–10% (light membrane fraction); F2, interface of 10–15% (early endosome rich fraction); F3, interface of 15–20% (early plus late endosomes). The 20–40% fraction was not taken. (C–I) Double immuno-EM analysis of sciatic nerve sections shows that the dynein motor protein associates with retrolinkin-labeled vesicles in WT (D–F) but fails to dock onto the same type of vesicles in BPAG1 null (G–I). Negative controls were performed with secondary antibodies alone in C. White arrows indicate colocalization of retrolinkin (15 nm) and DIC (5 nm). White boxes in H indicate retrolinkin labeling on fused and accumulated vesicles (high magnification in I), and the black arrow indicates DIC labeling (I) in BPAG1 null axons. [Scale bar: 100 nm (C–E), 50 nm (F), 65 nm (G and I), and 600 nm (H).]
In recent years, defects in axonal transport in human neurological diseases have become increasingly evident (24–32). However, how the cargo specificity is achieved in the retrograde transport pathway remains poorly understood. Our results suggest that, in combination with BPAG1n4, retrolinkin links vesicles to the retrograde motor complex. The identification of retrolinkin as an endosomal vesicular receptor may significantly advance our understanding of cargo–motor interaction in retrograde axonal transport. It may represent an important step toward identification of a family of vesicle adaptors/receptors responsible for cargo selection and specificity in different cell types and subcellular compartments.
Materials and Methods
Plasmid Construction.
The yeast two-hybrid bait construct was generated by inserting a PCR-amplified DNA fragment encoding amino acids 4680–5416 of BPAG1n4 into the pGBKT7 vector (Clontech, Mountain View, CA). Prey constructs for interaction site mapping were generated by inserting PCR-amplified fragments encoding various regions of retrolinkin into the pGADT7 vector (Clontech). Flag-tagged full-length retrolinkin was cloned into pcDNA3.1D/V5-TOPO (Invitrogen, Carlsbad, CA). Retrolinkin-cyto (amino acids 173–406) was cloned into pEGFP-N2 (Clontech) to create a GFP fusion mammalian expression construct. EGFP and retrolinkin-cyto-GFP were cloned into pLenti6/V5-D-TOPO (Invitrogen). The bacterial expression constructs for the retrolinkin N terminus (amino acids 31–460) were generated with pET-28a(+) (Novagen, San Diego, CA) and pGEX4T-1 (Amersham, Piscataway, NJ). The sequences of all DNA inserts generated from PCRs were confirmed by sequencing.
Antibodies.
We used the following antibodies: rabbit anti-GFP (Santa Cruz Biotechnology, Santa Cruz, CA), mouse anti-α-tubulin (Sigma, St. Louis, MO), mouse anti-β-actin (Sigma), mouse anti-p150Glued (BD Transduction Laboratories, Franklin Lakes, NJ), mouse anti-DIC (Santa Cruz Biotechnology), rabbit anti-Rab5B (Santa Cruz Biotechnology), rabbit anti-Rab7 (Santa Cruz Biotechnology), mouse anti-EEA1 (BD Transduction Laboratories), mouse anti-APP N terminus (Roche Molecular Biochemicals, Indianapolis, IN), mouse anti-APP C terminus (Zymed, Carlsbad, CA), rabbit anti-Trk (B-3; Santa Cruz Biotechnology), mouse anti-NF-68 (Sigma), anti-β-COP (BD Transduction Laboratories), anti-Bip (BD Transduction Laboratories), mouse anti-TGN 58K (Sigma), mouse anti-GST (Chemicon, Temecula, CA), secondary antibodies conjugated to Alexa-594 and Alexa-488, Cy5 (Molecular Probes, Carlsbad, CA), secondary antibodies conjugated to horseradish peroxidase (Jackson ImmunoResearch, West Grove, PA), and secondary antibodies conjugated to gold (Ted Pella Inc., Redding, CA). Rabbit and guinea pig polyclonal antibodies were raised against the N terminus (amino acids 31–460) of retrolinkin.
Yeast Two-Hybrid Screen.
We used the ERM2 domain of BPAG1n4 (amino acids 4680–5416) as bait in a yeast two-hybrid screen of a human fetal brain cDNA library (Matchmaker cDNA library, Clontech).
Deglycosylation Using Endo H.
The manufacturers' instructions were generally followed with minor modifications. Trichloroacetic acid (TCA) precipitation was performed on the total brain lysates, which were washed with acetone three times to remove the residual TCA. The pellet was dried and resuspended in 1× glycoprotein denaturing buffer (0.5% SDS/0.04% Triton X-100). The resuspended protein was denatured at 100°C for 10 min. After the addition of 1× G5 Buffer (50 mM sodium citrate), 2 μl of endo H (New England BioLabs, Ipswich, MA) was added, and the reaction mix was incubated for 1–2 h at 37°C. After the treatment, the reactions were subjected to immunoblot analysis.
Differential Fractionation and Sucrose Gradient.
Adult mouse spinal cord and sciatic nerves were homogenized in 250 mM sucrose, 3 mM imidazole, 1 mM EDTA, and protease inhibitor mixture. Lysates were centrifuged at 1,000 × g for 10 min, and the pellet was saved as nuclear fraction. The supernatant (postnuclear fraction) was centrifuged at 100,000 × g for 1 h at 4°C. The pellet and supernatant separated by ultracentrifugation were saved as membrane (P100) and cytosol fractions, respectively. The total membrane fraction (P100) was then adjusted to 40% sucrose and loaded on the bottom of a step sucrose gradient of 40–35–8%, or 25–20–15–5%. Gradients were centrifuged at 150,000 × g for 2 h at 4°C. Endosomal membranes were recovered at the interfaces of 8–35% or 10–15% and 15–20%, whereas heavy membranes (ER and Golgi) were at the 35%–40% interfaces.
Antibody Competition Assays.
The specificity of antibodies against retrolinkin is confirmed by competition assays with the recombinant protein (His-tagged mouse retrolinkin fragment, amino acids 31–460) used to raise the same antibodies. Antibodies were incubated with the recombinant protein (molar ratio 1:5) for 1 h at room temperature before being used for Western blotting or indirect immunofluorescence staining.
Pull-Down Assay of His-Tagged Recombinant Proteins.
Mouse brain was homogenized and lysed in 50 mM Tris·Cl (pH 7.4), 10 mM Hepes (pH 7.4), 150 mM NaCl, 0.5 mM EDTA, 1% Triton X-100, and protease inhibitors. Aliquots of the postnuclear fraction were incubated with rabbit polyclonal antibodies and Protein A-Sepharose 4B conjugate (Zymed) overnight at 4°C. Antibody-conjugated Protein A-Sepharose 4B was then washed with PBS buffer to remove nonspecific protein binding before incubation with purified His-tagged protein for 8 h at 4°C. Proteins bound to Sepharose 4B were eluted after intensive wash and analyzed by SDS/PAGE and Western blotting. The unbound supernatants were loaded as controls.
Viral Production and Transduction of Primary DRG Cells.
Viral production and transduction of primary neurons was performed according to the manufacturer's instructions (Invitrogen). Briefly, DRG cells from newborn mice (postnatal days 0–3) were cultured in Neurobasal medium with B27 supplement, glutamax, antimitotic, nerve growth factor (50 ng/ml) and gentamycin for 5 days. Cells were then incubated with viral stock harvested from 293FT cells overnight at 37°C. Transduced cells were cultured in fresh medium for 5–7 days before the transferrin (12) and mitochondria (22) transport assays.
In Vivo Imaging of Transferrin-Labeled Vesicles and Mitochondria.
For live imaging of mitochondria movement in neurons, cells were incubated with 200 nM MitoTracker Red CMXRos (Invitrogen) for 10 min at 37°C. After washing out the free dye in solution, cells were mounted on a TE2000U inverted microscope (Nikon, Tokyo, Japan) equipped with a ×100 oil immersion objective lens (N.A. = 1.45; Nikon) and illuminated with a mercury lamp (DCLP565, EX546/12, EM605/20). Time-lapse fluorescence images (two frames per s) were collected with a CCD camera (CoolSNAP HQ; Tucson, AZ) with 12-bit dynamic range. Images were scaled to fit the 8-bit color table. Transferrin experiments were performed on the same setup.
Supplementary Material
Acknowledgments
This work was supported by a Basil O'Connor Starter Scholar Research Award (March of Dimes) and National Institutes of Health Grants NS42791 and NS43281 (to Y.Y.) and NS24054 (to W.C.M.). J.-J.L. is a postdoctoral fellow supported by the Walter and Idun Berry Fellowship from the Stanford University School of Medicine.
Abbreviations
- endo H
endoglycosidase H
- ER
endoplasmic reticulum
- EEA1
early endosome antigen 1
- APP
amyloid precursor protein
- TBCB
tubulin folding cofactor B
- DRG
dorsal root ganglia
- DIC
dynein intermediate chain.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0602222104/DC1.
References
- 1.Goldstein LS, Yang Z. Annu Rev Neurosci. 2000;23:39–71. doi: 10.1146/annurev.neuro.23.1.39. [DOI] [PubMed] [Google Scholar]
- 2.Schliwa M, Woehlke G. Nature. 2003;422:759–765. doi: 10.1038/nature01601. [DOI] [PubMed] [Google Scholar]
- 3.Vale RD. Cell. 2003;112:467–480. doi: 10.1016/s0092-8674(03)00111-9. [DOI] [PubMed] [Google Scholar]
- 4.Nakagawa T, Setou M, Seog D, Ogasawara K, Dohmae N, Takio K, Hirokawa N. Cell. 2000;103:569–581. doi: 10.1016/s0092-8674(00)00161-6. [DOI] [PubMed] [Google Scholar]
- 5.Bowman AB, Kamal A, Ritchings BW, Philp AV, McGrail M, Gindhart JG, Goldstein LS. Cell. 2000;103:583–594. doi: 10.1016/s0092-8674(00)00162-8. [DOI] [PubMed] [Google Scholar]
- 6.Schroer TA. Annu Rev Cell Dev Biol. 2004;20:759–779. doi: 10.1146/annurev.cellbio.20.012103.094623. [DOI] [PubMed] [Google Scholar]
- 7.Holleran EA, Ligon LA, Tokito M, Stankewich MC, Morrow JS, Holzbaur EL. J Biol Chem. 2001;276:36598–36605. doi: 10.1074/jbc.M104838200. [DOI] [PubMed] [Google Scholar]
- 8.Muresan V, Stankewich MC, Steffen W, Morrow JS, Holzbaur EL, Schnapp BJ. Mol Cell. 2001;7:173–183. doi: 10.1016/s1097-2765(01)00165-4. [DOI] [PubMed] [Google Scholar]
- 9.Hoogenraad CC, Akhmanova A, Howell SA, Dortland BR, De Zeeuw CI, Willemsen R, Visser P, Grosveld F, Galjart N. EMBO J. 2001;20:4041–4054. doi: 10.1093/emboj/20.15.4041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koushika SP, Schaefer AM, Vincent R, Willis JH, Bowerman B, Nonet ML. J Neurosci. 2004;24:3907–3916. doi: 10.1523/JNEUROSCI.5039-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo L, Degenstein L, Dowling J, Yu QC, Wollmann R, Perman B, Fuchs E. Cell. 1995;81:233–243. doi: 10.1016/0092-8674(95)90333-x. [DOI] [PubMed] [Google Scholar]
- 12.Liu JJ, Ding J, Kowal AS, Nardine T, Allen E, Delcroix JD, Wu C, Mobley W, Fuchs E, Yang Y. J Cell Biol. 2003;163:223–229. doi: 10.1083/jcb.200306075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ding J, Liu JJ, Kowal AS, Nardine T, Bhattacharya P, Lee A, Yang Y. J Cell Biol. 2002;158:427–433. doi: 10.1083/jcb.200202055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liakopoulos TD, Pasquier C, Hamodrakas SJ. Protein Eng. 2001;14:387–390. doi: 10.1093/protein/14.6.387. [DOI] [PubMed] [Google Scholar]
- 15.Yang Y, Dowling J, Yu QC, Kouklis P, Cleveland DW, Fuchs E. Cell. 1996;86:655–665. doi: 10.1016/s0092-8674(00)80138-5. [DOI] [PubMed] [Google Scholar]
- 16.Dowling J, Yang Y, Wollmann R, Reichardt LF, Fuchs E. Dev Biol. 1997;187:131–142. doi: 10.1006/dbio.1997.8567. [DOI] [PubMed] [Google Scholar]
- 17.Yamanaka K, Vande Velde C, Eymard-Pierre E, Bertini E, Boespflug-Tanguy O, Cleveland DW. Proc Natl Acad Sci USA. 2003;100:16041–16046. doi: 10.1073/pnas.2635267100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martin-Zanca D, Oskam R, Mitra G, Copeland T, Barbacid M. Mol Cell Biol. 1989;9:24–33. doi: 10.1128/mcb.9.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mu FT, Callaghan JM, Steele-Mortimer O, Stenmark H, Parton RG, Campbell PL, McCluskey J, Yeo JP, Tock EP, Toh BH. J Biol Chem. 1995;270:13503–13511. doi: 10.1074/jbc.270.22.13503. [DOI] [PubMed] [Google Scholar]
- 20.Selkoe DJ. Curr Opin Neurobiol. 1994;4:708–716. doi: 10.1016/0959-4388(94)90014-0. [DOI] [PubMed] [Google Scholar]
- 21.Wunderlich W, Fialka I, Teis D, Alpi A, Pfeifer A, Parton RG, Lottspeich F, Huber LA. J Cell Biol. 2001;152:765–776. doi: 10.1083/jcb.152.4.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vanden Berghe P, Hennig GW, Smith TK. Am J Physiol. 2004;286:G671–G682. doi: 10.1152/ajpgi.00283.2003. [DOI] [PubMed] [Google Scholar]
- 23.Tai AW, Chuang JZ, Bode C, Wolfrum U, Sung CH. Cell. 1999;97:877–887. doi: 10.1016/s0092-8674(00)80800-4. [DOI] [PubMed] [Google Scholar]
- 24.Williamson TL, Cleveland DW. Nat Neurosci. 1999;2:50–56. doi: 10.1038/4553. [DOI] [PubMed] [Google Scholar]
- 25.Dupuis L, de Tapia M, Rene F, Lutz-Bucher B, Gordon JW, Mercken L, Pradier L, Loeffler JP. Neurobiol Dis. 2000;7:274–285. doi: 10.1006/nbdi.2000.0292. [DOI] [PubMed] [Google Scholar]
- 26.Zhao C, Takita J, Tanaka Y, Setou M, Nakagawa T, Takeda S, Yang HW, Terada S, Nakata T, Takei Y, et al. Cell. 2001;105:587–597. doi: 10.1016/s0092-8674(01)00363-4. [DOI] [PubMed] [Google Scholar]
- 27.Reid E, Kloos M, Ashley-Koch A, Hughes L, Bevan S, Svenson IK, Graham FL, Gaskell PC, Dearlove A, Pericak-Vance MA, et al. Am J Hum Genet. 2002;71:1189–1194. doi: 10.1086/344210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hafezparast M, Klocke R, Ruhrberg C, Marquardt A, Ahmad-Annuar A, Bowen S, Lalli G, Witherden AS, Hummerich H, Nicholson S, et al. Science. 2003;300:808–812. doi: 10.1126/science.1083129. [DOI] [PubMed] [Google Scholar]
- 29.Puls I, Jonnakuty C, LaMonte BH, Holzbaur EL, Tokito M, Mann E, Floeter MK, Bidus K, Drayna D, Oh SJ, et al. Nat Genet. 2003;33:455–456. doi: 10.1038/ng1123. [DOI] [PubMed] [Google Scholar]
- 30.LaMonte BH, Wallace KE, Holloway BA, Shelly SS, Ascano J, Tokito M, Van Winkle T, Howland DS, Holzbaur EL. Neuron. 2002;34:715–727. doi: 10.1016/s0896-6273(02)00696-7. [DOI] [PubMed] [Google Scholar]
- 31.Stokin GB, Lillo C, Falzone TL, Brusch RG, Rockenstein E, Mount SL, Raman R, Davies P, Masliah E, Williams DS, Goldstein LS. Science. 2005;307:1282–1288. doi: 10.1126/science.1105681. [DOI] [PubMed] [Google Scholar]
- 32.Cavalli V, Kujala P, Klumperman J, Goldstein LS. J Cell Biol. 2005;168:775–787. doi: 10.1083/jcb.200410136. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.