Abstract
Photorhabdus is a virulent pathogen that kills its insect host by overcoming immune responses. The bacterium also secretes a range of antibiotics to suppress the growth of other invading microorganisms. Here we show that Photorhabdus produces a small-molecule antibiotic (E)-1,3-dihydroxy-2-(isopropyl)-5-(2-phenylethenyl)benzene (ST) that also acts as an inhibitor of phenoloxidase (PO) in the insect host Manduca sexta. The Photorhabdus gene stlA encodes an enzyme that produces cinnamic acid, a key precursor for production of ST, and a mutation in stlA results in loss of ST production and PO inhibitory activity, which are both restored by genetic complementation of the mutant and also by supplying cinnamic acid. ST is produced both in vitro and in vivo in sufficient quantities to account for PO inhibition and is the only detectable solvent-extractable inhibitor. A Photorhabdus stlA− mutant is significantly less virulent, proliferates slower within the host, and provokes the formation of significantly more melanotic nodules than wild-type bacteria. Virulence of the stlA− mutant is also rescued by supplying cinnamic acid. The proximate cause of the virulence effect, however, is the inhibition of PO, because the effect of the stlA− mutation on virulence is abolished in insects in which PO has been knocked down by RNA interference (RNAi). Thus, ST has a dual function both as a PO inhibitor to counter host immune reactions and also as an antibiotic to exclude microbial competitors from the insect cadaver.
Keywords: Photorhabdus luminescens, RNA interference, stilbene, virulence
Pathogens face the twin challenges of avoiding host defenses and suppressing the growth of competitor microorganisms. Here we show that a specialist insect pathogen, Photorhabdus, achieves both of these feats by using a single small-molecule chemical. Gram-negative Photorhabdus bacteria are pathogens of a wide range of insects, as well as symbionts of entomopathogenic heterorhabditid nematodes (1). Infective juvenile-stage nematodes in the soil locate and enter potential host insects, then releasing Photorhabdus cells from their gut (2). The bacteria proliferate in the host's body cavity, especially in association with the gut (3), eventually causing death of the host. A number of genes encoding lethal toxins have been identified in Photorhabdus luminescens (4–11); however, the mechanisms whereby the bacteria persist in the face of the insect immune system are poorly understood.
In the case of the model lepidopteran Manduca sexta, RNAi knockdown experiments have shown that when experimentally infected with a biologically relevant number (100) of Photorhabdus cells [a single nematode releases 50–250 cells (12)], the host insect's immune system recognizes the presence of the bacteria and mounts antimicrobial defenses that include the transcription of several immune-related genes (13). Although these host immune responses are eventually unsuccessful, they restrain the progress of the infection, and in their absence, the invading bacteria proliferate more quickly and the insect dies sooner (13). It is therefore clear that natural selection will act on Photorhabdus to promote the acquisition of defenses against host immune responses. Some Photorhabdus toxins are known to target host immune cells such as hemocytes (14). But because some host defenses are soluble agents present in the host's hemolymph (blood), it is likely that at least some of the pathogen's virulence genes encode not cytotoxins, but agents directed against such humoral defenses.
An important component of the insect immune system is the phenoloxidase (PO) system (15). Prophenoloxidase (PPO) is present in hemolymph plasma, being activated by a protease cascade that is initiated after recognition of invading microbes (16), leading to production of melanotic nodules around invading microbes (17). Photorhabdus infection has been shown to be associated with PO inhibition (10, 18).
A major secreted product of P. luminescens both in vitro and in vivo is (E)-1,3-dihydroxy-2-(isopropyl)-5-(2-phenylethenyl)benzene (ST). This hydroxystilbene compound is known to possess broad-spectrum antimicrobial activity (19), and it has been suggested (20) that the function of ST is to defend the dead insect against invasion by other microbes. But compounds of this type have been reported to inhibit tyrosinase, an enzyme similar to PO (21). We show here that ST is not only a potent inhibitor of activated insect PO, but also that PO inhibition leads to increased host susceptibility. It therefore appears that ST has a dual function, both in suppressing an important host defense and also by inhibiting the growth of microbial competitors.
Results
Photorhabdus Produces an Inhibitor of Activated PO.
P. luminescens bacteria were grown in liquid culture, and after 48 h both cells and medium strongly inhibited Manduca PO that had been activated by exposure in vitro to Escherichia coli LPS (Fig. 1A).
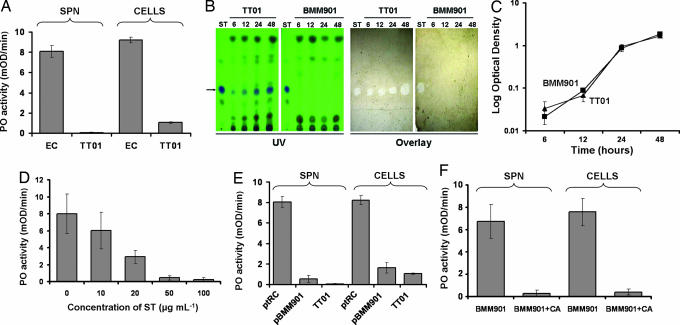
Fig. 1.
PO inhibitory activity in P. luminescens cultures. (A) Inhibitory activity of Photorhabdus TT01 and E. coli culture supernatants (SPN) and bacterial cells. (B) Separation by TLC of ST from Photorhabdus culture supernatants, extracted at 6, 12, 24, or 48 h, is shown next to overlays of the TLC plates with control hemolymph containing activated PO. Inhibition of the enzyme is associated only with ST. (C) Growth in culture of WT (TT01) and stlA− (BMM901) bacteria. (D) Dose–response curve of PO inhibition by purified ST. (E) Rescue of PO inhibition in BMM901 by genetic complementation with the WT stlA gene in pBMM901 (ptRC indicates the BMM901 mutant with the control vector ptRC; TT01 is WT). (F) Rescue of PO inhibition in BMM901 by addition of cinnamic acid (CA) to the bacterial culture medium.
ST Inhibits Activated Insect PO.
We subjected a solvent extract of the culture supernatants to thin-layer chromatography (TLC) to separate ST from other metabolites, confirming that ST was produced in culture (Fig. 1B). ST was produced as early as 6 h and increased in concentration thereafter. We overlaid the TLC plates with Manduca hemolymph to show that PO inhibitory activity was associated only with the ST spots and that PO inhibition was detectable by this method as early as 6 h after inoculation of the culture (Fig. 1B), corresponding to exponential bacterial growth (Fig. 1C). ST was present in both culture supernatant and bacterial cells (data not shown).
To quantitatively assess its ability to inhibit PO, we obtained pure ST from culture filtrates and confirmed its identity (see Materials and Methods). Authentic ST was a potent and dose-dependent inhibitor of activated insect PO, inhibiting PO activity by 50% at ≈15 μg·ml−1, or 60 μM (Fig. 1D).
A Mutant That Does Not Produce ST Does Not Inhibit PO.
We used a genetic approach to show that production of ST accounts for all significant inhibition by Photorhabdus of activated insect PO. The BMM901 mutant of P. luminescens does not produce ST because the stlA gene, disrupted in BMM901, is required for the synthesis of cinnamic acid, an essential precursor for the synthesis of ST (19). Using TLC, we confirmed the lack of ST in BMM901 supernatants and also showed that they did not inhibit activated PO (Fig. 1B). This failure to produce ST was not due to altered growth kinetics, because BMM901 grew at the same rate as the wild-type (WT) control (Fig. 1C). Production of ST by BMM901 has been shown to be restored to normal levels by genetic complementation with the WT stlA gene or by addition of cinnamic acid to the culture medium (19). We found that production of the PO inhibitor at 48 h was completely rescued by genetic complementation with an in trans copy of WT stlA (Fig. 1E) and also by addition of cinnamic acid (Fig. 1F). These results show that ST, as a related metabolite of cinnamic acid, is responsible for inhibition of activated insect PO by Photorhabdus.
ST Acts to Counter Host Defenses in Vivo.
We next investigated whether ST production affects host defenses in vivo during infections of Manduca. We measured immune response to the P. luminescens BMM901 mutant by counting the number of melanotic nodules that were formed after experimental infection (Fig. 2A). WT Photorhabdus cells from a culture of the same age served as controls. To ensure that our experiments were realistic simulations of natural infections, we injected a number of Photorhabdus cells (100) that is similar to that introduced by the nematode vector (12). The number of nodules formed after infection with BMM901 was significantly greater (Mann–Whitney test, P < 0.0001) than when WT bacteria were used. When the mutation in BMM901 was genetically complemented by an intact copy of the stlA gene the number of nodules was significantly less than for BMM901 and not significantly different from WT Photorhabdus. These results imply that Photorhabdus normally suppresses melanotic nodule formation by the insect host and that this suppression is due to the production of ST by the bacterium.
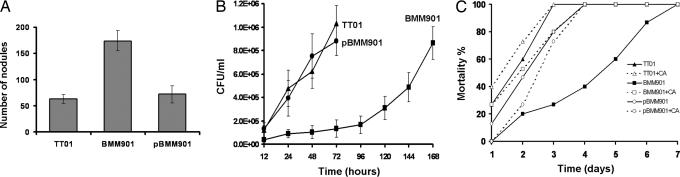
Fig. 2.
Production of ST by Photorhabdus inhibits host nodule formation, enhances bacterial growth in the insect, and speeds the death of infected insects. (A) Formation of melanotic nodules in Manduca infected with WT TT01 P. luminescens, the non-ST-producing stlA− strain BMM901, or the genetically complemented stlA+ strain pBMM901. Insects were injected with 100 cells, left for 24 h, and dissected to determine the number of nodules located in the hemocoel. Shown are means ± SE for experiments, each of five insects. (B) Proliferation within hemolymph of P. luminescens, WT TT01, BMM901, or pBMM901 after injection (at time t = 0) into Manduca larvae. Shown are means ± SE; n = 3 insects per point. (C) Mortality after infection with WT P. luminescens (TT01), the non-ST-producing mutant BMM901, and BMM901 complemented with the intact stlA gene in pBMM901. Mortality is shown in each case with and without addition of cinnamic acid (CA); n = 10 insects per treatment.
ST Production Contributes Significantly to the Virulence of Photorhabdus.
If ST production by Photorhabdus contributes significantly to virulence, it would be expected that when production of ST is disrupted the ability of the bacterium to proliferate within Manduca would be diminished, and the survival of the host insects would be enhanced. We confirmed this prediction by measuring the number of bacteria that could be recovered from hemolymph, as well as host survival, at various times after injecting 100 cells of WT Photorhabdus, the ST nonproducer BMM901, or the rescued mutant pBMM901. WT bacteria proliferated within the insect markedly faster (Fig. 2B), and significantly more WT colonies were recovered 72 h after infection (t = 9.30, df = 4, P = 0.001) than for BMM901. Insects infected with BMM901 took longer to die, so that the number of surviving insects on day 4 was significantly greater (χ2 = 8.57, df = 1, P = 0.003) than when infected with WT bacteria (Fig. 2C). When the ST nonproducing mutant was rescued with pBMM901, however, the survival time of infected insects was similar to that of insects infected with WT bacteria. Further, the survival-enhancing effect of the mutation (Fig. 2C) was completely reversed by providing cinnamic acid; when this compound was provided, all of the insects were dead by 4 days after infection, whether they were infected with BMM901 or TT01. These results show unequivocally that production of ST contributes significantly to the virulence of Photorhabdus.
RNAi Shows That the Effects of ST on Virulence Are Mediated Only Through Its Effects on PO.
Next, we showed that the ability of ST to inhibit activated PO and the effects of the chemical on virulence are causally related. To do so, we used RNAi to knock down expression of the gene encoding the host insect's PPO, by injecting dsRNA specific to the M. sexta PPO gene 6 h before injecting either WT Photorhabdus or BMM901. We first confirmed that the RNAi pretreatment effectively removed mRNA encoding PPO from both fat body and hemocytes (Fig. 3A). We also verified by PAGE combined with an in-gel assay for PO activity (Fig. 3B) that the RNAi pretreatment successfully reduced the amount of activatable PO protein present in hemolymph plasma. When we used our standard in vitro PO assay to determine the amount of activatable PO in hemolymph of insects infected with Photorhabdus (Fig. 3C), we found, as expected, that insects given WT bacteria showed little activatable PO activity, whether or not PPO had been knocked down, because Photorhabdus in these insects had produced ST that would have inhibited any PO that was present. Those insects receiving BMM901, conversely, showed high levels of activatable PO unless they had been pretreated with dsPPO. In the latter case, the level of PO was not significantly different from that seen in the insects infected with TT01. These results show that infection of Manduca with Photorhabdus induces the host insect to produce more PPO and that the additional enzyme is completely inhibited by ST in the case of WT, but not the mutant BMM901.
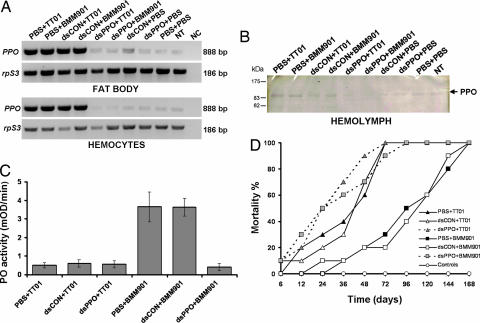
Fig. 3.
RNAi knockdown of PPO mRNA levels in Manduca. Insects were preinjected (time 0) with PBS (control), a control dsRNA (dsCON), or a dsRNA specific to PPO (dsPPO) and 6 h later were infected with P. luminescens TT01 or BMM901. Tissue was collected 18 h after infection. (A) RT-PCR was used to measure mRNA levels in fat body and hemocytes. The constitutively transcribed gene rpS3 was used as a loading control. Details of the various treatments are given in the text. NT, no treatment; NC, negative control (no template). (B) SDS/PAGE gel of Manduca hemolymph proteins after various treatments including RNAi knockdown of PPO. PO activity was detected by an in-gel activity assay. (C) Reduction of LPS-activatable PO activity in hemolymph samples after RNAi treatment by using dsRNA specific to M. sexta PPO. After PPO knockdown, hemolymph of insects infected with BMM901 no longer differed significantly in PO activity from those given TT01. Shown are means ± SE; n = 8. (D) Survival of Manduca larvae pretreated with RNAi to knock down PPO (dsPPO) or treated with a control dsRNA (dsCON). In each case, pretreatment with dsRNA was 6 h before injection of 100 Photorhabdus bacteria. n = 10 insects per treatment.
Insects treated with dsPPO, now with much reduced levels of activatable PO in their hemolymph, were significantly more susceptible to infection by WT Photorhabdus and died significantly faster than insects injected with a control RNAi reagent (dsCON) (Fig. 3D); the number of insects dead at 72 h was significantly different between the dsPPO and dsCON treatments (χ2 = 9.89, df = 1, P = 0.002). Remarkably, however, there was now no difference in survival between PPO-knockdown insects infected with BMM901 bacteria and WT bacteria. Thus, in the absence of activatable PO, it makes no difference whether the bacteria secrete ST. Therefore, the effect of ST on virulence is mediated only through its effect on PO.
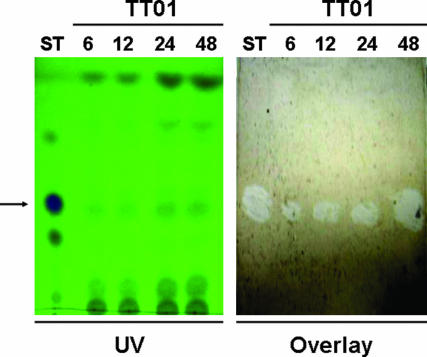
Finally, we determined that ST is produced during Photorhabdus infections of Manduca caterpillars in sufficient concentration to explain these results, by subjecting solvent extracts of hemolymph to TLC. Spots corresponding to ST were present as early as 6 h after infection, and hemolymph overlays confirmed that these spots inhibited PO (Fig. 4). By comparing the intensity of the TLC spots to dilutions of a known standard, we found that by 24 h after infection, the concentration of ST in infected hemolymph samples had risen to between 275 and 550 μg·ml−1, confirming that the concentration of ST was more than sufficient to inhibit PO completely (see Fig. 1D).
Fig. 4.
Production of ST by Photorhabdus in vivo during infection of Manduca caterpillars. Depicted is a TLC plate showing presence of ST in hemolymph of infected insects at times as short as 6 h after infection (Left) and an overlay of the TLC plate with control hemolymph containing activated PO (Right), to show inhibition of the activated enzyme by materials present in the treated insects.
Discussion
We have shown that production by the insect-specific pathogen P. luminescens of the phenylpropanoid chemical ST makes a significant contribution to the virulence of the bacterium to its model lepidopteran host M. sexta. The bacterium inhibits two key immune defenses of the insect: activity of the antimicrobial enzyme PO and formation of melanotic nodules. We have further shown that the effect of ST on virulence requires the presence in the host of activatable PO.
The fact that the hemolymph does not melanize during infections of lepidopteran hosts by Photorhabdus and Xenorhabdus bacteria has been previously noted and attributed to the production of a PO inhibitor by the bacterium (10, 22). This inhibition is remarkable because lepidopteran hemolymph normally melanizes readily in response to even minute amounts of microbial pattern molecules (23).
The two host defenses targeted by ST, the enzyme PO and nodule formation, are functionally linked; activation of PO is a necessary step for the production of melanotic nodules (15). In another study (24), it was noted that during infection of Manduca by the related bacterium Xenorhabdus nematophila the formation of melanotic nodules was reduced. This effect was attributed to inhibition of host eicosanoid signaling, which is known to be involved in hemocyte aggregation (25), but the authors apparently did not examine whether PO was inhibited. In this work, we have clearly shown that inhibition of PO by ST is the crucial factor that mediates the effects of Photorhabdus infection on both melanotic nodule formation and bacterial virulence. Our results do not exclude the possibility that Photorhabdus infection might also have effects on eicosanoid signaling in Manduca, but if such effects occur, they are a minor contribution to virulence compared with the effects of ST mediated by PO inhibition.
The inhibition by ST of activated insect PO is in accord with a previous report that a number of hydroxystilbenes are inhibitors of mammalian tyrosinase (21). The activity of ST against insect PO found here (IC50 ≈ 60 μM) is slightly greater than, but consistent with, the activities of other hydroxystilbenes against tyrosinase (21).
Photorhabdus and related insect pathogenic bacteria are formidable producers of antibiotics (26). ST was identified as a product of P. luminescens in vitro (27, 28), is produced during infections in vivo (20, 29), and has broad-spectrum antimicrobial and antihelminthic activity against Gram-positive bacteria, fungi, and nematodes (19, 26, 30, 31). It was suggested (20, 27) that the principal function of ST is to prevent or restrain the growth of competing microbial pathogens or saprotrophs. The present work shows that ST is additionally used by the pathogen to abrogate the defenses of the insect host. In accord with previous work (20), we found that production of ST by Photorhabdus during a natural infection begins early, with ST being detectable in hemolymph extracts as early as 6 h. Melanotic nodule formation normally occurs in Manduca within 24 h of infection by bacteria (25). Accordingly, we found that by 24 h, the concentration of ST in hemolymph had risen to >1 mM. ST concentration in hemolymph during natural Photorhabdus infections may reach values approaching 10–15 mM by 4 days (20). The concentration of ST in the hemolymph is thus many times that necessary to inhibit all insect PO activity. Moreover, we found that when cultured in vitro, a part of the total extractable PO inhibitory activity was associated with Photorhabdus cells (Fig. 1). This cell-associated PO-inhibiting activity is likely to be ST because it is not present in BMM901 cells. We speculate that ST is bound to the surface of the bacterial pathogen's cell envelope, where it is ideally placed to inhibit PO that has been activated by the microbial pattern-sensitive cascade of PPO-activating proteases.
Although we have shown that ST accounts for all significant PO inhibition present in solvent extracts of P. luminescens TT01 culture medium, it remains possible that the bacteria produce other minor PO inhibitory compounds. A related compound, ST epoxide, is also produced by a different isolate of Photorhabdus and is a powerful antibiotic as well (32). We did not detect ST epoxide either in culture supernatants or in infected hemolymph, and the overlay experiments indicate that all detectable PO inhibitory activity comigrates with ST, but we cannot rule out the possibility that the epoxide is produced as a minor or highly labile component. Photorhabdus may also produce other solvent-inextractable PO inhibitors (I.E., S.B., R.H.f.-C., and S.E.R., unpublished data). Other associated nematode bacterial pathogens of insects also inhibit PO (22). Moreover, it has also been shown that the symbiotic nematode Steinernema glaseri (associated with Xenorhabdus poinarii) produces factors that inhibit host nodule formation (33). Because we did not use nematodes in the present work, this finding is not directly relevant to our results, but the principle that PO inhibitors may be important during infections by specialist insect pathogens may be generally relevant in understanding pathogenesis in insects.
Finally, we have previously noted that the stlA mutant BMM901 does not affect the virulence of P. luminescens toward Galleria mellonella larvae (19). Because ST also inhibits activated PO from Galleria (result not shown), this lack of effect on virulence implies that inhibition of PO is not required for Photorhabus pathogenesis in Galleria. Thus, the PO response, although widespread in insects, may be more active/important in the defense systems of some insects than in others. Alternatively, Photorhabdus may have additional ways of overcoming the host defenses of Galleria that are ineffective in Manduca. It is well known that Photorhabdus bacteria possess a large number of genes that contribute to pathogenicity (10); the diversity of insect defenses has presumably contributed to the evolution of this pathogen's formidable armory of virulence factors.
Materials and Methods
Insects and Bacteria.
Bacterial strains used were P. luminescens subsp. laumondii strain TT01 (11); a mutant of this strain, BMM901 (TT01 stlA::Kn) (19); and E. coli DH5α [(F−, ϕ80dlacZΔM15, endA1, recA1, hsdR17 (rk−, mk+), supE44, thi-1, gyrA96, relA1, Δ(lacZYA-argF)U169, λ)]. All were grown in shake cultures in Luria–Bertani medium. P. luminescens was cultured at 30°C and E. coli at 37°C. In some experiments as noted, BMM901 was transformed with the pTRC99A-based plasmid pBMM901, bearing the intact WT stlA gene (19).
Larvae of M. sexta (L.) (Lepidoptera: Sphingidae) were maintained on artificial diet (34). Newly molted (day 0) fifth-stage larvae were injected and then bled to collect hemolymph as described (13). In some experiments, cinnamic acid was added to the culture medium and also to the resuspending medium at a final concentration of 33 μg·ml−1 (19).
After injection, larvae were held individually with diet at 25°C, and nodule formation was assessed after 24 h. To document numbers of recoverable bacteria, insects were bled at fixed time points and up to 7 days after injection with 100 washed cells of TT01, BMM901, or pBMM901. Aliquots of hemolymph (50 μl) were immediately added to prechilled Grace's insect medium (450 μl), and serial dilutions were then plated in duplicate onto appropriate nutrient agar. Plates were incubated at 30°C for 48 h, and individual colonies were counted. Colony-forming units per insect were calculated, assuming that the hemolymph volume of the insect was 300 μl. Mortality, defined as inability to react to a needle poke, was scored at intervals up to 7 days after bacterial injection. Ten insects were used for each treatment.
Chemicals.
ST was purified from 100-ml cultures of P. luminescens TT01. Incubation was at 30°C, shaking at 200 rpm on an orbital incubator. After 48 h, the cells were removed by centrifugation. The combined supernatant was extracted into EtOAc (3 × 100 ml), dried (MgSO4), and evaporated to dryness by using a rotary evaporator. Flash chromatography (20:80 EtOAc:Petrol, Rf 0.4) of the crude residue afforded ST as an off-white crystalline solid (1.5 mg). δH(400 MHz, DMSO-d6): 1.21 ppm (6H, d, 3JHH 8); 3.30 (1H, m), 6.45 (2H, s); 6.87 (1H, d, 3JHH 16); 7.00 (1H, d, 3JHH 16); 7.34 (2H, d, 3JHH 8); 7.37 (1H, t, 3JHH 8); 7.56 (2H, dt, 3JHH 8 3JHH 7). m/z (EI+, %): 254 (60); 239 (100); 105 (30). m/z (CI+, %): 255 (100). [MH+]: 239 (50); 213 (30); 107 (25). All other chemicals and media were from Sigma (Andover, U.K.) unless otherwise stated.
PO Assay.
PO activity of Manduca hemolymph was quantified by using a microplate enzyme assay as described (13). Putative inhibitors (20 μl of bacterial supernatant or cells) were prepared from bacterial cultures by centrifugation of cells, which were then washed in PBS; supernatants were sterile filtered through a 0.2-μm filter.
TLC Analysis of Photorhabdus Culture Supernatants and Manduca Hemolymph Samples.
Extraction and TLC analysis of culture supernatants from P. luminescens TT01 or BMM901 was conducted as described (19). For the presence of ST in vivo, insects were injected with 50 μl of 100 washed TT01 cells and then bled at fixed timepoints (6, 12, 24, or 48 h). Hemolymph plasma was obtained by centrifugation of hemocytes.
Hemolymph overlays from naïve insects were carried out by placing TLC plates in 15-ml diluted hemolymph plasma (1:3 vol/vol dilution with PBS) containing 1 μl of E. coli LPS (5 mg ml−1) and 3 μl of 4-methylcatechol (20 mM) solution. TLC plates were then incubated at room temperature for 1 h, and PO inhibition was observed as inhibition of melanization over the stilbene bands.
RNA inhibition of Manduca PPO.
PPO cDNA was amplified from fat body total RNA extracted from insects injected 24 h previously with E. coli to elicit an immune response. Primers were designed from the sequence of the M. sexta PPO cDNA (GenBank accession no. L42556) (35). Sequences of the primers were: (forward) 5′-AAACAACTCCCAAACGATGC-3′ and (reverse) 5′-TGTGCATGTTGTTGTGGATG-3′. The resulting PCR product (888 bp) was cloned into the pCR4-TOPO vector (Invitrogen, Paisley, U.K.) and used as a template to generate dsRNA specific for the PPO gene (dsPPO). Synthesis of dsRNA and RNAi procedures have been published (13, 36). The success of the RNAi procedure was assessed by RT-PCR to test for the presence of PPO mRNA in fat body and hemocytes. RT-PCR control reactions for ribosomal protein S3 (rpS3) (37) were also performed (see refs. 13 and 36 for primers and PCR conditions) as a loading control.
PPO protein levels after RNAi were assessed by subjecting hemolymph plasma samples to SDS/PAGE and then staining the gels overnight by using the specific PO substrate 4-methylcatechol (20 mM). PPO expression was assessed by using two insects for each treatment, which invariably showed the same result.
Acknowledgments
We thank Sandra Barns for rearing Manduca. This work was supported by a grant from the U.K. Biotechnology and Biological Sciences Research Council (to D.J.C., S.E.R., and R.H.f.-C.).
Abbreviations
- ST
(E)-1,3-dihydroxy-2-(isopropyl)-5-(2-phenylethenyl)benzene
- PO
phenoloxidase
- PPO
prophenoloxidase.
Footnotes
The authors declare no conflict of interest.
References
- 1.Forst S, Dowds B, Boemare N, Stackebrandt E. Annu Rev Microbiol. 1997;51:47–72. doi: 10.1146/annurev.micro.51.1.47. [DOI] [PubMed] [Google Scholar]
- 2.Daborn PJ, Waterfield N, Blight MA, ffrench-Constant RH. J Bacteriol. 2001;183:5834–5839. doi: 10.1128/JB.183.20.5834-5839.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Silva CP, Waterfield NR, Daborn PJ, Dean P, Chilver T, Au CPY, Sharma S, Potter U, Reynolds SE, ffrench-Constant RH. Cell Microbiol. 2002;4:329–339. doi: 10.1046/j.1462-5822.2002.00194.x. [DOI] [PubMed] [Google Scholar]
- 4.Yang G, Dowling AJ, Gerike U, ffrench-Constant RH, Waterfield NR. J Bacteriol. 2006;188:2254–2261. doi: 10.1128/JB.188.6.2254-2261.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.ffrench-Constant R, Waterfield N. Adv Applied Microbiol. 2006;58:169–183. [PubMed] [Google Scholar]
- 6.Brown SE, Cao AT, Dobson P, Hines ER, Akhurst RJ, East PD. Appl Environ Microbiol. 2006;72:1653–1662. doi: 10.1128/AEM.72.2.1653-1662.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Waterfield N, Kamita SG, Hammock BD, ffrench-Constant R. FEMS Microbiol Lett. 2005;245:47–52. doi: 10.1016/j.femsle.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 8.Brugirard-Ricaud K, Duchaud E, Givaudan A, Girard PA, Kunst F, Boemare N, Brehelin M, Zumbihl R. Cell Microbiol. 2005;7:363–371. doi: 10.1111/j.1462-5822.2004.00466.x. [DOI] [PubMed] [Google Scholar]
- 9.Marokhazi J, Waterfield N, LeGoff G, Feil E, Stabler R, Hinds J, Fodor A, ffrench-Constant RH. J Bacteriol. 2003;185:4648–4656. doi: 10.1128/JB.185.15.4648-4656.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.ffrench-Constant R, Waterfield N, Daborn P, Joyce S, Bennett H, Au C, Dowling A, Boundy S, Reynolds S, Clarke D. FEMS Microbiol Rev. 2003;26:433–456. doi: 10.1111/j.1574-6976.2003.tb00625.x. [DOI] [PubMed] [Google Scholar]
- 11.Duchaud E, Rusniok C, Frangeul L, Buchrieser C, Givaudan A, Taourit S, Bocs S, Boursaux-Eude C, Chandler M, Charles JF, et al. Nat Biotechnol. 2003;21:1307–1313. doi: 10.1038/nbt886. [DOI] [PubMed] [Google Scholar]
- 12.Ciche TA, Ensign JC. Appl Environ Microbiol. 2003;69:1890–1897. doi: 10.1128/AEM.69.4.1890-1897.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eleftherianos I, Millichap PJ, ffrench-Constant RH, Reynolds SE. Dev Comp Immunol. 2006;30:1099–1107. doi: 10.1016/j.dci.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 14.Au C, Dean P, Reynolds SE, ffrench-Constant RH. Cell Microbiol. 2004;6:89–95. doi: 10.1046/j.1462-5822.2003.00345.x. [DOI] [PubMed] [Google Scholar]
- 15.Cerenius L, Soderhall K. Immunol Rev. 2004;198:116–126. doi: 10.1111/j.0105-2896.2004.00116.x. [DOI] [PubMed] [Google Scholar]
- 16.Kanost MR, Jiang HB, Yu XQ. Immunol Rev. 2004;198:97–105. doi: 10.1111/j.0105-2896.2004.0121.x. [DOI] [PubMed] [Google Scholar]
- 17.Gillespie JP, Kanost MR, Trenczek T. Annu Rev Entomol. 1997;42:611–643. doi: 10.1146/annurev.ento.42.1.611. [DOI] [PubMed] [Google Scholar]
- 18.da Silva C, Dunphy GB, Rau ME. Dev Comp Immunol. 2000;24:367–379. doi: 10.1016/s0145-305x(99)00063-4. [DOI] [PubMed] [Google Scholar]
- 19.Williams JS, Thomas M, Clarke DJ. Microbiology-SGM. 2005;151:2543–2550. doi: 10.1099/mic.0.28136-0. [DOI] [PubMed] [Google Scholar]
- 20.Hu K, Webster JM. FEMS Microbiol Lett. 2000;189:219–223. doi: 10.1111/j.1574-6968.2000.tb09234.x. [DOI] [PubMed] [Google Scholar]
- 21.Ohguchi K, Tanaka T, Kido T, Baba K, Iinuma M, Matsumoto K, Akao Y, Nozawa Y. Biochem Biophys Res Commun. 2003;307:861–863. doi: 10.1016/s0006-291x(03)01284-1. [DOI] [PubMed] [Google Scholar]
- 22.Yokoo S, Tojo S, Ishibashi N. J Insect Physiol. 1992;38:915–924. [Google Scholar]
- 23.Ji CY, Wang Y, Guo XP, Hartson S, Jiang HB. J Biol Chem. 2004;279:34101–34106. doi: 10.1074/jbc.M404584200. [DOI] [PubMed] [Google Scholar]
- 24.Park YJ, Kim Y, Putnam SM, Stanley DW. Arch Insect Biochem Physiol. 2003;52:71–80. doi: 10.1002/arch.10076. [DOI] [PubMed] [Google Scholar]
- 25.Miller JS, Nguyen T, Stanley-Samuelson DW. Proc Natl Acad Sci USA. 1994;91:12418–12422. doi: 10.1073/pnas.91.26.12418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Akhurst RJ. J Gen Microbiol. 1982;128:3061–3065. doi: 10.1099/00221287-128-12-3061. [DOI] [PubMed] [Google Scholar]
- 27.Li JX, Chen GH, Wu HM, Webster JM. Appl Environ Microbiol. 1995;61:4329–4333. doi: 10.1128/aem.61.12.4329-4333.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Richardson WH, Schmidt TM, Nealson KH. Appl Environ Microbiol. 1988;54:1602–1605. doi: 10.1128/aem.54.6.1602-1605.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu KJ, Li JX, Wang WJ, Wu HM, Lin H, Webster JM. Can J Microbiol. 1998;44:1072–1077. [Google Scholar]
- 30.Hu KJ, Li JX, Webster JM. Nematology. 1999;1:457–469. [Google Scholar]
- 31.Han RC, Ehlers RU. Nematology. 1999;1:687–693. [Google Scholar]
- 32.Hu KJ, Li JX, Li B, Webster JM, Chen GH. Bioorg Med Chem. 2006;14:4677–4681. doi: 10.1016/j.bmc.2006.01.025. [DOI] [PubMed] [Google Scholar]
- 33.Wang Y, Gaugler R. Biol Control. 1999;14:45–50. [Google Scholar]
- 34.Reynolds SE, Nottingham SF, Stephens AE. J Insect Physiol. 1985;31:119–127. [Google Scholar]
- 35.Hall M, Scott T, Sugumaran M, Soderhall K, Law JH. Proc Natl Acad Sci USA. 1995;92:7764–7768. doi: 10.1073/pnas.92.17.7764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eleftherianos I, Marokhazi J, Millichap PJ, Hodgkinson AJ, Sriboonlert A, ffrench-Constant RH, Reynolds SE. Insect Biochem Mol Biol. 2006;36:517–525. doi: 10.1016/j.ibmb.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 37.Jiang H, Wang Y, Kanost MR. Insect Mol Biol. 1996;5:31–38. doi: 10.1111/j.1365-2583.1996.tb00038.x. [DOI] [PubMed] [Google Scholar]