Abstract
The crystal structure of the RCK-containing MthK provides a molecular framework for understanding the ligand gating mechanisms of K+ channels. Here we examined the macroscopic currents of MthK in enlarged Escherichia coli membrane by patch clamp and rapid perfusion techniques and showed that the channel undergoes desensitization in seconds after activation by Ca2+ or Cd2+. Additionally, MthK is inactivated by slightly acidic pH only from the cytoplasmic side. Examinations of isolated RCK domain by size-exclusion chromatography, static light scattering, analytical sedimentation, and stopped-flow spectroscopy show that Ca2+ rapidly converts isolated RCK monomers to multimers at alkaline pH. In contrast, the RCK domain at acidic pH remains firmly dimeric regardless of Ca2+ but restores predominantly to multimer or monomer at basic pH with or without Ca2+, respectively. These functional and biochemical analyses correlate the four functional states of the MthK channel with distinct oligomeric states of its RCK domains and indicate that the RCK domains undergo oligomeric conversions in modulating MthK activities.
Keywords: inactivation, desensitization, channel structure, giant spheroplast, patch clamp
K+ channels are found in organisms of all three kingdoms and are even found to be encoded by certain viruses. Despite their diverse biological roles, the fundamental molecular mechanisms underlying the ion-selective conductance and channel gating are universal (1–6). Each K+ channel has four subunits that converge to form an ion permeation pathway encircled by gating elements such as voltage- or ligand-activation modules. One such ligand-activation module called RCK can be found at the cytoplasmic carboxyl (C) terminus of the large-conductance, Ca2+-gated K+ channels (BKs) of animals (7) and a large number of prokaryotic K+ channels (8). In animal BK channels the RCK domains act as Ca2+ or pH sensors for modulating the voltage-gating properties (9–11). Among prokaryotic ones, the MthK channel of the archaeon Methanobacterium thermoautotrophicum is activated by millimolar concentrations of Ca2+ through binding to its RCK domains (3).
MthK consists of a two-span transmembrane pore domain of ≈100 aa and a C-terminal cytoplasmic RCK domain of ≈230 aa. Critical insights into the general mechanism of K+ channel gating have been made based on the comparison of the crystal structures of KcsA and MthK, in which the pore-lining helices (TM2) bend to sterically control access to the ion permeation pathway (6, 12). By comparing the crystal structures of the RCK domains of Escherichia coli Kch (7) and MthK channels, an octameric RCK “gating ring” assembly has been proposed as the gating machinery (3). An intriguing feature of the gating ring is the existence of four free RCK domains (M107–A336) associated with the four membrane-anchored RCK domains. The gating ring conformation is experimentally based on the crystal structure of a MthK mutant, M107I, in which the membrane-anchored RCK domains of two M107I channels, lacking their free RCK domains, are arranged by a tail-to-tail (RCK-to-RCK) assembly between two M107I channels. Therefore, it is assumed that the four free RCK domains of MthK would assemble noncovalently with the four membrane-anchored RCK domains in the same manner (3, 13).
The mode of the dimeric assembly of the RCK domain of MthK resembles that of the RCK dimer of Kch (7), and KTN (K+ Transport and Nucleotide binding) dimers of Mja218 and Bsu222 (14). Together, RCK and KTN domains form a unique structurally related family herein referred to as RCK/KTN family. The structure of the canonical RCK/KTN dimer is most notably characterized by a swapping exchange of the sixth helix (αF, or α6 of KTN) between the two subunits stabilizing the dimeric assembly. Near the dimer interface, referred to as “flexible” or “hinged” interface, lies the Ca2+- or NAD(H)-binding pockets of the RCK or KTN domain dimers, respectively. The scaffold of the canonical RCK/KTN dimer displays a wide variation of hinge angles (14). The gating ring model predicts that Ca2+ binding to each RCK narrows the hinge angle and, in turn, expands the diameter of the gating ring and thus the pore entryway (3, 13).
Despite the elegant simplicity of the model, there are experimental data suggesting that the gating machinery may not require free RCK domains (15, 16). Moreover, the isolated KTN domain forms a variety of assembly modes of the canonical dimers, including tetramer in solution (14) and also octamers in the crystals (17), indicating that the flexibility of the RCK/KTN domain assembly can result in different modes of oligomers.
To better understand how the RCK domain mediates ion channel activities, we characterized the functional properties of MthK and found that the ligand-mediated activation of MthK is followed by a slow desensitization despite the continuous presence of the ligands. Interestingly, the channel is also sensitive to the pH on the cytoplasmic side. Additionally, our biochemical examinations of the isolated RCK protein reveal striking structure–function correlations between the oligomeric states of the RCK domain and the functional states of the channel in responses to Ca2+ and pH. These data therefore illustrate that the transitions among the closed, open, desensitized, and inactivated states of MthK are mediated by ligand- and pH-induced oligomeric conversions of the RCK domain assembly.
Results
MthK Undergoes Desensitization After Ca2+ or Cd2+ Activation.
The activities of purified MthK were previously examined after incorporation into planar lipid bilayers (3, 18, 19). Here we investigated its activity in inside-out membrane patches directly excised from giant E. coli spheroplasts (20). A K+-specific current was readily observed upon 20 mM Ca2+ perfusion at pH 7.5 (Fig. 1A, bottom trace). Patches from control transformants never exhibited this Ca2+-inducible current (n > 20) (Fig. 1A, top trace), even though they readily displayed the activities of the nanoSiemens conductances of the native mechanosensitive channels when suction was applied (21) (data not shown). The number of unitary conductances in a single patch varied between 100 and 600. Similar to those reported previously, the unitary current rectifies inwardly (Fig. 1B), and the Ca2+ dose–response of the macroscopic current has a half-effective concentration (EC50) of ≈8.5 mM (Fig. 1C). However, the Hill coefficient, ≈2.4 (Fig. 1C), and the unitary conductance, ≈50 pS at −100 mV (Fig. 1B), are somewhat smaller than those determined in the synthetic lipid bilayer (3, 18, 19). By rapidly switching between Ca2+ and EGTA solution, we found both activation and deactivation being half complete in <200 msec (t1/2 = 128 ± 26 msec and 91 ± 33 msec, respectively; n = 12) (Fig. 1D).
Fig. 1.
MthK currents recorded from inside-out membrane patches of giant E. coli spheroplasts at pH 7.5. (A) A Ca2+-inducible K+ current is observed from a patch excised from an E. coli expressing MthK (bottom trace) but not one bearing the control empty vector (top trace). Unitary currents are evident after the bulk desensitization (Inset). (B) Unitary current–voltage plot. The pipette and bath are filled with symmetric solutions. With Ca20K (filled circle), the Ca2+-inducible current rectifies inwardly. No current was observed when K+ was replaced with Na+ (empty circles, Ca20Na) (n = 5 patches; SDs are shown). (C) Ca2+ dose–response curve. The macroscopic peak amplitudes were normalized with the unitary current at each Ca2+ concentration. The Hill equation fitting curve is shown in smooth line. Each test episode took place after a rest period of ≈8 min in EGTA solution (n = 6 patches; means and SDs are shown). (D) Rapid Ca2+ activation and deactivation. (E) MthK desensitization can be fit with a single-exponential equation (smooth curve). (F) Recovery from the Ca2+-desensitized state, as examined in a continued recording trace from a single patch. After excision, the membrane patch was perfused with EGTA solution for over 10 min before the first activation by Ca2+ (far left spike). After full desensitization, Ca2+ activations (second to eighth spikes) were repeatedly tested in an increasing time intervals, during which the patch was perfused with EGTA solution to recover the desensitized channels. Signals were digitized at 250 Hz. (G) A model of MthK functional states.
Interestingly, a slow decline in MthK activity is evident when Ca2+ perfusion continues. The current reaches its peak rapidly and then gradually decays to zero in seconds, indicating that the MthK undergoes desensitization. Although the kinetics may be more complex, this desensitization process can be approximated as a single-exponential curve with a time constant of 4.5 ± 1.9 sec (n = 11) (Fig. 1E). The loss of activity in the prolonged Ca2+ perfusion is not permanent. The desensitized channels recover after Ca2+ is removed (tau ≈ 110 sec) (Fig. 1F). These findings entail at least three functional states of MthK upon Ca2+ binding: closed, open, and desensitized states (Fig. 1G).
With the excised MthK-containing membrane patch for ligand screening, we found that Cd2+ is more effective than Ca2+ to activate MthK. The rates of Cd2+ activation and deactivation are similar to those of Ca2+ (t1/2 = 105 ± 9 and 116 ± 34 msec, respectively; n = 5) (Fig. 2A). Millimolar concentrations of Cd2+ from the cytoplasmic side also block MthK and reduces the unitary current (Fig. 2C Right) as Ca2+ does (Fig. 2C Left) (3, 19). The Cd2+-activated MthK also undergoes desensitization, but with a slower time constant than the Ca2+-activated one (tau = 18.0 ± 1.0 sec, n = 5, single-exponential fit) (Fig. 2B). The macroscopic Cd2+ dose–response reveals a left-shift with an EC50 of ≈3.5 mM and a Hill coefficient of ≈1.4 (Fig. 2D). Because of the similarity in charge and ionic radius between Cd2+ and Ca2+ (0.97 Å and 0.99 Å, respectively), Cd2+ presumably binds to the same acidic amino acid side chains coordinating Ca2+.
Fig. 2.
MthK currents activated by cadmium. (A) A typical trace shows the rapid Cd2+ activation and deactivation. (B) The Cd2+-activated MthK also undergoes desensitization. The smooth curve shows a single-exponential fitting. (C) Traces of unitary currents show that millimolar concentrations of Ca2+ and Cd2+ from the cytoplasmic side reduce the unitary conductance of MthK. The signals were digitized at 10 kHz. (D) Macroscopic Cd2+ dose–response. The Hill equation fitting curve is shown by the smooth curve (n = 5). The Ca2+ dose–response curve is shown by the smooth dashed curve.
Neutral to Slightly Acidic pH Inactivates MthK.
Interestingly, the activity of MthK is profoundly affected by the pH of the perfusion solutions. In contrast to the nearly full activation by 20 mM Ca2+ at pH 7.5, MthK cannot be activated when the pH of the Ca2+ solution is slightly shifted toward acidic (pH 6.5 or lower) [Fig. 3A Left, dark blue (pH 6.5) and black (pH 6.0) traces]. The H+ dose–response curve reveals a half-inactivation pH (IC50) of ≈7.1 and a Hill coefficient of ≈1.6 (Fig. 3A Right). The acidic pH not only inactivates the resting closed channels, but also the Ca2+-activated (open) channels. Fig. 3B shows that the Ca2+-activated channels (at pH 7.5) can be rapidly inactivated when the pH of the Ca2+ perfusion solution is switched to 6.5 or lower (t1/2 = 268 ± 89, pH 6.5, and 128 ± 64 msec, pH 6.0; n = 5). Unlike the Ca2+-desensitized MthK, however, the acid-inactivated channels are readily activated by Ca2+ when the pH is restored to 7.5 (Fig. 3C, arrows). Furthermore, the acid inactivation happens only when the low pH is applied from the cytoplasmic side because MthK remain fully active when the pipette is filled with a pH 6.0 solution (Fig. 3D). These results together indicate that the acid inactivation and the ligand-mediated desensitization are two distinct kinetic processes (Fig. 3E). Because acid inactivation acts only from the cytoplasmic side of the channel, the RCK assembly is the primary candidate of the pH-sensing modules.
Fig. 3.
MthK is inactivated by neutral to slightly acidic pH only from the cytoplasmic side. (A Left) Lowering the pH (X) of the Ca20K solution reduces the activation peak amplitude [X = pH 8.5 (gray), 8.0 (red), 7.5 (green), 7.0 (blue), 6.5 (dark blue), and 6.0 (black)]. (A Right) The means of each relative peak amplitude (circles) at each pH were fit with the Hill equation (smooth curve; n = 5 patches; SDs are shown). Each test episode took place after a rest period of ≈8 min in EGTA (pH 7.5) solution to ensure full recovery (red). (B Left) Superimposed episodes show that the Ca2+-activated MthK (Ca20K, pH 7.5 for 1 sec) can also be inactivated rapidly when the pH is dropped to 6.5 or 6.0 (dark blue and black traces, respectively). The activation peaks are normalized to the same amplitude. (B Right) t1/2 of the pH inactivation on the Ca2+-activated channels (n = 5; SDs are shown). (C) Sequential recording traces show that the acid-inactivated channels (at pH 6.0 or 6.5) can be reactivated by Ca2+ (arrows) when the pH of the Ca20K solution is returned to 7.5. (D) Lowering the pH of the pipette solution (filled with Ca20K, pH 6.0) does not inactivate the channel. (E) The acidic inactivation is a functionally distinct step from the desensitization.
Dynamic Oligomeric States of the Free RCK Depend on Ca2+ and pH.
To examine how RCK domain modulates the functional transitions between different states, isolated RCK domain (M107–A336) of MthK was separately overexpressed and purified for biochemical analyses. Interestingly, the isolated RCK domain does not form a stable octameric assembly throughout, but exists in various oligomeric states in response to both Ca2+ and pH (Fig. 4). In the absence of Ca2+ (Fig. 4A, red lines and dots), the domain is completely dimeric at pH 6.0 (Fig. 4A, first panel) but associates into high-order oligomers (hexamers) at pH 6.5 (Fig. 4A, second panel). Further increase of the pH to 8.0 or 8.5, however, dissociates the domain into predominantly monomers (Fig. 4A, third and fourth panels, respectively, and Fig. 4B). When Ca2+ is present (Fig. 4A, blue lines and dots), the domain remains dimeric at pH 6.0 (Fig. 4A, first panel) and associates into high-order oligomers (tetramers) at pH 6.5 (Fig. 4A, second panel). Interestingly, at pH 8.0 or 8.5, the domain does not dissociate into monomer as it does with no Ca2+, but remains as high-order oligomers (largely hexamers, Fig. 4A, third and fourth panels, respectively). These results together demonstrate the dynamic oligomeric nature of the RCK domain in solution and indicate that the conversions between these conformational states can account for the functional transitions of the channel in response to Ca2+ and pH.
Fig. 4.
The oligomeric states of the isolated RCK protein depend on Ca2+ and pH. (A) Size determinations in different pH solutions in the presence of 5 mM Ca2+ (blue) or EGTA (red) using size-exclusion HPLC (smooth lines, 280 nm absorption) and static light scattering (dots, the molecular masses on the y axes). Arrowheads indicate the molecular mass of each oligomeric state calculated from the amino acid sequence. (B) Sedimentation equilibrium analysis of the isolated RCK domain in pH 8.5 EGTA solution reveals that the domain is at equilibrium between monomer and hexamer, which also match the sizes derived from static light scattering measurement. (C) Summary of the oligomeric states of the isolated RCK protein in different pHs in the presence (blue) or absence (red) of Ca2+. (D) Fluorescence intensity of Trp-123 changes over time upon Ca2+ binding to the isolated RCK protein. The fluorescence measurement is indicated with gray dots. Single-exponential (tau = 5.7 sec) and double-exponential fits are shown in blue and red, respectively.
Ca2+ Converts RCK Monomers into Oligomers in Two Kinetic Steps.
To correlate the Ca2+ activation and subsequent desensitization with the dynamic conformational changes in the RCK domain at alkaline pH, we monitored the kinetics of RCK association from Ca2+-free monomers to Ca2+-bound hexamers by stopped-flow fluorescence spectroscopy. The only Trp residue (Trp-123) in the RCK domain, located close to the Ca2+-coordinating residues and the dimer hinge (Fig. 5), provides a nearly ideal fluorescence probe to monitor subtle changes in its environment. The Trp fluorescence of isolated RCK shows a rapid increase upon Ca2+ addition, which then slowly reaches its plateau (Fig. 4D). This fluorescence change does not fit well with a single-exponential equation (blue line) and requires double-exponential fitting (red line), indicating that the RCK undergoes two distinct conformational changes between the two boundary conditions, from monomers to hexamers, upon Ca2+ binding.
Fig. 5.
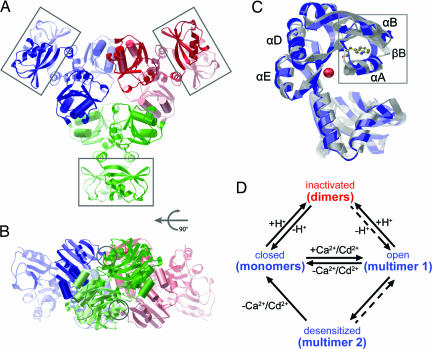
The crystal structure and oligomeric states of the RCK assembly. (A) Top view of RCK hexameric assembly. Hydrophobic αD (longer and closer to each other) and αE highlighted as cylinders at the hydrophobic interface between neighboring dimers mediate higher order assemblies. The subdomain (E259–A336) is boxed. Each canonical dimer is shown in a different color. (B) Side view of RCK hexameric assembly. The capping interdimer interaction areas centered around Asn-147 are circled. (C) Conformational changes upon Ca2+ binding are shown between Ca2+-free (blue) and Ca2+-bound (gray) RCK structures. Major differences are highlighted inside a box. The position of Trp-123 in a yellow ball-and-stick model shows its proximity to the Ca2+-binding site. Ca2+ is shown as a red sphere. (D) A model diagram to illustrate the oligomeric conversion model. Changes in RCK oligomeric states underlie transitions between four functional states of MthK: closed, open, desensitized, and acid-inactivated states. At alkaline pH (blue), the RCK domains are monomeric and the channel stays at an activatable closed state. Ca2+ converts the RCK monomers to a multimer (conformation 1), which opens the channel. The multimer undergoes a slow conformational change into another form of multimer (conformation 2), which desensitizes the channel. Removing the ligands dissociates the desensitized RCK multimer into monomers and resets the channel to the closed state. At acidic pH (red), the RCK domains are structurally locked into dimers and cannot be converted to the multimer (conformation 1) by Ca2+/Cd2+, thus rendering the channel inactivated.
In this two-step oligomerization, the initial time constant of ≈1.4 sec is followed by a slower time constant of ≈9.3 sec. Surprisingly, these two rates match close to the rates of channel activation (t1/2 ≈ 120 msec) and desensitization (tau ≈ 5 sec). Although the oligomeric state of the intermediate cannot be characterized, the close matches of the two rates implicate that the fast Ca2+ activation is mediated by an oligomeric association of RCK monomers, whereas the desensitization after Ca2+ activation is mediated by a subsequent slower conformational change to another form of multimer. The larger discrepancy (≈10-fold) between the fast rate of isolated RCK conversion and the t1/2 of channel activation can be accounted for by the entropic gain in favor of the membrane-anchored RCK of intact channel.
Three RCK Dimers Assembled into a Hexamer in Crystal Structure.
The dynamic oligomeric nature of the RCK domain in solution is consistent with a crystallographic observation, in which three canonical dimers pack into a hexamer in the crystal (Fig. 5, packed in space group R32 with a monomer in the asymmetric unit; see Materials and Methods). The core scaffold of the RCK dimer in the hexamer structure is the canonical RCK/KTN dimer, and the additional subdomains of the dimer are spatially separated outwards from the main assembly core (lined boxes enclosing the subdomains in Fig. 5A). The canonical dimer is the common building block for both the hexamer described herein and for the octameric gating ring of MthK (M107I) and isolated RCK (D184N) (3, 13), and they show little difference in hinge angle (<5°; data not shown). They are all similar such that the hexamer assembly is a tightly packed triangle-shaped “trimer” of dimers (trimer axis being vertically aligned in the viewpoint of Fig. 5B), whereas the octameric assembly in the MthK (M107I) and the RCK (D184N) is a square-shaped “tetramer” of dimers. For all cases, the “exposed hydrophobic” surface is used commonly (cylinders in Fig. 5 A and B) for the high-order assembly interface. Although the hexameric assembly of the isolated RCK protein cannot represent a physiological conformation for a tetrameric channel, this observation instead reiterates that the exposed hydrophobic surface acts as a common flexible interface for different high-order assemblies, which may provide a ground for the assembly of the observed RCK tetramers in solution (Fig. 4A) or KTN tetramers (14).
Discussion
Multiple Functional States of MthK and Oligomeric States of Its RCK Domain.
In the present study, we characterized the electrophysiological properties of MthK using the excised membrane patches of giant E. coli spheroplasts with rapid perfusion technique. We find that the functionalities of MthK are far more complex than previously thought and entail at least closed, open, desensitized, and acid-inactivated states. In parallel, we showed that the isolated RCK domain can exist in monomeric, dimeric, tetrameric, and hexameric states depending on both Ca2+ and pH. The striking kinetic correlation between the functional states of the channel and the oligomeric states of the isolated RCK domain provides the link in understanding the structure–function relationship underlying the gating machinery of the MthK channel.
Modulating MthK Activity by Oligomeric RCK Conversions.
The RCK domain exists in various oligomeric states in accordance to Ca2+ and pH. This indicates that the oligomeric conversions most likely accompany functional transitions of the intact channel. Although the structure–function relationship may be more complicated around the pH titration midpoint (pH 7.1), where the channels are partially inactivated and thus may exist in at least three states, clear correlations can be made at two pH boundaries: At pH 8.5, where MthK can be nearly 100% activated, the Ca2+causes a rapid RCK association from monomers to a multimer; at pH 6.0, where the channels are completely insensitive to Ca2+, the acidity traps the RCK domains in dimers regardless of Ca2+ (Fig. 5D). Titration of key residues at the subunit interface of RCK (13, 22), or with the inner surface of the cell membrane (16), may account for the structural changes underlying different oligomeric states. Functionally, shifting toward acidic pH is nonproductive, where the RCK domain is structurally “locked” at dimeric state and the channel remains inactivated, which is comparable to the state viewed as a nonfunctional unstable assembly (13). However, when shifting toward basic pH, the RCK domain becomes “unlocked” to monomeric state that is readily convertible to a high-order oligomer by ligand binding, to activate the channel (presumed multimer 1 in Fig. 5D). In the present study, it is not possible to conclude whether the multimers (multimers 1 and 2) are the physiological tetramer or octamer. The isolated wild-type RCK domain tends to form a hexamer, whereas the octameric assembly have been observed in the membrane-tethered RCK (3) or in the free RCK (D184N) domain (13). Tetrameric assembly can also be observed experimentally in solution by narrowly balancing the two opposing assembly forces: pH shifting to basic pH leading to lower order assembly and Ca2+-binding leading to the higher order assembly (Fig. 4A, pH 6.5, blue). It is important to note that the isolated RCK proteins have more degrees of freedom than those membrane tethered ones in doman assembly, and other interactions may retain the free RCKs physically associated with an intact channel.
When we compared atomic positions of our Ca2+-free RCK and the Ca2+-bound RCK of MthK (M107I) (Protein Data Bank ID code 1LMQ), small but significant conformational differences in two regions primarily near the Ca2+-binding site can be identified (boxed in Fig. 5C). The first region (S124–V139, average rmsd = 3.2 Å) involves αA for which the Cα of Ser-124 and Ser-126 in the Ca2+-bound RCK display the main-chain shifts up to 3.6 Å. The second region (E144–N158, average rmsd = 3.0 Å) consists of αB and the loop preceding it. αA and αB shield Trp-123 from the solvent. The αA also extensively contacts the swapped helix αF and thus likely contributes to dimer stability. Therefore, the Ca2+-triggered multimerization may indeed be initiated near αA and αB, propagate through αF, and thus stabilize RCK dimers leading to high-order assemblies adjoined through the common exposed hydrophobic surfaces.
Physiological Implications of MthK in Nature.
pH homeostasis is an important aspect in microbial cell physiology. In E. coli, for instance, the internal pH (pHi) is maintained at ≈7.4–7.8 for optimum growth and growth ceases when pHi drops below 6.6 (23–25). The fact that the pH sensitivity of MthK is parallel to the pHi variation that affects cell growth entails physiological relevance. Further pertaining to the microbial survival is the Cd2+, which is generally toxic to microbes when present in micromolar or millimolar concentrations (26). The activation of MthK by submillimolar concentrations of Cd2+ suggests that the channel may participate in heavy metal detoxification. Whether MthK can be activated by other small molecules remains to be characterized. The common nucleotide cofactors known to bind the KTN domain [e.g., NAD(H), NADP(H), or ATP (14, 17)] apparently have no effects on the activity of MthK (data not shown).
Because channels passively dissipate the electrochemical gradients when open, most animal channels have elaborate mechanisms to prevent wasteful leakage by inactivating it or to reach closure rapidly after opening. In this study, we showed that MthK, like animal channels, both slowly desensitizes and rapidly inactivates over a very narrow range of pH. Even though Methanobacterium is an archaeon, which branched off from bacteria and eukaryotes billions of years ago, the conserved features demonstrate that they share a common evolutionary origin of K+ channels.
Materials and Methods
Electrophysiology and Data Analysis.
The MthK gene was cloned from the genome of Methanobacterium thermautotrophicus strain Delta H (ATCC) into the pB11d vector (15) with a C-terminal hexhistidine tag. Giant E. coli spheroplasts were made following the protocol previously described (20) with minor modifications. For patch clamp, a glass pipette (no. 2-000-100; Drummond Scientific, Broomall, PA) was pulled to a tip size ≈4.5–5.0 bubble number with a P-97 Micropipette Puller (Sutter Instrument, Novato, CA) and filled with the Ca20K (pH 7.5) solution (10 mM Hepes·Tris, pH 7.5/500 mM sucrose/150 mM KCl/20 mM CaCl2) in all of the recordings except Fig. 1B [filled with Ca20Na (pH 7.5) containing 150 mM NaCl instead of 150 mM KCl] and Fig. 3D (Ca20K, pH 6.0). The same solution was also added to the chamber for seal formation. A seal of ≈2–3.5 GOhm was usually achieved. Gentle suctions were applied to the patch to confirm the presence of mechanosensitive channels, which serve as an internal control. Perfusion solutions contain basic components of 500 mM sucrose, 10 mM buffer (Mes·Tris for pH 6.0 and 6.5; Hepes·Tris for 7.0 and 7.5; Tris·Hepes for 8.0 and 8.5), and 150 mM KCl or NaCl. The recovery (EGTA) solution contains an additional 5 mM EGTA and 20 mM MgCl2. The activation solutions contain additional CaCl2 or CdCl2. The perfusion solutions were gravity-fed to a single-walled, three-barrel glass tubing (0.7-mm i.d. tubes) of a SF-77B perfusion system (Warner Instruments, Hamden, CT). The currents were recorded by using an EPC7 Patch Clamp Amplifier (HEKA Elektronik) and digitized by using a Digidata1322A digitizer and Clampex8.1 software (Axon Instruments, Sunnyvale, CA) at 1 kHz unless otherwise specified. The signals were low-pass-filtered at a frequency equal to or less than half of the digitizing frequency. All of the recordings were done at a holding membrane potential of −50 mV except in Fig. 1B. To calculate the EC50, IC50, and Hill coefficients (nH), the averaged data set of the relative ion channel activity (from Ca2+ or Cd2+ activation), and the relative peak amplitude (PpHX/PpH8.5 from acidic inactivation) were fitted with Hill equation: r = rmax/(1 + EC50/[L])nH, where rmax is the maximum number of relative activity and [L] is the concentration of the ligand (Ca2+, Cd2+, or H+).
Protein Expression and Purification.
The RCK domain (M107–A336) was separately expressed in the pHis9 vector (a Gateway-adapted derivative of pET-28a). After Ni-affinity chromatography, the N-terminal 9×His tag was cleaved by thrombin overnight at 4°C with dialysis in 25 mM Tris·HCl (pH 8.0), 150 mM KCl, 5 mM EGTA, and 1 mM DTT. For size-exclusion HPLC, light scattering, and sedimentation equilibrium, the protein was further purified by a Superdex-200 column in the same buffer, and the pH was titrated to 5.0, then run through a Superdex-200 column again in a K·citrate·HCl (pH 5.0) buffer. The purified RCK (≈2 mg/ml) was then dialyzed into various solutions, containing 25 mM buffer (Mes·HCl for pH 6.0 and 6.5 and Tris·HCl for pH 8.0 and 8.5), 150 mM KCl, 1 mM DTT, and 5 mM Ca2+ or EGTA. For crystallization, the protein was exchanged into 20 mM Tris·HCl (pH 8.0), 100 mM KCl, 1 mM DTT, and 1 mM EDTA, then concentrated to ≈8 mg/ml.
Size-Exclusion HPLC, Static Light Scattering, and Sedimentation Equilibrium Analyses.
Static light scattering was done with a Wyatt Minidawn in line with HPLC size-exclusion chromatography (G4000PWXL column; Tosoh Bioscience, Montgomeryville, PA). The molecular weights were calculated according to the Wyatt instruction with an extinction coefficient of 10,810 M−1·cm−1, derived by using Gill's method (27). Sedimentation equilibrium analytical ultracentrifugation was carried out on a Beckman XL-I centrifuge. The sample in pH 8.5 EGTA solution was run with three concentrations and spun at five different speeds (11,300, 16,000, 19,700, 22,700, and 25,400 × g) overnight at 20°C. The data of 15 scans of each sample were globally fit with the two-component, noninteracting model (fitting variance of 3.22 × 10−5) by using Ultrascan 6.2 software, whereas a single-component model does not generate a respectable fit.
Stopped-Flow Fluorescence Spectroscopy Analysis.
Fluorescence measurements were made on a Bio Logic MOS-450 spectrometer. The purified RCK (50 μM) in 25 mM Tris·HCl (pH 8.0–8.5), 150 mM KCl, 1 mM DTT, and 5 mM EDTA was rapidly mixed (estimated dead time of 3 msec) with 25 mM Tris·HCl, pH 8.0–8.5, 150 mM KCl, 1 mM DTT, and 65 mM CaCl2 by using a stop-flow device with a total shot volume of 150 μl to give a final free Ca2+ concentration of 30 mM (excitation at 280 nm and cutoff filter at 320 nm).
X-Ray Crystallography.
Crystals of the isolated RCK protein were obtained by hanging drop over a well solution of 0.1 M Na cacodylate (pH 6.5), 1.3–1.5 M LiSO4, and 2% benzamidine·HCl. Crystals were flash-frozen in well solution supplemented with 0.2 M LiSO4 and 12% xylitol. Data from a single crystal were collected at Stanford Synchrotron Radiation Laboratory beamline 1-5 (Stanford, CA) (0.97944 Å) and processed by using HKL2000 (28) and Truncate (29). Crystals belong to the space group R32 (a = b = 165.89 Å, c = 82.22 Å, α = β = 90°, γ = 120°, monomer per asymmetric unit). Phases were determined by molecular replacement by using two subdomains of RCK from the previous structure of MthK (M107I) (Protein Data Bank ID code 1LMQ) as a search model. The program Phaser (30) was used for a sequential molecular replacement search using subdomain 1 (R116–I229) then subdomain 2 (E259–A336) individually to ensure chain connectivity between the two subdomains. A solution was found with a log(likelihood) gain of 382. Maps were calculated with this model and the remainder of the molecule (S230–E258) built into the resulting maps. Refinement consisted of alternating rounds of manual model building using the program O (31) and automated refinement using the programs CNS (32) and Refmac (29). Refmac with three TLS groups defined (H117–P231, F232–S260, and T261–A336) was used in the final rounds of refinement. The final model consists of amino acids R116–A336 of RCK and includes no solvent molecules. The Matthews coefficient is 4.52 and solvent content is 72.6%. Crystallographic data range from 30.0 to 3.2 Å (3.31–3.20 Å for the highest resolution shell) with the following statistics: Rmerge (%) = 6.8 (29.3), I/σ = 21.5 (5.2), redundancy = 6.4 (6.4), completeness (%) = 91.7 (59.7), Rwork/Rfree = 23.2/29.9, rmsdbonds (Å) = 0.012, and rmsdangles (°) = 1.355.
Acknowledgments
We thank C. Kung and Y. Saimi for critical comments on the manuscript, S. Loukin and P. Slesinger for suggestions on data analysis, W. Kwiatkowski and J. Kelber for helping with analytical sedimentation studies, and P. O'Maille for the pHIS9 vector. K.A.B. was supported by a fellowship from the American Heart Association.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
Data deposition: The atomic coordinates and structure factors have been deposited in the Protein Data Bank, www.pdb.org (PDB ID code 2OGU).
References
- 1.Cordero-Morales JF, Cuello LG, Zhao Y, Jogini V, Cortes DM, Roux B, Perozo E. Nat Struct Mol Biol. 2006;13:311–318. doi: 10.1038/nsmb1069. [DOI] [PubMed] [Google Scholar]
- 2.Doyle DA, Cabral JM, Pfuetzner RA, Kuo AL, Gulbis JM, Cohen SL, Chait BT, MacKinnon R. Science. 1998;280:69–77. doi: 10.1126/science.280.5360.69. [DOI] [PubMed] [Google Scholar]
- 3.Jiang YX, Lee A, Chen JY, Cadene M, Chait BT, MacKinnon R. Nature. 2002;417:515–522. doi: 10.1038/417515a. [DOI] [PubMed] [Google Scholar]
- 4.Jiang Y, Lee A, Chen J, Ruta V, Cadene M, Chait BT, MacKinnon R. Nature. 2003;423:33–41. doi: 10.1038/nature01580. [DOI] [PubMed] [Google Scholar]
- 5.Mayer ML, Olson R, Gouaux E. J Mol Biol. 2001;311:815–836. doi: 10.1006/jmbi.2001.4884. [DOI] [PubMed] [Google Scholar]
- 6.Perozo E, Cortes DM, Cuello LG. Science. 1999;285:73–78. doi: 10.1126/science.285.5424.73. [DOI] [PubMed] [Google Scholar]
- 7.Jiang YX, Pico A, Cadene M, Chait BT, MacKinnon R. Neuron. 2001;29:593–601. doi: 10.1016/s0896-6273(01)00236-7. [DOI] [PubMed] [Google Scholar]
- 8.Kuo MM-C, Haynes JW, Loukin SH, Kung C, Saimi Y. FEMS Microbiol Rev. 2005;29:961–985. doi: 10.1016/j.femsre.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 9.Schreiber M, Wei A, Yuan A, Gaut J, Saito M, Salkoff L. J Biol Chem. 1998;273:3509–3516. doi: 10.1074/jbc.273.6.3509. [DOI] [PubMed] [Google Scholar]
- 10.Xia X-M, Zeng X, Lingle CJ. Nature. 2002;418:880–884. doi: 10.1038/nature00956. [DOI] [PubMed] [Google Scholar]
- 11.Xia X-M, Zhang X, Lingle CJ. J Neurosci. 2004;24:5585–5591. doi: 10.1523/JNEUROSCI.1296-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang YX, Lee A, Chen JY, Cadene M, Chait BT, MacKinnon R. Nature. 2002;417:523–526. doi: 10.1038/417523a. [DOI] [PubMed] [Google Scholar]
- 13.Ye S, Li Y, Chen L, Jiang Y. Cell. 2006;126:1161–1173. doi: 10.1016/j.cell.2006.08.029. [DOI] [PubMed] [Google Scholar]
- 14.Roosild TP, Miller S, Booth IR, Choe S. Cell. 2002;109:781–791. doi: 10.1016/s0092-8674(02)00768-7. [DOI] [PubMed] [Google Scholar]
- 15.Kuo MM-C, Saimi Y, Kung C. EMBO J. 2003;22:4049–4058. doi: 10.1093/emboj/cdg409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ptak CP, Cuello LG, Perozo E. Biochemistry. 2005;44:62–71. doi: 10.1021/bi048390f. [DOI] [PubMed] [Google Scholar]
- 17.Albright RA, Ibar J-LV, Kim CU, Gruner SM, Morais-Cabral JH. Cell. 2006;126:1147–1159. doi: 10.1016/j.cell.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 18.Zadek B, Nimigean CM. J Gen Physiol. 2006;127:673–685. doi: 10.1085/jgp.200609534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parfenova LV, Crane BM, Rothberg BS. J Biol Chem. 2006;281:21131–21138. doi: 10.1074/jbc.M603109200. [DOI] [PubMed] [Google Scholar]
- 20.Blount P, Sukharev SI, Moe PC, Martinac B, Kung C. Methods Enzymol. 1999;294:458–482. doi: 10.1016/s0076-6879(99)94027-2. [DOI] [PubMed] [Google Scholar]
- 21.Martinac B, Buechner M, Delcour AH, Adler J, Kung C. Proc Natl Acad Sci USA. 1987;84:2297–2301. doi: 10.1073/pnas.84.8.2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dong J, Shi N, Berke I, Chen L, Jiang Y. J Biol Chem. 2005;280:41716–41724. doi: 10.1074/jbc.M508144200. [DOI] [PubMed] [Google Scholar]
- 23.Booth I. Microbiol Rev. 1985;49:359–378. doi: 10.1128/mr.49.4.359-378.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ingraham J, Marr A. In: Escherichia coli and Salmonella Cellular and Molecular Biology. Neidhardt FC, editor. Washington, DC: Am Soc Microbiol; 1996. pp. 1570–1578. [Google Scholar]
- 25.Slonczewski J, Foster J. In: Escherichia coli and Salmonella Cellular and Molecular Biology. Neidhardt FC, editor. Washington, DC: Am Soc Microbiol; 1996. pp. 1539–1549. [Google Scholar]
- 26.Nies DH. FEMS Microbiol Rev. 2003;27:313–339. doi: 10.1016/S0168-6445(03)00048-2. [DOI] [PubMed] [Google Scholar]
- 27.Gill SC, von Hippel PH. Anal Biochem. 1989;182:319–326. doi: 10.1016/0003-2697(89)90602-7. [DOI] [PubMed] [Google Scholar]
- 28.Otwinowski Z, Minor W. Methods in Enzymology. New York: Academic; 1997. pp. 307–326. [DOI] [PubMed] [Google Scholar]
- 29.Collaborative Computational Project, Number 4. Acta Crystallogr D. 1994;50:760–763. [Google Scholar]
- 30.McCoy AJ, Grosse-Kunstleve RW, Storoni LC, Read RJ. Acta Crystallogr D. 2005;61:458–464. doi: 10.1107/S0907444905001617. [DOI] [PubMed] [Google Scholar]
- 31.Jones TA, Zou JY, Cowan SW, Kjeldgaard M. Acta Crystallogr A. 1991;47:110–119. doi: 10.1107/s0108767390010224. [DOI] [PubMed] [Google Scholar]
- 32.Brunger AT, Adams PD, Clore MG, DeLano WL, Gros P, Grosse-Kunstleve RW, Jiang J, Kuszewski J, Nilges M, Pannu NS, et al. Acta Crystallogr D. 1998;54:905–921. doi: 10.1107/s0907444998003254. [DOI] [PubMed] [Google Scholar]