Abstract
One of the central tasks of stem cell biology is to understand the molecular mechanisms that control self-renewal in stem cells. Several cytokines are implicated as crucial regulators of hematopoietic stem cells (HSCs), but little is known about intracellular signaling for HSC self-renewal. To address this issue, we attempted to clarify how self-renewal potential is enhanced in HSCs without the adaptor molecule Lnk, as in Lnk-deficient mice HSCs are expanded in number >10-fold because of their increased self-renewal potential. We show that Lnk negatively regulates self-renewal of HSCs by modifying thrombopoietin (TPO)-mediated signal transduction. Single-cell cultures showed that Lnk-deficient HSCs are hypersensitive to TPO. Competitive repopulation revealed that long-term repopulating activity increases in Lnk-deficient HSCs, but not in WT HSCs, when these cells are cultured in the presence of TPO with or without stem cell factor. Single-cell transplantation of each of the paired daughter cells indicated that a combination of stem cell factor and TPO efficiently induces symmetrical self-renewal division in Lnk-deficient HSCs but not in WT HSCs. Newly developed single-cell immunostaining demonstrated significant enhancement of both p38 MAPK inactivation and STAT5 and Akt activation in Lnk-deficient HSCs after stimulation with TPO. Our results suggest that a balance in positive and negative signals downstream from the TPO signal plays a role in the regulation of the probability of self-renewal in HSCs. In general, likewise, the fate of stem cells may be determined by combinational changes in multiple signal transduction pathways.
Keywords: c-mpl, p38 MAPK, STAT5, Akt
Manipulation of stem cell self-renewal is a necessity for the development of stem cell-based regenerative and transplantation medicine. To this end, we need to understand molecular mechanisms underlying self-renewal in stem cells. In hematopoietic stem cells (HSCs), the best-studied mammalian stem cells, self-renewal has been demonstrated by in vivo assays (1–4). However, molecular mechanisms regulating self-renewal remain poorly understood. In particular, despite numerous studies of cytokines and cytokine receptors, little is known about intracellular signaling events in self-renewal of HSCs (5–7). Major difficulties have been the paucity of HSCs and the in vitro recapitulation of self-renewal (8, 9). We have approached this issue by analyzing Lnk-deficient mice (Lnk−/−) in comparison with WT mice.
Lnk is an adaptor protein containing a proline-rich domain, a pleckstrin homology domain, and a Src homology 2 domain (10). In Lnk−/− mice, long-term marrow repopulating activity is markedly elevated because of increases in both absolute number and self-renewal activity of HSCs (4, 11). These results suggest that Lnk negatively regulates the key signaling pathways of HSC self-renewal. Lnk is expressed in various hematopoietic lineages, in which some of its functions have been reported (12–15). Lnk is thought to regulate stem cell factor (SCF) signaling pathways negatively in immature B cells (12, 13). Recent reports indicated that Lnk negatively regulates thrombopoietin (TPO) signaling in megakaryocytes and erythropoietin signaling in erythroblasts (14, 15). Although the functions of Lnk as a negative regulator of cytokine signaling are shared by these lineages, the target signaling pathways appear to differ among these lineages. We therefore attempted to determine Lnk target signaling pathways in HSCs.
In both WT and Lnk−/− mice, CD34-negative or low, c-Kit-positive, Sca-1-positive, lineage marker-negative (CD34−KSL) cells within adult mouse bone marrow (BM) are highly enriched in HSCs (4, 16). When single-cell transplantation with CD34−KSL cells was performed, rates of long-term reconstitution were similar in WT and Lnk −/− mice, indicating similar degrees of HSC enrichment in this population. Using these highly enriched HSC populations, we first studied cytokine-induced division of CD34−KSL cells and found that Lnk is involved in the TPO signaling pathway. We then investigated how HSCs self-renew in culture with TPO by competitive repopulation and paired daughter cell assays. Furthermore, we developed single-cell immunostaining procedures for signal transduction analysis to examine Lnk-interacting intracellular signaling pathways in TPO-stimulated CD34−KSL cells.
Results
In Vitro Survival and Division of Single CD34−KSL Cells.
Direct effects of cytokines on both survival and proliferation of HSCs were evaluated to see which cytokine signals are influenced by the absence of Lnk. Serum-free culture of single WT or Lnk−/− CD34−KSL cells was performed in the presence of various cytokines at 100 ng/ml. Every 24 h after initiation of culture, cells in each well were examined under the microscope. At 72 h of culture, no CD34−KSL cells survived without a cytokine. In contrast, >70% of cells survived in the presence of SCF or TPO, and <20% of cells survived in the presence of IL-3, IL-6, or IL-11 (Fig. 1A). When cells were cultured with SCF, frequencies of cell division did not differ between Lnk−/− CD34−KSL cells (47.9 ± 5.3%) and WT CD34−KSL cells (54.6 ± 9.1%) (P = 0.197). In contrast, when cells were cultured with TPO, the frequency of cell division in Lnk−/− CD34−KSL cells (66.7 ± 8.0%) was significantly greater than that in WT CD34−KSL cells (49.8 ± 7.6%) (P = 0.009) (Fig. 1B). These data indicate that SCF or TPO, but not the other cytokines studied, can support survival and division of significant proportions of WT and Lnk−/− CD34−KSL cells, and that TPO promotes division of Lnk−/− CD34−KSL cells more efficiently than division of WT CD34−KSL cells.
Fig. 1.
In vitro survival and division of single WT or Lnk−/− CD34−KSL cells in the presence of a cytokine. WT or Lnk−/− CD34−KSL cells (n = 96) underwent single-cell serum-free culture in the presence of SCF, TPO, IL-3, IL-6, or IL-11. At 72 h of culture, the number of cells in each well was counted under an inverted microscope. Wells containing one or more cell(s) were judged to exhibit “survival” and wells containing two or more cells were judged to exhibit “division.” Frequencies of survival (A) and division (B) from five independent experiments are shown as mean ± SD. ∗, P = 0.009.
Hypersensitivity of Lnk−/− CD34−KSL Cells to TPO Stimulation.
Sensitivity to SCF or TPO stimulation was compared between WT and Lnk−/− CD34−KSL cells with respect to dose–response of cell division. WT or Lnk−/− CD34−KSL cells were subjected to single-cell serum-free culture with graded doses of SCF (1, 5, 10, 50, or 100 ng/ml) or TPO (0.1, 0.5, 1, 5, 10, 50, or 100 ng/ml). After 72 h of culture, frequencies of cell division at each concentration were determined. As shown in Fig. 2A, dose–response curves were plotted by nonlinear regression. Midpoint effective doses (ED50s) were obtained for SCF and TPO. When WT or Lnk−/− CD34−KSL cells were cultured with SCF, respective ED50s were 8.03 ± 0.92 and 7.17 ± 2.09 ng/ml (P = 0.73). When WT or Lnk−/− cells were cultured with TPO, respective ED50s were 1.89 ± 0.55 and 0.69 ± 0.06 ng/ml (P = 0.04). SCF dose–response behaviors were quite similar in WT and Lnk−/− CD34−KSL cells. In contrast, the concentrations of TPO that induced cell division were lower for Lnk−/− CD34−KSL cells than for WT CD34−KSL cells.
Fig. 2.
Hypersensitivity of Lnk−/− CD34−KSL cells to TPO stimulation. Dose–response curves to SCF, TPO, or both are shown for WT and Lnk−/− CD34−KSL cells. (A) (Left) When cultured with SCF, WT or Lnk−/− cells had ED50s of 8.03 ± 0.92 or 7.17 ± 2.09 ng/ml, respectively (P = 0.73). (Right) When cultured with TPO, WT or Lnk−/− cells had ED50s of 1.89 ± 0.55 or 0.69 ± 0.06 ng/ml, respectively (P = 0.04). (B) Synergistic effects of SCF and TPO on division of CD34−KSL cells. Percentages of cells having undergone division by 72 h of culture among WT (Left) or Lnk−/− (Right) CD34−KSL cells in the presence of SCF plus TPO are presented as isobolograms. Contours show percent division at intervals of 10%.
The synergistic effects of SCF and TPO on cell division were compared between WT and Lnk−/− CD34−KSL cells. Single-cell serum-free cultures were performed in the presence of SCF and TPO at various combinations of concentrations. At 72 h of culture, frequencies of cell division were determined and demonstrated by isobolography (17, 18) (Fig. 2B). When concentrations of both SCF and TPO were >5 ng/ml, > 80% of both WT and Lnk−/− CD34−KSL cells underwent cell division. When the concentration of SCF was greater than that of TPO, dose–response behaviors of WT and Lnk−/− CD34−KSL cells were similar. In contrast, when the TPO concentration was greater than that of SCF and the SCF concentration was <5 ng/ml, Lnk−/− CD34−KSL cells divided more frequently than did WT CD34−KSL cells. These results indicate that in CD34−KSL cells the absence of Lnk causes hypersensitivity to TPO but not to SCF even when both TPO and SCF are present.
In addition, the kinetics of cytokine-induced cell division was compared between WT and Lnk−/− CD34−KSL cells [supporting information (SI) Fig. 5]. When cultured with SCF or SCF and TPO, WT and Lnk−/− CD34−KSL cells showed quite similar first-division kinetics. Of note is that the frequencies of cell division were similar in WT and Lnk−/− CD34−KSL cells when those cells were cultured with a combination of SCF and TPO at saturating concentrations (Fig. 2B and SI Fig. 5). In contrast, when cultured with TPO, it became apparent after 24 h of culture that Lnk−/− CD34−KSL cells underwent a first division more frequently than did WT CD34−KSL cells (SI Fig. 5).
Repopulating Activity Increases in Lnk−/− CD34−KSL Cells in Response to TPO.
We assumed that TPO might be able to induce self-renewal in Lnk−/− HSCs more efficiently than in WT HSCs. To address this issue, 40 WT or Lnk−/− CD34−KSL cells were cultured in serum-free medium in the presence of cytokines for 72 h and underwent competitive repopulation assay (SI Fig. 6A). Twelve weeks after transplantation, repopulating units (RUs) were determined by analyzing peripheral blood donor chimerism. The RU is a quantitative index of repopulating activity, and 1 RU is defined as the amount of repopulating activity in 105 unfractionated BM cells from WT mice, based on competitive repopulation assay (19).
As shown in Fig. 3, RUs did not change in WT CD34−KSL cells after a 72-h culture period in the presence of SCF, TPO, or SCF plus TPO. RUs did not change significantly in Lnk−/− CD34−KSL cells after culture with SCF alone. However, RUs were significantly increased in Lnk−/− CD34−KSL cells after culture with TPO alone or SCF plus TPO. These repopulating activities were transplantable into secondary recipient mice (SI Table 2), suggesting that self-renewal potential was maintained in cultured cells. On average, RU increased 3-fold in both cases. Based on the positive correlation between RUs and HSC numbers (SI Fig. 6B), it was estimated that the number of HSCs proportionately increased 3-fold. To increase in number, HSCs should have undergone symmetrical self-renewal divisions.
Fig. 3.
RU increase in Lnk−/− CD34−KSL cells after culture. Forty WT (Left) or Lnk−/− (Right) CD34−KSL cells were transplanted (n = 8). Similar cells alternatively were cultured with SCF (50 ng/ml) and/or TPO (50 ng/ml) for 3 days, and then transplanted (n = 8). RUs before (n = 8) and after culture were compared 12 weeks after transplantation. Data are shown in terms of fold increase (average RU before culture = 1.0). RUs significantly increased in Lnk−/− CD34−KSL cells after culture with TPO (P = 0.025) or SCF plus TPO (P = 0.046). ∗, P < 0.05 vs. before culture.
Symmetrical Self-Renewal Division of Lnk−/− HSCs in Culture.
To know whether increased RUs in effect resulted from symmetrical self-renewal in Lnk−/− HSCs, paired daughter cell experiments were performed (SI Fig. 6C). Single CD34−KSL cells were directly transplanted into lethally irradiated mice or individually cultured in the presence of SCF and TPO. When cells in culture gave rise to two daughter cells, each daughter cell was separated from the other by micromanipulation techniques. Individual daughter cells were transplanted into lethally irradiated mice.
As shown in Table 1, both daughter cells were detected as long-term repopulating cells in 3 of 20 pairs of daughters of single Lnk−/− CD34−KSL cells and in none of 28 pairs of daughters of single WT CD34−KSL cells. One of the two daughter cells was detected as a long-term repopulating cell in 3 of 20 pairs of daughters of Lnk−/− CD34−KSL cells and in 3 of 28 pairs of daughters of WT CD34−KSL cells. When symmetrical self-renewal is defined as division resulting in generation of two daughter cells with long-term repopulation potential, and asymmetrical self-renewal is defined as division resulting in generation of one daughter cell with long-term repopulating potential and another without long-term repopulation potential, these data support the conclusion that in culture with SCF and TPO, Lnk−/− HSCs undergo symmetrical self-renewal division more frequently than do WT HSCs.
Table 1.
Symmetrical self-renewal in culture of Lnk −/− CD34− KSL cells but not WT CD34− KSL cells
| Single CD34− KSL cells | Daughter cells |
No. of pairs (%) | % Chimerism | |
|---|---|---|---|---|
| One | The other | |||
| WT | + | + | 0/28 (0) | |
| WT | + | − | 3/28 (11) | 0.6, 0.6, 0.6 |
| Lnk−/− | + | + | 3/20 (15) | (2.0; 5.9), (50.9; 72.9), (42.9; 81.4) |
| Lnk−/− | + | − | 3/20 (15) | 0.7, 2.4, 23.0 |
Single WT or Lnk−/− CD34−KSL cells were cultured with SCF and TPO. After a single cell gave rise to paired-daughter cells, each daughter cell was individually transplanted (SI Fig. 6C). Twelve weeks after transplantation reconstitution was observed in 4 of 25 mice or 3 of 16 mice that, respectively, had received single WT or Lnk−/− CD34−KSL cells at day 0. Successful reconstitution is shown as +, and undetectable reconstitution is shown as −, for each transplanted daughter cell.
Development of Signal Transduction Analysis for HSCs.
Because of the paucity of HSCs (a single mouse yielded ≈1,000 CD34−KSL cells) to apply commonly used signal transduction assays to this study was difficult. To circumvent this problem, we combined multicolor fluorescence-activated cell sorting, fluorescent immunostaining, confocal laser scanning, and computational quantification of fluorescent intensities. One of the two keys to success in this assay was in-droplet immunostaining procedures. Their principal steps are illustrated in SI Fig. 7. From the initial cell purification step to the final analysis step, cells were maintained in a droplet of medium to avoid cell loss and cell damage. The other key was measurement of signal intensity in individual cells. In this study, we focused on phosphorylation kinetics of signaling molecules because Lnk is an adaptor protein containing a Src homology 2 domain. As demonstrated in SI Fig. 8, phosphorylation intensity of each CD34−KSL cell was evaluated by using NIH ImageJ software. We named this method the single-cell imaging of phosphorylation (SCIPhos) assay.
To validate the SCIPhos assay, signal transduction in a cytokine-dependent cell line was examined simultaneously by both SCIPhos assays and conventional Western blot analysis. After cytokine deprivation, TPO-dependent 32D/Mpl cells were stimulated with TPO, and phosphorylation of STAT5 and Jak2 was quantitatively measured. As representatively shown in SI Fig. 9, SCIPhos and Western blot analyses gave similar kinetics of STAT5 phosphorylation. Interestingly, STAT5 phosphorylation increased in a dose-dependent manner for TPO stimulation, and SCIPhos data correlated well with Western blot data (SI Fig. 9C). Good correlation between results of these two assay techniques was also observed for Jak2 phosphorylation (data not shown).
Phosphorylation of Signaling Molecules in WT and Lnk−/− CD34−KSL Cells.
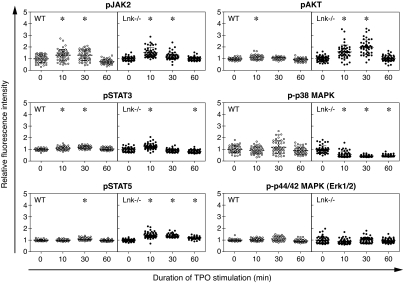
To identify the key signal pathways involved in HSC self-renewal, phosphorylation kinetics of JAK2, STAT3, STAT5, Akt, p38 MAPK, and p44/42 MAPK after TPO or SCF stimulation was compared between WT and Lnk−/− CD34−KSL cells. As shown in Fig. 4, after stimulation with TPO JAK2 was similarly phosphorylated in WT and Lnk−/− CD34−KSL cells. However, STAT5 and Akt were more intensely phosphorylated in Lnk−/− CD34−KSL cells than in WT CD34−KSL cells. In contrast, phosphorylation levels of p38 MAPK in Lnk−/− CD34−KSL cells were down-regulated after TPO stimulation, whereas those in WT CD34−KSL cells remained unchanged. Significant phosphorylation of p44/42 MAPK was not detected in either cell type. On the other hand, after stimulation with SCF, we detected no significant difference between WT and Lnk−/− CD34−KSL cells in phosphorylation patterns of these signaling molecules (data not shown). Because these comparisons were made with actively self-renewing HSCs, enhanced up-regulation of STAT5 and Akt pathways and enhanced down-regulation of p38 MAPK pathways can be inferred to be associated with initiation of HSC self-renewal.
Fig. 4.
TPO-mediated signal transduction in WT and Lnk−/− CD34−KSL cells. Phosphorylation kinetics of JAK2, STAT3, STAT5, Akt, p38 MAPK, and p44/42 MAPK in TPO-stimulated WT and Lnk−/− CD34−KSL cells. All fluorescence intensities of individual cells were computationally quantified, normalized, and compared against the mean intensity of unstimulated cells. Each dot shows normalized fluorescence intensity of individual cells (n = 50). Error bars indicate mean ± SD. ∗, P < 0.001 vs. unstimulated cells (t = 0).
Discussion
To understand why self-renewal potential is greater in HSCs without Lnk, we focused our attention on a first division of CD34−KSL cells because self-renewal is likely to be progressively reduced in the following divisions (7, 8). Analysis of their first division revealed that Lnk−/− CD34−KSL cells are more sensitive to TPO than are WT CD34−KSL cells. SCF and TPO synergistically acted on both WT and Lnk−/− CD34−KSL cells and efficiently induced their division. Over 80% of both WT and Lnk−/− CD34−KSL cells divided once by day 3 of culture in the presence of saturating amounts of SCF and TPO (Fig. 2 and SI Fig. 5). Finding that the frequency of dividing cells among Lnk−/− CD34−KSL cells did not differ from that among WT CD34−KSL cells led us to the hypothesis that outcomes of a first division might differ between WT and Lnk−/− CD34−KSL cells. This hypothesis was supported by competitive repopulation data and was verified by paired-daughter cell experiment data. During 3-day culture with TPO or with SCF and TPO, RUs increased 3-fold in Lnk−/− cells, whereas RUs did not significantly increase in WT cells (Fig. 3A). To increase RUs, Lnk−/− CD34−KSL cells must have undergone symmetrical self-renewal division during the culture period. As far as first divisions were examined, both symmetrical and asymmetrical self-renewal divisions were detected in Lnk−/− CD34−KSL cells (Table 1). In contrast, symmetrical self-renewal division was not detected in WT CD34−KSL cells. The results clearly indicate that Lnk−/− HSCs self-renew better than do WT HSCs in response to TPO.
Nolan and colleagues (20–22) have reported an intracellular phospho-protein analysis technique using flow cytometry (Phospho Flow). This technique enables signal transduction analysis of target cells in heterogeneous cell populations. The cells that surround CD34-KSL cells, however, have produced many cytokines before a particular experimental stimulus is applied to CD34-KSL cells. The unavoidable prestimulatory effects of these cytokines impair the utility of the Phospho Flow method in the analysis of CD34−KSL cells. Furthermore, internalization of c-Kit in response to SCF makes phenotypic identification of CD34-KSL cells difficult. To overcome these problems, in the SCIPhos assay cells are first sorted and then stimulated with cytokines under defined conditions, followed by single-cell immunostaining. The SCIPhos assay enables quantitative measurement of phosphorylation levels of signal transduction molecules in individual cells, as verified by comparison with Western blotting data using only 50 cells (SI Fig. 5). Moreover, intracellular localization of signal molecules can be examined simultaneously (23).
Using SCIPhos assays, we attempted to identify signal transduction pathways that in self-renewing Lnk−/− HSCs are activated or inactivated differently from those in self-renewing WT HSCs. Because RUs in cultured Lnk−/− cells increased in the presence of TPO alone, but not SCF alone, to the same extent as that seen in the presence of SCF plus TPO (Fig. 3), we simply compared SCF- or TPO-mediated signal transduction between Lnk−/− and WT HSCs. Lnk−/− HSCs in the process of self-renewal revealed enhancement of combinatorial change in signal transduction, i.e., activation of both STAT5 and Akt signal transduction and inactivation of p38 MAPK (Fig. 4). Phoshorylation of p38 MAPK in freshly isolated HSCs was possibly caused by stress in cell preparation procedures. However, its dephosphorylation was significantly enhanced in Lnk−/− HSCs. Kato et al. (24) have recently reported that continuous activation of STAT5, but not STAT3, in CD34−KSL cells results in myeloproliferative disease accompanied with increase of a broad range of myeloid progenitors in addition to HSCs. Akt has been shown to facilitate cell cycle progression and suppression of apoptosis (25). The p38 MAPK pathway has been implicated as regulating cell cycle progression negatively by activating transcription of the Ink4a-Arf locus and inhibiting the expression of D-type cyclins (26). A p38 MAPK inhibitor reportedly prevents decline of self-renewal potential in HSCs through serial transplantation (27). These signal modifications together may give HSCs advantages in maintaining self-renewal potential. How Lnk controls the probability of symmetrical self-renewal in HSCs is extremely intriguing.
No hematopoietic malignancy has been observed to date in Lnk-deficient mice (11, 28). Lnk may provide a suitable molecular target for enhancement of self-renewal capacity of HSCs. Lnk inhibitors should give selective advantage to HSCs and should be useful for stem cell transplantation or gene therapy targeting HSCs. Alternatively, Lnk inhibitors can be used for ex vivo expansion of HSCs. Takizawa et al. (29) have reported that transient inhibition of endogenous Lnk activity by introduction of a dominant-negative form of Lnk can increase engraftment rates of HSCs. Interestingly, inhibition of Lnk function increases cell adhesion ability in HSCs so that HSCs can efficiently home to a BM niche. These results suggest that Lnk interacts with multiple signaling cascades related to cytokine signal transduction and cell mobility.
In this study, we showed that Lnk negatively regulates TPO-mediated signaling pathways in HSCs. Comparison of HSC numbers among Lnk−/−, TPO−/−, and Lnk−/−TPO−/− mice has recently been reported (28). Increases in numbers of HSCs in Lnk−/− mice have been shown to be overridden by decreases in numbers of HSCs in TPO−/− mice (28). Interestingly, HSC numbers are greater in Lnk−/−TPO−/− mice than in TPO−/− mice, suggesting that not only TPO signaling is involved. We conclude that Lnk negatively interacts with signaling pathways downstream of TPO/c-Mpl that play an important role in HSC fate decision, namely, whether or not HSCs self-renew. We finally propose that self-renewal of HSC is regulated by a balance in positive and negative signals from multiple pathways rather than by self-renewal-specific signals. In this regard, self-renewal signaling may be much more complicated than generally thought.
Materials and Methods
Mice.
C57BL/6 mice congenic for the Ly5 locus (B6-Ly5.1) and Lnk−/− B6-Ly5.1 mice were bred and maintained at the Animal Research Center of the Institute of Medical Science, University of Tokyo. The Animal Experiment Committee of the Institute of Medical Science, University of Tokyo, approved animal care and use. B6-Ly5.1/5.2 (B6-F1) mice were obtained from mating pairs of B6-Ly5.1 and B6-Ly5.2 mice. B6-Ly5.2 mice were purchased from Nihon SLC (Shizuoka, Japan).
Purification of CD34−KSL Cells.
CD34−KSL cells were purified from BM cells of 2-month-old WT or Lnk−/− B6-Ly5.1 mice as described (16, 30). In brief, low-density cells were isolated on Ficoll-Paque PLUS (Amersham Bioscience, Uppsala, Sweden). The cells were stained with an antibody mixture consisting of biotinylated anti-Gr-1, anti-Mac-1, anit-B220, anti-CD4, anti-CD8, and anti-Ter-119 antibodies (Pharmingen, San Diego, CA). The cells were subsequently labeled with MACS goat anti-rat IgG microbeads. Lineage-positive cells were then depleted by using the Midi-MACS system (Miltenyi Biotec, Bergisch Gladbach, Germany). The cells were further stained with FITC-conjugated anti-CD34, phycoerythrin-conjugated anti-Sca-1, and allophycocyanin (APC)-conjugated anti-c-Kit antibodies (Pharmingen). Biotinylated antibodies were detected with streptavidin-APC-Cy7 (Molecular Probes, Eugene, OR). Four-color analysis and sorting were performed on a MoFlo Cell Sorter (DakoCytomation, Glostrup, Denmark).
Single-Cell Serum-Free Culture.
Single-cell cultures of CD34−KSL cells were performed under serum-free conditions as described (9, 31). Cells were individually deposited into single wells of a 96-well round-bottom microtiter plate and cultured in S-clone SF-O3 medium (Sanko-Junyaku, Tokyo, Japan) supplemented with 0.5% BSA and the following cytokines: 0.1–100 ng/ml mouse SCF, 0.1–100 ng/ml human TPO, 100 ng/ml mouse IL-3, 100 ng/ml human IL-6, and 100 ng/ml human IL-11 (PeproTech, Rocky Hill, NJ). After cell sorting, the presence of one cell per well was verified under an inverted microscope. The cells were incubated at 37°C in a humidified atmosphere with 5% CO2 in air. At several time points, numbers of cells per well were counted under an inverted microscope. Each frequency of cell division was obtained from 96 wells.
Transplantation Assays.
Competitive repopulation assays were performed with the Ly5 congenic mouse system. Forty CD34−KSL cells from WT B6-Ly5.1 mice were transplanted into a WT B6-Ly5.2 mouse irradiated at a dose of 9.5 Gy with 4 × 105 competitor cells from WT B6-F1 mice. Forty CD34−KSL cells from Lnk−/− B6-Ly5.1 mice were transplanted into a WT B6-Ly5.2 mouse irradiated at a dose of 9.5 Gy with 8 × 105 competitor cells from WT B6-F1 mice. Concurrently, 40 WT or Lnk−/− CD34−KSL cells were cultured in the presence of cytokines for 3 days, and then transplanted, with 4 × 105 or 8 × 105 WT B6-F1 competitor cells, respectively, into a lethally irradiated (9.5 Gy) WT B6 mouse. When one CD34−KSL cell or each one of its paired-daughter cells generated in culture was transplanted, 2 × 105 competitor cells were used. Twelve weeks after transplantation, peripheral blood cells of the recipients were stained with FITC-conjugated anti-Ly5.2 (104) and biotinylated anti-Ly5.1 (A4) (Pharmingen). The cells were simultaneously stained with phycoerythrin (PE)-Cy7-conjugated anti-B220 antibody and a mixture of allophycocyanin-conjugated anti-Mac-1 and -Gr-1 antibodies and a mixture of PE-conjugated anti-CD4 and -CD8 antibodies (Pharmingen). The biotinylated antibody was detected with streptavidin-Texas red. Cells were analyzed on a FACS Vantage (Becton Dickinson, San Jose, CA). Percentage chimerism was calculated as (percent Ly5.1-donor cells) × 100/(percentage Ly5.1-donor cells + percentage F1-competitor cells). RUs were calculated with Harrison's method (19) as follows: RU = (percentage chimerism) × (number of competitor cells) × 10−5/(100 − percentage chimerism). In the case of single-cell transplantation, when percentage chimerism was >0.5, test donor cells were considered to be long-term repopulating cells. Secondary transplantation was performed 4 months after primary transplantation. After peripheral blood was analyzed for chimerism once again, 2 × 106 BM cells from selected recipient mice were transplanted into mice irradiated at a dose of 9.5 Gy.
Cell Lines.
32D cells (ATCC, Manassas, VA) were maintained in RPMI medium 1640 with 10% FCS and 10% conditioned medium from the culture of WEHI-3B cells. Human c-MPL cDNA was ligated to the pMY-IRES-EGFP retroviral vector (a gift of T. Kitamura, University of Tokyo, Tokyo, Japan). The recombinant viruses were produced by transfecting pMY-Mpl-IRES-EGFP into 293gp cells with pcDNA3-VSV-G. 32D cells were then infected with these viruses. TPO-dependent 32D (32D/Mpl) cells were selected, cloned, and maintained with 10 ng/ml TPO.
SCIPhos Assay.
Phosphorylation of cytokine signaling molecules was analyzed by fluorescent immunocytostaining (SI Fig. 7). WT or Lnk−/− CD34−KSL cells were directly sorted by flow cytometry into droplets of medium on poly-l-lysine-coated glass slides (Matsunami Glass, Osaka, Japan). Cells were stimulated with cytokine(s) at indicated concentrations for indicated times, and then fixed with 4% paraformaldehyde and permeabilized with 0.1% Triton X-100. Subsequently, the cells were stained with phosphorylation-specific anti-JAK2 (Tyr-1007/1008), anti-STAT3 (Tyr-705), anti-STAT5 (Tyr-694), anti-Akt (Ser-473), anti-p38 MAPK (Thr-180/Tyr-182), and anti-p44/42 MAPK (Thr-202/Tyr-204) antibodies (Cell Signaling Technology, Beverly, MA). After washing with PBS containing 2% goat serum, cells were stained with Alexa Fluor 488- or 647-conjugated goat anti-rabbit IgG antibody (Molecular Probes) and DAPI.
To avoid quenching of fluorescence, cells were scanned only once, at the cells' centers, by a TCS SP2 AOBS confocal laser-scanning microscope (Leica, Wetzlar, Germany). Cell images were obtained at 100 × 100 pixels resolution by using a ×63 objective lens. Fluorescence intensities of individual cells (n = 50) were computationally quantified by using ImageJ 1.33 software (http://rsb.info.nih.gov/ij) and were normalized against the mean intensity of unstimulated cells.
Western Blot Analysis.
32D/Mpl cells were starved in RPMI medium 1640 with 10% FCS for 12 h. A total of 2 × 105 such cells were stimulated with 50 ng/ml TPO for 0, 1, 5, 10, or 60 min or 0, 0.8, 1.6, 3.2, 6.4, or 12.8 ng/ml TPO for 10 min. Cells were lysed in buffer consisting of 50 mM Tris·HCl, 5 mM EDTA, 150 mM NaCl, 1% Triton X-100, 1 mM Na3VO4, 1 mM NaF, and protease inhibitor in water (Roche Molecular Biochemicals, Basel, Swizerland). Cell lysates in 2× SDS sample buffer (BioRad Laboratories, Hercules, CA) were subjected to SDS/PAGE, blotted, and probed with antiphosphorylated STAT5 (Tyr-694) or anti-phosphorylated JAK2 (Tyr-1007/1008) antibodies. Primary antibodies were detected with the SuperSignal West Pico system (Pierce Biotechnology, Rockford, IL).
Statistical Analysis and Nonlinear Regression.
Mean values of two groups were compared by two-tail unpaired t testing. Nonlinear regression by the four-parameter logistic method was performed for the dose–response and division kinetics curves. The ED50s of the two groups were compared by F testing. All statistical analyses were performed on Prism 4 software (GraphPad, San Diego, CA).
Supplementary Material
Acknowledgments
We thank T. Kitamura for providing human c-MPL cDNA and pMY-IRES-EGFP retroviral vector, A. S. Knisely for critical reading of the manuscript, A. Iwama for helpful discussions, and Y. Yamazaki for excellent assistance in analysis and sorting on a flow cytometer. This work was supported in part by grants from the Naito Foundation, the Terumo Lifescience Foundation, and the Ministry of Education, Culture, Sport, Science, and Technology of Japan.
Abbreviations
- HSC
hematopoietic stem cell
- SCF
stem cell factor
- TPO
thrombopoietin
- BM
bone marrow
- RU
repopulating unit
- SCIPhos
single-cell imaging of phosphorylation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0606238104/DC1.
References
- 1.Dick JE, Magli MC, Huszar D, Phillips RA, Bernstein A. Cell. 1985;42:71–79. doi: 10.1016/s0092-8674(85)80102-1. [DOI] [PubMed] [Google Scholar]
- 2.Lemischka IR, Raulet DH, Mulligan RC. Cell. 1986;45:917–927. doi: 10.1016/0092-8674(86)90566-0. [DOI] [PubMed] [Google Scholar]
- 3.Keller G, Snodgrass R. J Exp Med. 1990;171:1407–1418. doi: 10.1084/jem.171.5.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ema H, Sudo K, Seita J, Matsubara A, Morita Y, Osawa M, Takatsu K, Takaki S, Nakauchi H. Dev Cell. 2005;8:907–914. doi: 10.1016/j.devcel.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 5.Ogawa M. Blood. 1993;81:2844–2853. [PubMed] [Google Scholar]
- 6.Eaves C, Miller C, Conneally E, Audet J, Oostendorp R, Cashman J, Zandstra P, Rose-John S, Piret J, Eaves A. Ann NY Acad Sci. 1999;872:1–8. doi: 10.1111/j.1749-6632.1999.tb08447.x. [DOI] [PubMed] [Google Scholar]
- 7.Nakauchi H, Sudo K, Ema H. Ann NY Acad Sci. 2001;938:18–24. doi: 10.1111/j.1749-6632.2001.tb03570.x. discussion 24–25. [DOI] [PubMed] [Google Scholar]
- 8.Ema H, Takano H, Sudo K, Nakauchi H. J Exp Med. 2000;192:1281–1288. doi: 10.1084/jem.192.9.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takano H, Ema H, Sudo K, Nakauchi H. J Exp Med. 2004;199:295–302. doi: 10.1084/jem.20030929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takaki S, Watts JD, Forbush KA, Nguyen NT, Hayashi J, Alberola-Ila J, Aebersold R, Perlmutter RM. J Biol Chem. 1997;272:14562–14570. doi: 10.1074/jbc.272.23.14562. [DOI] [PubMed] [Google Scholar]
- 11.Takaki S, Morita H, Tezuka Y, Takatsu K. J Exp Med. 2002;195:151–160. doi: 10.1084/jem.20011170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takaki S, Sauer K, Iritani BM, Chien S, Ebihara Y, Tsuji K, Takatsu K, Perlmutter RM. Immunity. 2000;13:599–609. doi: 10.1016/s1074-7613(00)00060-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Velazquez L, Cheng AM, Fleming HE, Furlonger C, Vesely S, Bernstein A, Paige CJ, Pawson T. J Exp Med. 2002;195:1599–1611. doi: 10.1084/jem.20011883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tong W, Lodish HF. J Exp Med. 2004;200:569–580. doi: 10.1084/jem.20040762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tong W, Zhang J, Lodish HF. Blood. 2005;105:4604–4612. doi: 10.1182/blood-2004-10-4093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Osawa M, Hanada K-i, Hamada H, Nakauchi H. Science. 1996;273:242–245. doi: 10.1126/science.273.5272.242. [DOI] [PubMed] [Google Scholar]
- 17.Gessner PK. Toxicology. 1995;105:161–179. doi: 10.1016/0300-483x(95)03210-7. [DOI] [PubMed] [Google Scholar]
- 18.Tallarida RJ. J Pharmacol Exp Ther. 2001;298:865–872. [PubMed] [Google Scholar]
- 19.Harrison DE, Jordan CT, Zhong RK, Astle CM. Exp Hematol. 1993;21:206–219. [PubMed] [Google Scholar]
- 20.Krutzik PO, Nolan GP. Cytometry A. 2003;55:61–70. doi: 10.1002/cyto.a.10072. [DOI] [PubMed] [Google Scholar]
- 21.Krutzik PO, Hale MB, Nolan GP. J Immunol. 2005;175:2366–2373. doi: 10.4049/jimmunol.175.4.2366. [DOI] [PubMed] [Google Scholar]
- 22.Perez OD, Nolan GP. Nat Biotechnol. 2002;20:155–162. doi: 10.1038/nbt0202-155. [DOI] [PubMed] [Google Scholar]
- 23.Yamazaki S, Iwama A, Takayanagi S, Morita Y, Eto K, Ema H, Nakauchi H. EMBO J. 2006;25:3515–3523. doi: 10.1038/sj.emboj.7601236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kato Y, Iwama A, Tadokoro Y, Shimoda K, Minoguchi M, Akira S, Tanaka M, Miyajima A, Kitamura T, Nakauchi H. J Exp Med. 2005;202:169–179. doi: 10.1084/jem.20042541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alvarez B, Martinez AC, Burgering BM, Carrera AC. Nature. 2001;413:744–747. doi: 10.1038/35099574. [DOI] [PubMed] [Google Scholar]
- 26.Bulavin DV, Phillips C, Nannenga B, Timofeev O, Donehower LA, Anderson CW, Appella E, Fornace AJ., Jr Nat Genet. 2004;36:343–350. doi: 10.1038/ng1317. [DOI] [PubMed] [Google Scholar]
- 27.Ito K, Hirao A, Arai F, Takubo K, Matsuoka S, Miyamoto K, Ohmura M, Naka K, Hosokawa K, Ikeda Y, Suda T. Nat Med. 2006;12:446–451. doi: 10.1038/nm1388. [DOI] [PubMed] [Google Scholar]
- 28.Buza-Vidas N, Antonchuk J, Qian H, Mansson R, Luc S, Zandi S, Anderson K, Takaki S, Nygren JM, Jensen CT, Jacobsen SE. Genes Dev. 2006;20:2018–2023. doi: 10.1101/gad.385606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takizawa H, Kubo-Akashi C, Nobuhisa I, Kwon SM, Iseki M, Taga T, Takatsu K, Takaki S. Blood. 2006;107:2968–2975. doi: 10.1182/blood-2005-05-2138. [DOI] [PubMed] [Google Scholar]
- 30.Sudo K, Ema H, Morita Y, Nakauchi H. J Exp Med. 2000;192:1273–1280. doi: 10.1084/jem.192.9.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iwama A, Oguro H, Negishi M, Kato Y, Morita Y, Tsukui H, Ema H, Kamijo T, Katoh-Fukui Y, Koseki H, et al. Immunity. 2004;21:843–851. doi: 10.1016/j.immuni.2004.11.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.