Abstract
Notch receptors control differentiation and contribute to pathologic states such as cancer by interacting directly with a transcription factor called CSL (for CBF-1/Suppressor of Hairless/Lag-1) to induce expression of target genes. A number of Notch-regulated targets, including genes of the hairy/enhancer-of-split family in organisms ranging from Drosophila to humans, are characterized by paired CSL-binding sites in a characteristic head-to-head arrangement. Using a combination of structural and molecular approaches, we establish here that cooperative formation of dimeric Notch transcription complexes on promoters with paired sites is required to activate transcription. Our findings identify a mechanistic step that can account for the exquisite sensitivity of Notch target genes to variation in signal strength and developmental context, enable new strategies for sensitive and reliable identification of Notch target genes, and lay the groundwork for the development of Notch pathway inhibitors that are active on target genes containing paired sites.
Keywords: Mastermind, Notch signaling, signal transduction, dimerization, gene expression
Notch proteins constitute the receptors of essential signaling pathways that influence a broad range of cell fate decisions in metazoan organisms (1, 2). Notch signals are used iteratively at different decision points and have functional outcomes that depend heavily on gene dose and context. Both deficiencies and abnormal increases of Notch signaling are associated with human developmental abnormalities and cancer, emphasizing the importance of precisely regulating Notch signal strength (3–9).
The intracellular portion of the Notch receptor (ICN) is the primary effector of canonical Notch signaling. ICN is proteolytically released from the membrane upon ligand stimulation, translocates to the nucleus, and induces transcription of target genes by driving the assembly a Notch transcriptional activation complex (NTC) that includes a DNA-bound transcription factor CBF-1/Suppressor of Hairless/Lag-1 (CSL) (10) and a coactivator protein of the Mastermind-like (MAML) family (11, 12). Structures of NTC cores, now solved for both human Notch1 and Caenorhabditis elegans LIN12, reveal that the Notch ankyrin-repeat (ANK) domain and a Rel-homology region (RHR) within CSL combine to create a groove that binds the N-terminal part of MAML-1 as a kinked, 70-Å helix (13, 14). The CSL/ICN/MAML ternary complex then recruits additional factors through the C-terminal portion of MAML, such as p300/CBP, PCAF/GCN5, and the CDK8/mediator complex (15–18), that drive the transcription of target genes.
A key unresolved question in Notch signaling is to determine the factors that govern target gene activation in different developmental, physiologic, and pathophysiologic contexts. One clue is found in the promoter regions of a number of well characterized Notch-responsive genes (19), such as the hairy/enhancer-of-split genes in Drosophila and a number of their mammalian homologues, which contain dual “sequence-paired” binding sites (SPSs) (20, 21). SPSs consist of two CSL-binding sites oriented head-to-head that typically are separated by 16 or 17 nt, depending on the species examined [supporting information (SI) Fig. 6]. Both the directionality and the spacing between the paired sites influence Notch-dependent transcription in cell-based assays and transgenic flies, suggesting that the SPS is a specialized response element that modulates or tunes the response of target genes to varying doses of activated Notch (22, 23). Accordingly, the promoter of the HES-1 gene, which contains an SPS, responds to lower doses of ICN than does the HES-5 promoter, which lacks an SPS (23).
The evolutionary conservation of paired CSL recognition sites suggests that cooperative assembly of higher-order Notch complexes might have a role in regulating transcription of certain key Notch target genes. Yet, a molecular model for assembly of higher-order complexes has remained elusive. CSL does not dimerize on cognate DNA sites (22, 24, 25), and previous studies have failed to detect any evidence of cooperativity in the loading of intracellular Notch-derived polypeptides onto CSL/DNA complexes at SPSs (23).
We now find that assembled NTCs cooperatively dimerize on SPSs. Dimerization requires both CSL and MAML, depends on conserved residues from the convex face of the Notch ANK domain and is a general feature of the four mammalian Notch receptors. Dimerization-defective forms of ICN1 have a diminished capacity to activate promoter elements containing SPS sites and interfere with transcriptional induction by normal ICN1. These findings suggest that site cooperativity serves as a regulatory switch that controls the transcription of certain Notch target genes containing SPS elements and provide a model for context- and dose-dependent variation in the sensitivity of different Notch-responsive genes to Notch signals.
Results
Crystal Contacts Suggest a Mode of Dimerization at SPS Elements.
Cocrystals of a human NTC core (13), which consists of an N-terminal MAML-1 peptide, the ANK domain of human Notch1, and CSL on a DNA duplex derived from the HES-1 promoter, contain contacts between the convex surfaces of ANK domains from adjacent unit cells that also are seen in crystals of the ANK domain solved in isolation in several different crystallization conditions (13, 26). These contacts lie near a twofold symmetry axis in the crystals, such that the interacting complexes are positioned head-to-head at a distance roughly equal to that needed to occupy both recognition elements of an SPS (Fig. 1A). Primary sequence alignment of Notch ANK domains from different homologs shows that the key contacts are evolutionarily conserved (Fig. 1B). These conserved residues are not engaged in contacts within an individual MAML1/ANK/CSL/DNA complex, suggesting that the observed conservation reflects functional importance in mediating dimerization at SPS sites. The conservation among the four mammalian Notch receptors also predicts that each receptor should be capable of making interactions like those between the adjacent Notch1 complexes illustrated here (Fig. 1).
Fig. 1.
ANK–ANK interactions between conserved residues in the crystal lattice. (A) Structure of two copies of the MAML-1/ANK/CSL/DNA complex related by crystallographic symmetry. The protein subunits are rendered as ribbons (ANK, purple; CSL, gold; and MAML-1, green), and the DNA is rendered as blue sticks. Residues of ANK engaged in lattice contacts are shown as cyan sticks. (B) Sequence alignment of ANK in the region of the dimer interface. Key residues that participate in Notch1 lattice contacts are highlighted in yellow. (C) Specific contacts observed between two symmetry-related ANK molecules (one in gray and the other in cyan) in the crystal structure. (Left) Salt bridges in ankyrin repeat two between K1946 of one ANK subunit and E1950 of the other. (Right) Interactions in ankyrin repeat three between R1985 of one ANK subunit and three backbone carbonyl groups of the other.
The ANK–ANK contacts primarily are electrostatic and lie in the second and third ankyrin repeats. Key interactions consist of contacts between the guanidino group of Arg-1985 and at least three backbone carbonyl oxygen atoms, as well as interactions between Glu-1950 and Lys-1946 (Fig. 1C). Arg-1983 also forms hydrogen bonds with Ser-1952 and a backbone carbonyl. In addition to homotypic interactions between the ANK domains, unmodeled electron density in the MAML-1/ANK/CSL/DNA complex (13) also suggests the existence of interactions between the ANK domain of one complex and the N-terminal end of MAML-1 in the second complex (SI Fig. 7). Based on the architecture of the complex, and the evolutionary conservation of SPSs and the crystal contact residues, we postulated that the ANK domains of Notch receptors mediate dimerization of ternary complexes on SPSs found in Notch target gene promoters.
ANK–ANK Interactions Drive Dimerization of NTCs.
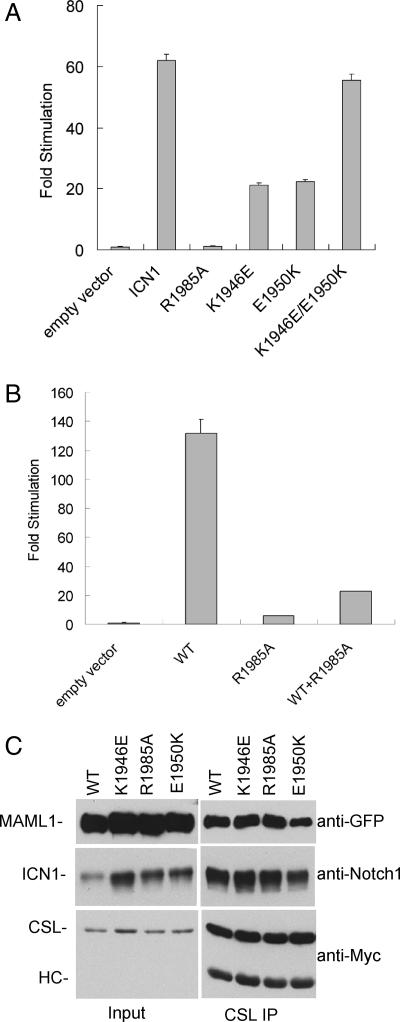
To test whether residues engaged in ANK–ANK contacts in the crystal contribute to transcriptional activation of SPS-bearing promoters, we compared the ability of different forms of ICN to induce transcription of a luciferase gene under control of the HES-1 promoter, which has a functionally important SPS element (22, 23). In contrast to normal ICN1, mutations that disrupt the predicted dimerization interface either abrogate (R1985A) or diminish (K1946E and E1950K) the ability of ICN1 to induce expression of the HES-1 reporter gene (Fig. 2A). Combining the K1946E and E1950K mutations in cis, however, rescues the defect in transcriptional activation, indicating that the putative dimerization interface is functionally important in regulating transcriptional activity at a promoter that contains an SPS. In addition, when coexpressed with ICN1, the R1985A mutation dominantly interferes with activation of the HES-1 promoter element by normal ICN1 (Fig. 2B). Importantly, when these mutants are scored on an artificial reporter that contains four CSL-binding sites oriented in the same direction and in tandem, there is no change in the ability of the mutants to activate transcription (SI Fig. 8). Moreover, in cotransfected cells, all ICN1 polypeptides with mutations that disrupt the predicted dimerization interface are expressed at similar levels to normal ICN1, and they coimmunoprecipitate in similar amounts with CSL and MAML-1 (Fig. 2C). Together, these findings indicate that the ability to form monomeric ternary complexes with MAML-1 and CSL is not affected by these mutations.
Fig. 2.
HES-1 reporter assays depend on an intact dimerization interface. (A) Effect of dimerization mutations on the ability of ICN1 to stimulate a HES-1 reporter gene. (B) Dominant negative activity of R1985A. In A, U2OS cells were cotransfected with the indicated pcDNA3 plasmids (1 ng per well), whereas in B, the cells were transfected with 10 ng of normal ICN1 plasmid and/or 100 ng of ICN1 R1985A plasmid. In both A and B, fold stimulation is expressed relative to the activity of the empty vector control, which is arbitrarily given a value of 1. All data points were obtained in triplicate; error bars correspond to standard deviations. (C) ICN1 polypeptides bearing dimerization mutations form stable complexes with CSL and MAML-1. 293 cells were cotransfected with plasmids encoding the indicated forms of ICN1, MAML-1 fused to GFP, and Myc-tagged CSL. Complexes were recovered from whole-cell lysates with an anti-Myc antibody and blotted for the presence of GFP-MAML-1, ICN1, and CSL-Myc as indicated.
To establish directly whether NTCs (consisting of one molecule each of MAML-1, ICN, and CSL) can cooperatively dimerize on DNA, we carried out electrophoretic mobility shift assays (EMSAs) on an oligonucleotide probe containing the HES-1 promoter SPS (Fig. 3A). Without Notch or MAML-1, CSL binds to each of the two sites independently. When present in excess, most probes bind a single CSL molecule (Fig. 3A), a finding consistent with previous studies showing that CSL binds its recognition element as a monomer without detectable cooperativity at paired sites (23–25). Adding RAMANK from Notch1 does not change the stoichiometric distribution of complexes bound per probe molecule. However, when MAML-1 is added, the stoichiometric distribution of the complexes changes dramatically: all of the probe is either free or bound by NTC dimers, indicating that NTC loading at one site leads to cooperative loading of the second site. As predicted, cooperative loading is abrogated by the R1985A mutation, which instead produces a smear corresponding to an ensemble of species that likely results from a weak residual tendency to self-associate. In contrast, the R1985A mutation does not detectably affect ternary complex formation on a probe containing only a single CSL-binding site (Fig. 3A Right), indicating that the R1985A mutation is a cooperativity mutant that specifically interferes with dimerization. The partial loss of activity of the K1946E and E1950K mutants in the HES-1 reporter assays is echoed in EMSA titrations, where the proteins undergo a cooperative transition to form dimers at a concentration ≈4-fold greater than normal ICN1 or the K1946E/E1950K double mutant (SI Fig. 9).
Fig. 3.
Cooperative dimerization of NTCs on HES-1 promoter DNA. (A) EMSAs performed with either normal or R1985A forms of human RAMANK1. (Left) Probe containing the SPS element of the human HES-1 promoter. (Right) Single-site probe. (B) Sequences used in EMSAs to determine the importance of DNA site integrity, spacing, and orientation in promoting cooperative dimerization of ternary complexes. The S and SPS probes from the human HES-1 promoter sequence are identical to those used in A. The consensus site is underlined, and alterations are noted with lowercase in red. (C) EMSAs performed by using RAMMANK-1, CSL, and MAML-1 on the DNA probes listed in B. Mutation of one site (mutA or mutB), inversion of the second site (invB), or an insertion of 5 bp between the two sites (ins) prevents cooperative dimerization of ternary complexes. The band that contains the dimer of ternary complexes on DNA is indicated to the left of the gel. (D) Ability of NTCs to undergo cooperative dimerization as a function of intersite spacer length. Oligonucleotide probes used to scan spacer length are listed in SI Table 1.
Proper Spacing and Orientation of CSL-Binding Sites Are Necessary for Cooperative Dimerization.
To test whether higher-order complexes exhibit specificity for the SPS architecture, we carried out additional EMSA assays on variant DNA sequences that eliminate the integrity of one of the SPS sites, flip the site orientation, or alter the spacing between the sites by a half-turn of helix (Fig. 3B). When either site A or site B is mutated so that it no longer corresponds to a CSL consensus site (YGTGDGAA) (27), cooperative assembly of the dimer is no longer observed (Fig. 3C, mutA and mutB). Moreover, cooperative dimerization is no longer detected when the second site is inverted (Fig. 3C, invB), and it is dramatically diminished when the second site is moved by a 5-base insertion (ins). Because the intrinsic affinity of a single ternary complex for DNA is not altered under the conditions of inversion or insertion, these studies show that the proper spatial arrangement of the two individual binding sites is needed for cooperative dimerization to occur.
We next asked what range of spacer lengths between sites is compatible with cooperative loading of dimeric complexes (Fig. 3D). The optimal spacing between consensus sites for cooperative dimerization is 16 bp, but cooperative dimerization still can occur on templates with spacers varying from 15 to 19 bp, implying that two NTCs can adjust their positions relative to each other (see below) to accommodate a modest range of spacer lengths between sites. This inferred flexibility is consistent with the different conformations of CSL seen in the crystal structures of the Notch ternary complexes formed with the human and worm proteins (13, 14) and with the enrichment of adenosine and thymidine in the spacer between the paired sites.
To determine whether the assembly of NTCs and their cooperative dimers is general among the human Notch homologues, we tested the ability of the RAMANK domains of Notch1–4 to form complexes on single and sequence-paired sites (Fig. 4). Despite qualitative differences in mobility on the EMSA, all four purified RAMANK polypeptides bind to CSL independent of MAML-1 and then recruit MAML-1 to ternary complexes on a single site probe. When the longer, paired site probe is provided, all RAMANK polypeptides mediate cooperative dimerization, as predicted from the conservation in primary sequence at the dimerization interface (Fig. 1B). Thus, a similar series of events takes place to assemble single and dimeric NTCs in all four mammalian Notch homologues.
Fig. 4.
Comparison of complexes formed by the RAMANK regions of human Notches 1–4. Equal concentrations of RAMANK and CSL were mixed with or without the MAML-1 polypeptide, and EMSAs were performed on a short probe with a single CSL-binding site (Left and Center) or the SPS element from the HES-1 promoter (Right).
Discussion
Gene transcription in eukaryotes relies on the combinatorial integration of inputs from different biological signals. Cooperative interactions among transcription factors and their associated proteins on promoter DNA constitutes one mechanism by which signal integration is achieved (28). The studies reported here elucidate a new mechanistic step in the regulation and assembly of Notch transcription complexes: the cooperative formation of NTC dimers when two CSL-binding sites are arranged in an SPS architecture. Protein–protein interactions that stabilize the dimer include a conserved region on the convex surface of the ANK domain, which is on the opposite face from the surface engaged in interactions with CSL and MAML-1. Additional interactions among ICN, CSL, and MAML-1 may exist in the context of the full-length proteins. Cooperative formation of NTC dimers is seen with all four human Notch proteins, and the evolutionary conservation of both the dimerization interface and the SPS elements suggests that cooperativity between two NTCs on DNA represents a mechanism for modulation of NTC activity that is conserved among a number of metazoan organisms (Fig. 1B).
Previous studies examining whether isolated ANK domains from various Notch receptors form dimers yielded conflicting results, which now are reconciled by the findings reported here. Two-hybrid studies suggested that the ANK domains of Drosophila Notch and C. elegans GLP-1 are capable of self-association (29, 30), yet when isolated and purified, Drosophila ANK and the ANK domain from human Notch1 can remain monomeric at concentrations as high as 200 μM (31) and 6 μM (25) respectively, indicating that any weak intrinsic tendency of the ANK domain to self-associate in isolation readily is suppressed by commonly used solution conditions (probably because the contacts are predominantly electrostatic and the surface area buried in the ANK–ANK interface is only ≈500 Å2). When CSL, MAML-1, and ICN all are present in a single complex, contacts with MAML and CSL restrict the conformational space accessible to ANK, positioning it to participate in homotypic interactions. Thus, when two NTCs are oriented properly and adjacent to each other on DNA, the effective concentration of one ANK domain for the second increases dramatically, revealing a capacity for self-association that is not apparent from bulk solution experiments. Indeed, the ANK residues implicated in cooperative dimerization of human Notch1 NTCs form crystal contacts both in the NTCs themselves and in several structures of isolated Notch ANK domains solved independently, consistent with the idea that the context of the crystal lattice may more effectively mimic the constrained environment on DNA than bulk solution. Unmodeled electron density in the crystallized NTC core complex (SI Fig. 7) and the proximity of modeled atoms suggest that additional interactions between MAML-1 of one NTC and ANK from the other NTC also contribute to stabilization of dimers and are consistent with the biochemical observation that cooperative dimerization is not seen until after addition of the MAML-1 polypeptide. The conservation of the ANK–ANK interface among the four human Notches also suggests that NTCs may form heterodimers that depend on the promoter context and the cellular concentrations of the various Notch homologues.
Dependence of NTC Dimerization on CSL and DNA Flexibility.
Although the optimum distance between CSL sites is 16 bp, all spacer lengths from 15 to 19 bp can support cooperative dimerization of NTCs (Fig. 3D). The existence of length variation in the intersite distance between individual CSL-binding sites in human and Drosophila SPS elements (SI Fig. 6), as well as the range of DNA sequences that can accommodate NTC dimers (Fig. 3D), implies a requirement for intrinsic flexibility in one or more of the components of the complex.
Some of the flexibility to accommodate different spacer lengths probably comes from CSL itself. CSL, which is related structurally to the Rel-homology family of transcription factors, has three domains, and the linkers connecting the domains may be the source of flexibility. In canonical Rel-family members like nuclear factor of activated T cells 1 (NFAT1), flexibility in the linkers connecting the RHR-N and RHR-C domains enables adaptation to different environments to bind different DNA sequences and cooperate with other factors on DNA (32–34). The ability of the C. elegans homolog LAG-1 to undergo a domain shift upon complexation with ICN and MAML (14) substantiates the notion that the worm CSL protein also can adapt to different environments (for example, different spacer lengths between paired sites) by domain movement without losing the ability to bind consensus site DNA, although the divergence of the C. elegans notches (Fig. 1B) also raises the possibility that they may not cooperatively dimerize like the human proteins. Further support for this proposal emerges directly from comparison of the human (13) and C. elegans (14) NTC structures: when the β-trefoil domains (BTDs) of the two structures are superimposed, the rest of the protein domains (RHR-N, RHR-C, ANK, and MAML) rotate as a single rigid body around a hinge region between the BTD and RHR-N (35).
Flexibility also is likely to derive from the DNA that intervenes between the two sites of an SPS. If the DNA were rigid B-form DNA, a change in spacer length of 5 bp would result in a rotation of ≈180° around the DNA helix, a change that would be difficult to accommodate even with intrinsic protein flexibility. Thus, the length and the intrinsic flexibility of a particular spacer DNA, in addition to the individual affinities of the two CSL-binding sites for CSL, also ought to contribute to the likelihood that a given promoter DNA sequence will recruit an NTC dimer. Dimerization-induced bending or distortion of DNA also could influence occupancy of neighboring transcription factor binding sites without requiring direct protein–protein interactions (36).
Implications for Notch-Dependent Gene Expression.
Notch-regulated genes known to contain SPS elements in their promoters include basic helix–loop–helix genes from the enhancer-of-split complex in Drosophila, as well as homologous hairy/enhancer-of-split genes in Xenopus, mouse, and humans (20–23, 37, 38). The cooperative homodimerization of NTCs at such paired sites provides a molecular mechanism to account for the varying sensitivity of different genes to Notch dose and context (Fig. 5). The sensitivity of a particular promoter to a given dose of Notch signal may be influenced by the affinity of individual binding sites for CSL, the length and intrinsic flexibility of the spacer DNA at SPS elements, and the availability of neighboring cis-regulatory elements that can cooperate with NTCs. NTC dimers can assemble on promoters with SPS elements according to a cooperative dose–response curve that resembles the genetic switch exhibited by proteins like lambda repressor (39). Cooperativity in the response to Notch at these promoters thus will ensure tight regulation of transcription, with activated Notch rapidly flipping the switch from off to on. On the other hand, promoters with only one CSL-binding site or multiple sites without the proper SPS architecture may exhibit a less sensitive and more graded response to the dose of active Notch and instead depend more heavily on cooperativity with other transcription factors (23). Of note, studies investigating the functional implications of dimerization in a well established mouse model for leukemogenesis show that normal ICN1 induces T cell acute lymphoblastic leukemia (T-ALL) with full penetrance by 12 weeks, whereas ICN1 bearing the R1985A mutation fails to induce leukemia (W.S.P., unpublished observations). Whether dimers exert their activating effect on transcription by increasing promoter occupancy, by generating novel recognition sites for other transcription factors, or by recruiting basal factors more effectively is still an open question, the answer to which may vary depending on the identity of the promoter. The experimental criteria for formation of dimeric NTCs reported here should facilitate identification of the most sensitive direct targets of activated Notch in both normal development and disease pathogenesis. Knowledge of the specific interactions that dictate the assembly and regulation of dimeric NTCs also should enable the identification of small molecules that abrogate Notch activation of target genes containing specific types of promoter response elements.
Fig. 5.
Model for assembly of higher-order Notch transcription complexes. RAM delivers ANK to CSL, while also competing against or displacing corepressors. ANK binds to CSL with weak affinity, but recruitment of MAML-1 locks it in place to form a stable ternary complex (NTC). Interactions between two properly oriented complexes then promote cooperative formation of NTC dimers. The formation of both homodimers and heterodimers of different Notch receptors is postulated, as is the potential for recruitment of additional DNA-binding factor(s) that can cooperate with NTCs to activate transcription.
Experimental Procedures
Molecular Graphics.
Fig. 1 A and C were prepared with the program PyMOL (DeLano Scientific, Palo Alto, CA). The symmetry related copy of the MAML-1/ANK/CSL/DNA complex in Fig. 1A was rendered by applying the symexp operation to the coordinates of the complex (PDB ID code 2F8X; ref. 13) and then choosing the copy that approaches to within 5 Å of residue 1985 of the ANK domain of the initial complex.
EMSAs.
EMSAs were performed as described in ref. 25. For expression of RAMANK, residues 1761–2127 of human Notch1, 1701–2103 of human Notch2, 1666–2038 of human Notch3, and 1473–1828 of human Notch4 were built into a modified pGEX-4T1 vector described in ref. 25. The mutations in the ANK domain of Notch1 were introduced by QuikChange Site-Directed Mutagenesis kit (Stratagene, La Jolla, CA). CSL, RAMANK, ANK, MAML, and RAM polypeptides were purified as described (13, 25). The oligonucleotides used as probes are shown in SI Table 1. The final protein concentration for each lane (total volume 15 μl) was 140 nM for CSL, 170 nM for different versions of RAMANK, 240 nM for ANK, 440 nM for MAML-1, and 450 nM for RAM.
Reporter Gene Assays.
Empty pcDNA3 or pcDNA3 expressing ICN1 with indicated mutations were transiently cotransfected in triplicate into human U2OS cells with (i) firefly luciferase reporter genes driven by either an element derived from the HES-1 promoter (40) or an artificial element containing four tandem repeats of the CSL-binding site (41) and (ii) an internal Renilla luciferase reporter gene driven by an element derived from the human thymidine kinase promoter. Luciferase assays were performed and analyzed ≈48 h posttransfection by using the Promega (Madison, WI) Dual Luciferase Kit. Firefly luciferase activities were normalized by using internal Renilla luciferase control values and expressed relative to the empty vector control lysate, which was arbitrarily given a mean value of 1.
Immunoprecipitation Assays.
293 cells in six-well dishes were cotransfected with pcDNA3-ICN1 (0.1 μg), pEGFP-MAML1 (0.5 μg), and pcDNA3-CSL-Myc (1 μg) plasmids. Two days posttransfection, cell lysates and immunoprecipitates were prepared with anti-Myc (clone 9E10) antibody as described (42). Western blots were stained with antibodies against the Myc epitope, GFP (clone JL8; Clontech, Mountain View, CA), and the intracellular domain of Notch1 (42).
Supplementary Material
Acknowledgments
We thank Gavin Histen for technical assistance. This work is supported by National Institutes of Health grants to S.C.B. (R01 CA92433), W.S.P., and J.C.A.
Abbreviations
- ICN
intracellular portion of the Notch receptor
- NTC
Notch transcriptional activation complex
- CSL
CBF-1/Suppressor of Hairless/Lag-1
- MAML
Mastermind-like
- ANK domain
ankyrin-repeat domain
- RHR
Rel-homology region
- SPS
“sequence-paired” binding site.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0611092104/DC1.
References
- 1.Artavanis-Tsakonas S, Rand MD, Lake RJ. Science. 1999;284:770–776. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- 2.Hansson EM, Lendahl U, Chapman G. Semin Cancer Biol. 2004;14:320–328. doi: 10.1016/j.semcancer.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 3.Oda T, Elkahloun AG, Pike BL, Okajima K, Krantz ID, Genin A, Piccoli DA, Meltzer PS, Spinner NB, Collins FS, Chandrasekharappa SC. Nat Genet. 1997;16:235–242. doi: 10.1038/ng0797-235. [DOI] [PubMed] [Google Scholar]
- 4.Li L, Krantz ID, Deng Y, Genin A, Banta AB, Collins CC, Qi M, Trask BJ, Kuo WL, Cochran J, et al. Nat Genet. 1997;16:243–251. doi: 10.1038/ng0797-243. [DOI] [PubMed] [Google Scholar]
- 5.Crosnier C, Driancourt C, Raynaud N, Hadchouel M, Meunier-Rotival M. Hum Mutat. 2001;17:72–73. doi: 10.1002/1098-1004(2001)17:1<72::AID-HUMU11>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 6.Joutel A, Corpechot C, Ducros A, Vahedi K, Chabriat H, Mouton P, Alamowitch S, Domenga V, Cecillion M, Marechal E, et al. Nature. 1996;383:707–710. doi: 10.1038/383707a0. [DOI] [PubMed] [Google Scholar]
- 7.Garg V. Curr Opin Cardiol. 2006;21:180–184. doi: 10.1097/01.hco.0000221578.18254.70. [DOI] [PubMed] [Google Scholar]
- 8.Weng AP, Ferrando AA, Lee W, Morris JPt, Silverman LB, Sanchez-Irizarry C, Blacklow SC, Look AT, Aster JC. Science. 2004;306:269–271. doi: 10.1126/science.1102160. [DOI] [PubMed] [Google Scholar]
- 9.Nicolas M, Wolfer A, Raj K, Kummer JA, Mill P, van Noort M, Hui CC, Clevers H, Dotto GP, Radtke F. Nat Genet. 2003;33:416–421. doi: 10.1038/ng1099. [DOI] [PubMed] [Google Scholar]
- 10.Fortini ME, Artavanis-Tsakonas S. Cell. 1994;79:273–282. doi: 10.1016/0092-8674(94)90196-1. [DOI] [PubMed] [Google Scholar]
- 11.Petcherski AG, Kimble J. Nature. 2000;405:364–368. doi: 10.1038/35012645. [DOI] [PubMed] [Google Scholar]
- 12.Wu L, Aster JC, Blacklow SC, Lake R, Artavanis-Tsakonas S, Griffin JD. Nat Genet. 2000;26:484–489. doi: 10.1038/82644. [DOI] [PubMed] [Google Scholar]
- 13.Nam Y, Sliz P, Song L, Aster JC, Blacklow SC. Cell. 2006;124:973–983. doi: 10.1016/j.cell.2005.12.037. [DOI] [PubMed] [Google Scholar]
- 14.Wilson JJ, Kovall RA. Cell. 2006;124:985–996. doi: 10.1016/j.cell.2006.01.035. [DOI] [PubMed] [Google Scholar]
- 15.Fryer CJ, Lamar E, Turbachova I, Kintner C, Jones KA. Genes Dev. 2002;16:1397–1411. doi: 10.1101/gad.991602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wallberg AE, Pedersen K, Lendahl U, Roeder RG. Mol Cell Biol. 2002;22:7812–7819. doi: 10.1128/MCB.22.22.7812-7819.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kurooka H, Honjo T. J Biol Chem. 2000;275:17211–17220. doi: 10.1074/jbc.M000909200. [DOI] [PubMed] [Google Scholar]
- 18.Fryer CJ, White JB, Jones KA. Mol Cell. 2004;16:509–520. doi: 10.1016/j.molcel.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 19.Rebeiz M, Reeves NL, Posakony JW. Proc Natl Acad Sci USA. 2002;99:9888–9893. doi: 10.1073/pnas.152320899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bailey AM, Posakony JW. Genes Dev. 1995;9:2609–2622. doi: 10.1101/gad.9.21.2609. [DOI] [PubMed] [Google Scholar]
- 21.Nellesen DT, Lai EC, Posakony JW. Dev Biol. 1999;213:33–53. doi: 10.1006/dbio.1999.9324. [DOI] [PubMed] [Google Scholar]
- 22.Cave JW, Loh F, Surpris JW, Xia L, Caudy MA. Curr Biol. 2005;15:94–104. doi: 10.1016/j.cub.2004.12.070. [DOI] [PubMed] [Google Scholar]
- 23.Ong CT, Cheng HT, Chang LW, Ohtsuka T, Kageyama R, Stormo GD, Kopan R. J Biol Chem. 2006;281:5106–5119. doi: 10.1074/jbc.M506108200. [DOI] [PubMed] [Google Scholar]
- 24.Chung CN, Hamaguchi Y, Honjo T, Kawaichi M. Nucleic Acids Res. 1994;22:2938–2944. doi: 10.1093/nar/22.15.2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nam Y, Weng AP, Aster JC, Blacklow SC. J Biol Chem. 2003;278:21232–21239. doi: 10.1074/jbc.M301567200. [DOI] [PubMed] [Google Scholar]
- 26.Ehebauer MT, Chirgadze DY, Hayward P, Martinez Arias A, Blundell TL. Biochem J. 2005;392:13–20. doi: 10.1042/BJ20050515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tun T, Hamaguchi Y, Matsunami N, Furukawa T, Honjo T, Kawaichi M. Nucleic Acids Res. 1994;22:965–971. doi: 10.1093/nar/22.6.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ogata K, Sato K, Tahirov TH. Curr Opin Struct Biol. 2003;13:40–48. doi: 10.1016/s0959-440x(03)00012-5. [DOI] [PubMed] [Google Scholar]
- 29.Roehl H, Bosenberg M, Blelloch R, Kimble J. EMBO J. 1996;15:7002–7012. [PMC free article] [PubMed] [Google Scholar]
- 30.Matsuno K, Go MJ, Sun X, Eastman DS, Artavanis-Tsakonas S. Development (Cambridge, UK) 1997;124:4265–4273. doi: 10.1242/dev.124.21.4265. [DOI] [PubMed] [Google Scholar]
- 31.Zweifel ME, Barrick D. Biochemistry. 2001;40:14344–14356. doi: 10.1021/bi011435h. [DOI] [PubMed] [Google Scholar]
- 32.Giffin MJ, Stroud JC, Bates DL, von Koenig KD, Hardin J, Chen L. Nat Struct Biol. 2003;10:800–806. doi: 10.1038/nsb981. [DOI] [PubMed] [Google Scholar]
- 33.Jin L, Sliz P, Chen L, Macian F, Rao A, Hogan PG, Harrison SC. Nat Struct Biol. 2003;10:807–811. doi: 10.1038/nsb975. [DOI] [PubMed] [Google Scholar]
- 34.Wolberger C. Annu Rev Biophys Biomol Struct. 1999;28:29–56. doi: 10.1146/annurev.biophys.28.1.29. [DOI] [PubMed] [Google Scholar]
- 35.Barrick D, Kopan R. Cell. 2006;124:883–885. doi: 10.1016/j.cell.2006.02.028. [DOI] [PubMed] [Google Scholar]
- 36.Panne D, Maniatis T, Harrison SC. EMBO J. 2004;23:4384–4393. doi: 10.1038/sj.emboj.7600453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lamar E, Kintner C. Development (Cambridge, UK) 2005;132:3619–3630. doi: 10.1242/dev.01937. [DOI] [PubMed] [Google Scholar]
- 38.Davis RL, Turner DL, Evans LM, Kirschner MW. Dev Cell. 2001;1:553–565. doi: 10.1016/s1534-5807(01)00054-5. [DOI] [PubMed] [Google Scholar]
- 39.Ptashne M. Trends Biochem Sci. 2005;30:275–279. doi: 10.1016/j.tibs.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 40.Jarriault S, Brou C, Logeat F, Schroeter EH, Kopan R, Israel A. Nature. 1995;377:355–358. doi: 10.1038/377355a0. [DOI] [PubMed] [Google Scholar]
- 41.Hsieh JJ, Henkel T, Salmon P, Robey E, Peterson MG, Hayward SD. Mol Cell Biol. 1996;16:952–959. doi: 10.1128/mcb.16.3.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aster JC, Robertson ES, Hasserjian RP, Turner JR, Kieff E, Sklar J. J Biol Chem. 1997;272:11336–11343. doi: 10.1074/jbc.272.17.11336. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.