Abstract
Honey bee queens produce a sophisticated array of chemical signals (pheromones) that influence both the behavior and physiology of their nest mates. Most striking are the effects of queen mandibular pheromone (QMP), a chemical blend that induces young workers to feed and groom the queen and primes bees to perform colony-related tasks. But how does this pheromone operate at the cellular level? This study reveals that QMP has profound effects on dopamine pathways in the brain, pathways that play a central role in behavioral regulation and motor control. In young worker bees, dopamine levels, levels of dopamine receptor gene expression, and cellular responses to this amine are all affected by QMP. We identify homovanillyl alcohol as a key contributor to these effects and provide evidence linking QMP-induced changes in the brain to changes at a behavioral level. This study offers exciting insights into the mechanisms through which QMP operates and a deeper understanding of the queen's ability to regulate the behavior of her offspring.
Keywords: Apis mellifera, biogenic amines, neuroethology, neuromodulation, pheromonal communication
Complex social interactions require systems of communication that are reliable and unambiguous. The honey bee, Apis mellifera, employs >50 substances to communicate with and to organize its colony, and the information that is conveyed between colony members is both subtle and sophisticated (1–3).
Maintaining colony organization is a primary role of the queen, whose pheromones enable her to regulate not only the behavior but also the physiology of her nest mates. The most striking effects are those of queen mandibular pheromone (QMP), a chemical blend that induces young workers to feed and groom the queen (Fig. 1) and primes bees to perform colony-related tasks (4, 5). The retinue of workers that attend the queen facilitates the distribution of QMP throughout the colony, where it inhibits the rearing of new queens (6), helps prevent the development of worker ovaries (7), influences comb-building activities, (8) and affects the biosynthesis of juvenile hormone (9) [and hence the age-related behavioral ontogeny of recipient workers (10)]. Despite QMP's central role in the normal functioning and organization of honey bee colonies, very little is known about the cellular mechanisms through which it operates.
Fig. 1.
Honey bee queen surrounded by a retinue of workers attracted to her by QMP. The schematics show that one component of QMP, HVA, bears a striking structural resemblance to dopamine.
We were intrigued by one of the key components of QMP, homovanillyl alcohol (HVA) (4). HVA (4-hydroxy-3-methoxyphenylethanol) bears a striking structural resemblance to dopamine (see Fig. 1 Right), a biogenic amine that plays a central role in insect behavioral regulation and motor control (11–18). The presence of this compound within the pheromone blend suggested to us that dopamine function in the brain of recipient bees might be affected by exposure to QMP. To test this hypothesis, we exposed young workers to QMP and examined its effects on brain dopamine levels, levels of dopamine receptor gene expression, and cellular responses to this amine. The results provide compelling evidence that QMP alters the functioning of modulatory pathways in the brain that play a central role in behavioral regulation and motor control.
Results
Brain Dopamine Levels in Young Worker Bees.
Under normal colony conditions, dopamine levels in the brains of bees of foraging age (generally >3 weeks old) are significantly higher than in young worker bees performing tasks within the colony (19, 20). If a colony is rendered queenless, however, dopamine in the brains of young workers increases to a level not significantly different from that found in foragers (21–23). We began our analysis of QMP's effects on dopamine pathways of the brain by asking whether changes in brain dopamine levels occur as a consequence of altered exposure to QMP.
Worker bees were collected from brood cells as they emerged as adults and transferred into one of two incubators. In one incubator, bees were exposed for 2 days to synthetic QMP (see Materials and Methods), whereas in the second incubator bees were held under identical conditions but without exposure to QMP (controls). All bees were maintained at 34°C under constant darkness and fed ad libitum. HPLC with electrochemical detection (19, 22, 24) was used to measure brain dopamine levels in 2-day-old QMP-treated bees and in control bees of the same age. We found that young workers exposed to QMP exhibited significantly lower levels of dopamine in the brain than control bees (Fig. 2A).
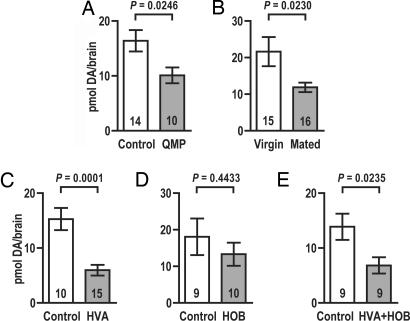
Fig. 2.
Brain dopamine (DA) levels in 2-day-old worker bees. (A) QMP exposure reduces levels of dopamine in the brain. (B) Young hive bees exposed to a mated queen show lower brain dopamine levels than those exposed to a virgin queen. (C) Reduction of brain dopamine levels after exposure to HVA. (D) Brain dopamine levels are not affected by exposure to HOB. (E) Reduction of brain dopamine levels from exposure to HVA and HOB combined. Data are expressed as means ± SEM. Numbers in each bar represent n values. P values show the significance of differences between groups as determined by two-tailed Student's t tests.
Expression of Dopamine Receptor Genes.
Earlier reports have shown that dopamine receptor densities (24), levels of dopamine receptor gene transcript (25, 26), and patterns of dopamine receptor gene expression in the brain (25, 27) change during the lifetime of the adult worker bee, and differential microarray analysis has tentatively identified dopamine receptor genes among several hundred genes proposed to be modulated by QMP (28). We used Northern blot analysis and quantitative RT-PCR (see Materials and Methods) to resolve whether levels of dopamine receptor gene expression are influenced by this pheromone.
Dopamine's actions in the bee are mediated by at least three different receptors, AmDOP1 receptors (29), AmDOP2 receptors (25), and AmDOP3 receptors (30), encoded by the genes Amdop1, Amdop2, and Amdop3, respectively. Before exploring QMP's effects, we looked for age-related changes in the expression of these genes in the absence of QMP. Levels of Amdop1, Amdop2, and Amdop3 mRNA in newly emerged, 1- and 2-day-old bees were compared with transcript levels detected in foragers. Our data show that each of the three dopamine-receptor genes exhibits a unique pattern of age-related changes in gene expression (Fig. 3).
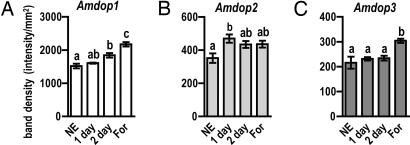
Fig. 3.
Age-related changes in dopamine receptor gene mRNA levels quantified by Northern blot analysis. Comparison of transcript levels in the brains of newly emerged adults (NE), 1-day-old workers (1 day), 2-day-old workers (2 day), and foragers (For; generally >21 days old) reveals age-related changes in the expression of Amdop1 (A), Amdop2 (B), and Amdop3 (C). Data are expressed as means ± SEM with a sample size of four independent RNA samples for each group. Overall significance was determined by one-way ANOVA with Tukey's tests used for post hoc comparisons. Letters above the bars indicate differences between groups. Groups that share letters are not significantly different.
But is the expression of these genes modulated by QMP? To answer this question, we exposed newly emerged adults to QMP for 2 days and compared the dopamine receptor gene transcript levels in these bees with those detected in controls of the same age that had not been exposed to the pheromone. Our results show that QMP alters dopamine receptor gene expression. After a 2-day treatment with QMP, Amdop1 transcript levels were significantly lower in QMP-treated bees than in controls (Fig. 4 A and D). Levels of Amdop2 and Amdop3 expression were highly variable, and differences between controls and QMP-treated bees in mean levels of Amdop2 (Fig. 4 B and E) and Amdop3 mRNA (Fig. 4 C and F) were not statistically significant.
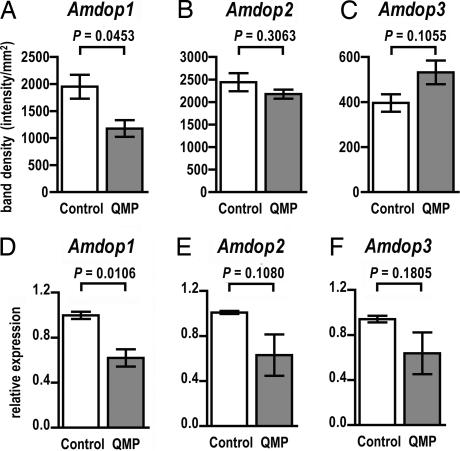
Fig. 4.
QMP modulation of dopamine receptor gene expression. (A–C) Northern blot analysis of gene transcript levels in 2-day-old workers. (A) Amdop1 mRNA levels are significantly lower in 2-day-old QMP-treated bees than in age-matched controls. (B and C) No significant differences in Amdop2 (B) or Amdop3 (C) mRNA levels were identified among QMP-treated bees and controls. (D–F) Gene expression levels determined by using quantitative RT-PCR. (D) Amdop1 transcript levels are selectively reduced by exposure to QMP. (E and F) Differences in Amdop2 (E) and Amdop3 (F) mRNA levels between QMP-treated bees and controls are not significant. Data are expressed as mean levels ± SEM with a sample size of three for each group. Statistical comparisons were performed by using two-tailed Student's t tests.
Responses to Dopamine.
Next, we asked whether QMP-induced changes affect the responsiveness of brain tissues to dopamine. Because dopamine receptor activation alters intracellular cAMP levels (25, 26, 29, 30), measurements of intracellular cAMP were used to monitor tissue responses to this amine (see Materials and Methods). We chose to examine dopamine-evoked responses in mushroom body calyces because these structures can be easily isolated from the brain. Moreover, intrinsic mushroom body neurons (Kenyon cells) express all three of the dopamine receptor genes identified in honey bees, Amdop1, Amdop2, and Amdop3 (25, 26, 29, 30), and mushroom bodies receive extensive input from dopamine-immunoreactive neurons (31).
Dopamine-evoked responses in tissue taken from bees treated for 2 days with QMP were strikingly different from those observed in tissue from control bees that had never been exposed to this pheromone (Fig. 5A). Calyces from control bees responded to 10 μM dopamine with a significant increase in cAMP levels (Fig. 5A, white bar). In calyces from bees exposed to QMP, however, dopamine caused a small but insignificant reduction in levels of cAMP (Fig. 5B, gray bar). Intriguingly, these effects could be mimicked by exogenous application of 10 μM HVA (Fig. 5, compare A and B), suggesting that dopamine receptors in the brain might be activated directly by this dopamine-like component of QMP. Is HVA also responsible for longer-term actions of QMP on the brain? We took advantage of the queen's own biology to investigate whether HVA contributes to QMP-induced reduction in brain dopamine levels.
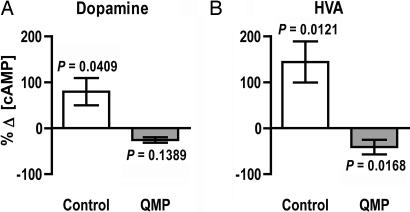
Fig. 5.
Responses of isolated mushroom body calyces to dopamine monitored by using measurements of intracellular cAMP. Calyces from QMP-exposed bees (gray bars) and from control bees (white bars) were exposed to either 10 μM dopamine (A) or 10 μM HVA (B). Note that dopamine-evoked responses are strikingly different in QMP-treated bees versus controls and that the effects of dopamine are mimicked by HVA. Data are expressed as mean levels ± SEM with a sample size of six for each group. P values refer to within-group differences between cAMP levels detected in dopamine-treated or HVA-treated tissues and those detected in tissues that were not exposed to dopamine or HVA.
Effects of HVA.
Virgin queens produce a form of QMP in which HVA levels are very low, often below detection levels (8, 32, 33). If HVA is responsible for effecting QMP-induced changes in brain dopamine levels (see Fig. 2A), levels of this amine in young bees taken from colonies occupied by a virgin queen might be expected to differ from those found in bees exposed to a mated queen. We released newly emerged adult workers into colonies containing either a mated queen or a virgin queen. Two days later, the same workers were recaptured, their brains were removed, and brain dopamine levels were analyzed by using HPLC with electrochemical detection. Our results show that the 2-day-old worker bees exposed to a mated queen exhibit significantly lower brain dopamine levels than bees of the same age taken from colonies occupied by a virgin queen (Fig. 2B). This finding is consistent with the hypothesis that HVA plays a direct role in mediating QMP's effects on dopamine levels in the brain.
To further examine HVA's ability to modulate brain dopamine levels, we exposed newly emerged worker bees to HVA for 2 days (see Materials and Methods) and compared the levels of dopamine in the HVA-treated bees with those found in 2-day-old control bees that had not been exposed to this compound. The results show that HVA alone mimics the effects of QMP on dopamine levels in the brain (Fig. 2C).
Are these effects specific to HVA? To begin to address this question, we exposed bees to a second major QMP component, methyl p-hydroxybenzoate (HOB), alone and together with HVA (see Materials and Methods). HOB alone had no effect on dopamine levels in the brain (Fig. 2D), and it did not alter the effects of HVA when added to this treatment (Fig. 2E). These results indicate that brain dopamine systems, although affected profoundly by HVA, are not affected by all components of the QMP blend.
Links to Behavior.
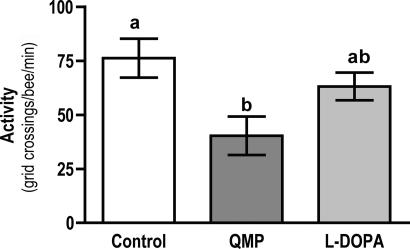
Taken together, these results provide clear evidence that QMP and, specifically, HVA modulate dopamine pathways in the brain. But what impact, if any, do these changes have on the behavior of young worker bees? Because a large body of evidence implicates dopamine in the regulation of locomotor activity in insects (13, 14, 16–18), we examined QMP's effects on activity levels in the bee. Individual bees were placed in a Petri dish with a grid pattern on the floor of the dish. A count was taken of the number of times a bee crossed a grid line over a 4-min period. This simple behavioral assay revealed that activity levels in young worker bees exposed to QMP from the time of adult emergence were significantly lower than in age-matched controls that had never been exposed to this pheromone (Fig. 6).
Fig. 6.
QMP-induced changes in activity. Activity levels in 4-day-old QMP-exposed bees were significantly lower than in age-matched control (untreated) bees. The effects of QMP were partially reversed by treating QMP-exposed bees with l-dopa. Data are expressed as mean activity levels ± SEM with a sample size of 20 for each group. Overall significance was determined by one-way ANOVA followed by Tukey's tests for post hoc comparisons. Letters above the bars indicate differences between groups. Groups that share a letter are not significantly different.
If this difference in activity occurs as a consequence of QMP-induced lowering of brain dopamine levels, pharmacological enhancement of brain dopamine levels might overcome the effects of QMP treatment. To explore this possibility, we fed QMP-treated bees with the dopamine precursor l-3,4-dihydroxyphenylalanine (l-dopa), a treatment shown to increase levels of dopamine in the brain (34, 35). l-dopa (Sigma–Aldrich, St. Louis, MO) was added to food at a final concentration of 3.25 mM. In contrast to bees treated with QMP alone, we found that activity levels in bees treated with l-dopa in addition to QMP were not significantly different from the levels of activity recorded in controls (Fig. 6). l-dopa reversed, at least partially, the effects of QMP.
Discussion
The results of this study reveal that honey bee queen pheromone has profound effects on dopamine pathways in the brain of young worker bees and that HVA, a compound that bestows a unique signature on QMP released by mated queens, plays a central role in mediating these effects.
The multidimensionality of QMP's effects on dopamine pathways is both striking and unexpected. Its effects at a behavioral level, however, are consistent with the central role that dopamine plays in behavioral regulation and motor control. In Drosophila, genetically targeted photostimulation of dopaminergic neurons (18) and pharmacological manipulation of dopamine pathways (16, 36) both have a significant impact on activity levels. Abnormalities in dopamine signaling also underlie aberrant activity levels in fumin. Fumin carries a mutation in the dopamine transporter gene dDAT (37), and reduced clearance of dopamine from the synaptic cleft is the likely cause of the hyperactivity it exhibits. (17). In young worker honey bees, QMP-induced lowering of activity levels can be reversed by treatment with the dopamine precursor l-dopa, presumably through restoration of dopamine levels in the brain.
In Drosophila, dopaminergic neurons that project to the mushroom bodies respond strongly to electric shock (38). Compromising the function of these neurons impairs aversion learning in the fruit fly (15), and pharmacological analyses suggest the same may be true in other insects (39), including bees (40), which raises the interesting possibility that QMP, through its actions on dopamine pathways, might have an impact not only on activity levels, but also on aversion learning in young workers. Preliminary work in our laboratory suggests that this is indeed the case (V.V. and A.R.M., unpublished data). Whether changes at the level of the mushroom bodies contribute to the QMP-induced changes in locomotor activity, however, remains unclear because many regions of the brain are densely innervated by dopaminergic neurons (31) and all are potential targets for modulation by QMP.
What is known about the cellular mechanisms through which dopamine operates in the brain of the bee? Although calcium-activated potassium currents have been identified as key targets of dopamine modulation in neurons within the primary olfactory centers (antennal lobes) of the brain (41), relatively little is known about dopamine's actions on the excitability and network properties of intrinsic mushroom body neurons. Previous work has shown, however, that in bees of foraging age dopamine applied iontophoretically into the mushroom body neuropil reduces and frequently reverses the sign of olfactory-evoked potentials recorded in this region of the brain (42). It is likely that more than one type of dopamine receptor contributes to these effects. In the present study, isolated calyces from bees not exposed to QMP responded to exogenously applied dopamine with a rise in cAMP levels, pointing to the presence of D1-like dopamine receptors in these tissues. AmDOP1 and AmDOP2 receptors both fall into this category, and the reduction in amplitude of dopamine-evoked responses in QMP-treated animals is consistent with the observed reduction in Amdop1 transcript levels in these bees. In some QMP-treated bees, cAMP levels fell slightly in response to dopamine application, suggesting that a D2-like dopamine receptor, such as AmDOP3, is present also in these tissues. In contrast with the actions of AmDOP1 and AmDOP2 receptors (26, 29), activation of AmDOP3 receptors reduces intracellular levels of cAMP (30).
That effects of exogenously applied dopamine were mimicked by HVA suggests that HVA might interact directly with one or more of the honey bee dopamine receptors. Could it contribute also to down-regulating the expression of Amdop1? The promoter region of Dmdop1, the Drosophila ortholog of the honey bee receptor gene Amdop1, contains a silencing region that harbors a cAMP-responsive element site (43). If increased levels of intracellular cAMP inhibit Amdop1 expression via such a mechanism, HVA activation of AmDOP1 receptors could potentially explain the QMP-induced down-regulation of Amdop1 expression observed in this study. In contrast to Amdop1, Amdop2, and Amdop3 expression levels were highly variable, suggesting that expression of this receptor may be affected by factors other than or in addition to QMP. QMP's effects on brain gene expression have been shown by Grozinger and colleagues to be highly dynamic (28). Therefore, it will be important for future studies to analyze QMP-induced changes in dopamine receptor gene expression over time.
It has long been known that QMP stimulates the olfactory system of the bee, and the behavioral consequences of its releaser actions are well documented (reviewed in ref. 3). Less is known, however, about the primer effects of this pheromone and, in particular, the cellular mechanisms through which QMP operates. This study shows that QMP acts directly on central neurons, altering their cellular properties and changing the output of neural circuits in the brain. Such dramatic consequences at the level of the CNS help to explain the magnitude of QMP's effects on the behavior and physiology of young worker bees. Dombroski et al. (44) have shown that feeding queenless worker bees with dopamine enhances ovary development. One interesting possibility, therefore, is that HVA regulation of dopamine systems contributes to QMP-induced inhibition of ovary development in young workers. It is tempting to speculate also that QMP-induced reduction of locomotor activity (and perhaps also aversion learning) might serve to enhance the performance of behaviors associated with duties, such as nursing, by ensuring that young workers remain close to the queen and brood.
Is QMP targeted at workers alone? Harano et al. (45) have recently shown that brain dopamine levels in mated queens are lower than in virgin queens. Interestingly, virgin queens also are more mobile within the colony than laying queens (2). These differences in brain physiology and behavior are consistent with the HVA-induced effects described in the present study, raising the intriguing possibility that QMP (and specifically HVA) may be regulating not only worker bee behavior but also the behavior and physiology of the queen herself.
Materials and Methods
QMP Treatment.
Synthetic QMP was obtained from PheroTech (Delta, BC, Canada) in the form of commercially available strips (BeeBoost). Each strip contains 10 queen equivalents of QMP, a dose that mimics a live queen (8). One queen equivalent contains 200 μg of (E)-9-oxodec-2-enoic acid, 80 μg of (E)-9-hydroxydec-2-enoic acid (15% +9-HDA and 85% −9-HDA), 20 μg of HOB, and 2 μg of HVA (33). Bees were placed in cages (8 cm × 3.5 cm × 1 cm) and exposed to QMP for 2 days. Fresh food and water were provided daily. It was considered unnecessary during this period to replace the pheromone strip because one strip in an average colony is reported to provide effective queen replacement for up to 3 weeks.
HPLC.
Bees were cold-anesthetized at 4°C. The brain was dissected from the head capsule and placed into a 1.5 ml Eppendorf tube, snap-frozen in liquid nitrogen, and stored in a −70°C freezer until analysis. Samples (one brain per sample) were sonicated for 8 s in 80 μl of ice-cold 0.4 M HClO4 containing 2.6 mM sodium metabisulfite and 2.7 mM EDTA. In addition, 20 μl of 3,4-dihydroxybenzylamine at a concentration of 5 ng/ml was included as an internal standard. Each sample was centrifuged at 9,000 × g for 20 min at 4°C. The supernatant (20 μl) was injected directly onto the HPLC column. The HPLC equipment used in this study consisted of an LC-10AD pump (Shimadzu, Tokyo, Japan), a Rheodyne (Rohnert Park, CA) injector, a Phenomenex (Torrance, CA) C18 column (4.6 × 100 mm, with 5-μm packing), and a model 5100A coulometric detector (ESA, San Francisco, CA). The mobile phase consisted of 32 mM sodium acetate, 100 mM sodium dihydrogen orthophosphate, 0.3 mM EDTA, 1.85 mM 1-octanesulfonic acid (sodium salt), and 8% (vol/vol) acetonitrile adjusted to pH 2.5. The working potential was set at +0.3 V, and a flow rate of 1 ml/min was used.
Calibration curves using dopamine HCl standards were determined at the beginning of each assay run. Standards were also included at intervals during the assay run to confirm sample peak retention times. Dopamine levels are expressed in picomoles per brain.
Quantification of Dopamine Receptor Gene Transcript Levels.
Northern blot analysis of total RNA (10 brains per sample) was performed as previously described (26). Band intensity was measured by using a Molecular Imager FX instrument (Bio-Rad, Hercules, CA) and Quantity One software (version 4.5.0). Gene transcript levels were also measured by using qRT-PCR (46) on an independently generated set of samples. Total RNA (10 bees per sample) was reverse transcribed with random hexamers and SuperScriptIII enzyme (Invitrogen, Carlsbad, CA), and receptor transcript levels were determined relative to the abundance of the EF-1α transcript (National Center for Biotechnology Information accession no. X52884) using 2−ΔΔCt methodology [Applied Biosystems (Foster City, CA) user bulletin #2, 1997] described by Bloch et al. (47). Gene-specific amplification products were generated with the following primers: Amdop1, 5′-TGAACGATCTCCTCGGCTAT (forward) and 5′-ACCCAACGACCGTATCTGAG (reverse); Amdop2, 5′-GGATCAACAGCGGAATGAAT (forward) and 5′-GCGAATCTTTGACTCGGTTT (reverse); Amdop3, 5′-CGTTGCAAACTGTCACCAAT (forward) and 5′-GACGTCCATTGCGATGTAAA (reverse); EF-1α, 5′-TGCAACCTACTAAG-CCGATG (forward) and 5′-GACCTTGCCCTGGGTATCTT (reverse) with an Applied Biosystems Prism 7000 Sequence Detector instrument, running version 1.6 SDS software and using QuantiTect SYBR green PCR reagent (Qiagen, Valencia, CA) according to standard protocols.
Responsiveness of Brain Tissue to Dopamine.
Mushroom body calyces were harvested from bees treated for 2 days with QMP as well as from controls of the same age that had not been exposed to this pheromone. In each case, measurements were taken of intracellular cAMP levels in tissues not exposed to dopamine as well as in tissues incubated for 20 min in this amine. Each sample of brain tissue assayed contained the calyces of two bees (two pairs of calyces per brain, eight calyces in total). Two measurements were taken from each sample, and six samples (replicates) were tested for each treatment.
Freshly dissected calyces were placed into Sf900II medium (Invitrogen) and kept on ice. Next, 3-isobutyl-1-methylxanthine (100 μM) was added either on its own or in conjunction with dopamine (10 μM) to give a final volume of 400 μl. Each sample of brain tissue was incubated with or without dopamine for 20 min at 30°C. The incubation medium was then carefully removed, and the tissue cAMP concentration was determined by using a Biotrak cAMP immunoassay (Amersham Biosciences, Piscataway, NJ). In Fig. 5, levels of cAMP in dopamine-treated tissues are expressed as a percentage of those detected in tissues receiving no dopamine treatment.
Responsiveness of Brain Tissue to HVA.
The procedures described above also were used to examine the responsiveness of brain tissue to exogenously applied HVA. In this case, tissues in the treatment group were exposed for 20 min to 10 μM HVA rather than to dopamine. HVA was obtained from K.N.S. and from Sigma–Aldrich.
Long-Term Treatment with HVA and HOB.
To examine the effects of HVA on brain dopamine levels, newly emerged bees were placed in incubators and allowed to feed ad libitum on either standard pollen patties containing 8% (wt/wt) lactalbumin, 16% brewer yeast, 42% sucrose, 17% pollen, and 17% water or pollen patties containing in addition either 0.012% (wt/wt) HVA, 0.11% (wt/wt) HOB, or both. HOB was used at a higher concentration because it is ≈10-fold more abundant than HVA in the QMP blend.
Statistical Analysis.
Two-tailed Student's t tests were used to analyze the significance of differences in brain dopamine levels, levels of dopamine receptor gene expression, and responsiveness to dopamine between control groups and groups treated with either QMP, HVA, and/or HOB. The significance of age-related differences in dopamine receptor gene expression and differences in activity levels among controls, QMP-treated bees, and bees treated with QMP plus l-dopa were tested by using one-way ANOVA followed by Tukey's tests for post hoc comparisons. Numbers of animals tested and P values obtained from the analyses are shown in the figures or provided in the figure legends.
Acknowledgments
We thank Kim Garrett for maintaining the honey bee colonies and Ken Miller for assisting with the formatting of Fig. 1. This research was supported by Royal Society of New Zealand Marsden Fund Grant UOO312.
Abbreviations
- QMP
queen mandibular pheromone
- HVA
homovanillyl alcohol
- HOB
methyl p-hydroxybenzoate
- l-dopa
l-3,4-dihydroxyphenylalanine.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
References
- 1.Winston ML, Slessor KN. Apidologie. 1998;29:81–95. [Google Scholar]
- 2.Winston M. The Biology of the Honey Bee. Cambridge, MA: Harvard Univ Press; 1987. [Google Scholar]
- 3.Slessor KN, Winston ML, Le Conte Y. J Chem Ecol. 2005;31:2731–2745. doi: 10.1007/s10886-005-7623-9. [DOI] [PubMed] [Google Scholar]
- 4.Slessor KN, Kaminski L-A, King GG, Borden JH, Winston ML. Nature. 1988;332:354–356. [Google Scholar]
- 5.Keeling CI, Slessor KN, Higo HA, Winston ML. Proc Natl Acad Sci USA. 2003;100:4486–4491. doi: 10.1073/pnas.0836984100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Winston ML, High HA, Colley SJ, Pankiw T, Slessor KN. Ann Entomol Soc Am. 1991;84:234–238. [Google Scholar]
- 7.Hoover SE, Keeling CI, Winston ML, Slessor KN. Naturwissenschaften. 2003;90:477–480. doi: 10.1007/s00114-003-0462-z. [DOI] [PubMed] [Google Scholar]
- 8.Ledoux MN, Winston ML, Keeling CI, Slessor KN, Le Conte Y. Insect Soc. 2001;48:14–20. [Google Scholar]
- 9.Kaatz H, Hildebrandt H, Engels W. J Comp Physiol B. 1992;162:588–592. [Google Scholar]
- 10.Pankiw T, Huang Z, Winston ML, Robinson GE. J Insect Physiol. 1998;44:685–692. doi: 10.1016/s0022-1910(98)00040-7. [DOI] [PubMed] [Google Scholar]
- 11.Mercer AR. In: Arthropod Brain: Its Evolution, Development, Structure and Functions. Gupta AP, editor. New York: Wiley; 1987. pp. 399–414. [Google Scholar]
- 12.Yellman C, Tao H, He B, Hirsh J. Proc Natl Acad Sci USA. 1997;94:4131–4136. doi: 10.1073/pnas.94.8.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cooper RL, Neckameyer WS. Comp Biochem Physiol B. 1999;122:199–210. doi: 10.1016/s0305-0491(98)10160-8. [DOI] [PubMed] [Google Scholar]
- 14.Pendleton RG, Rasheed A, Sardina T, Tully T, Hillman R. Behav Genet. 2002;32:89–94. doi: 10.1023/a:1015279221600. [DOI] [PubMed] [Google Scholar]
- 15.Schwaerzel M, Monastirioti M, Scholz H, Friggi-Grelin F, Birman S, Heisenberg M. J Neurosci. 2003;23:10495–10502. doi: 10.1523/JNEUROSCI.23-33-10495.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andretic R, van Swinderen B, Greenspan RJ. Curr Biol. 2005;15:1165–1175. doi: 10.1016/j.cub.2005.05.025. [DOI] [PubMed] [Google Scholar]
- 17.Kume K, Kume S, Park SK, Hirsh J, Jackson FR. J Neurosci. 2005;25:7377–7384. doi: 10.1523/JNEUROSCI.2048-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lima SQ, Miesenbock G. Cell. 2005;121:141–152. doi: 10.1016/j.cell.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 19.Taylor DJ, Robinson GE, Logan BJ, Laverty R, Mercer AR. J Comp Physiol B. 1992;170:715–721. doi: 10.1007/BF00198982. [DOI] [PubMed] [Google Scholar]
- 20.Schulz DJ, Robinson GE. J Comp Physiol A. 1999;184:481–488. doi: 10.1007/s003590000122. [DOI] [PubMed] [Google Scholar]
- 21.Harris JW, Woodring J. Comp Biochem Physiol C. 1995;111:271–279. [Google Scholar]
- 22.Purnell MT, Mitchell CJ, Taylor DJ, Kokay IC, Mercer AR. Brain Res. 2000;855:206–216. doi: 10.1016/s0006-8993(99)02337-9. [DOI] [PubMed] [Google Scholar]
- 23.Sasaki K, Nagao T. J Insect Physiol. 2001;47:1205–1216. doi: 10.1016/s0022-1910(01)00105-6. [DOI] [PubMed] [Google Scholar]
- 24.Kokay IC, Mercer AR. J Comp Physiol A. 1997;181:415–423. [Google Scholar]
- 25.Humphries MA, Mustard JA, Hunter SJ, Mercer A, Ward V, Ebert PR. J Neurobiol. 2003;55:315–330. doi: 10.1002/neu.10209. [DOI] [PubMed] [Google Scholar]
- 26.Mustard JA, Blenau W, Hamilton IS, Ward VK, Ebert PR, Mercer AR. Mol Brain Res. 2003;113:67–77. doi: 10.1016/s0169-328x(03)00091-3. [DOI] [PubMed] [Google Scholar]
- 27.Kurshan PT, Hamilton IS, Mustard JA, Mercer AR. J Comp Neurol. 2003;466:91–103. doi: 10.1002/cne.10864. [DOI] [PubMed] [Google Scholar]
- 28.Grozinger CM, Sharabash NM, Whitfield CW, Robinson GE. Proc Natl Aca Sci USA. 2003;100:14519–14525. doi: 10.1073/pnas.2335884100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blenau W, Erber J, Baumann A. J Neurochem. 1998;70:15–23. doi: 10.1046/j.1471-4159.1998.70010015.x. [DOI] [PubMed] [Google Scholar]
- 30.Beggs KT, Hamilton IS, Kurshan PT, Mustard JA, Mercer AR. Insect Biochem Mol Biol. 2005;35:873–882. doi: 10.1016/j.ibmb.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 31.Schäfer S, Rehder V. J Comp Neurol. 1989;280:43–58. doi: 10.1002/cne.902800105. [DOI] [PubMed] [Google Scholar]
- 32.Slessor KN, Kaminski L-A, King GG, Winston ML. J Chem Ecol. 1990;16:851–860. doi: 10.1007/BF01016495. [DOI] [PubMed] [Google Scholar]
- 33.Pankiw T, Winston ML, Plettner E, Slessor KN, Pettis JS, Taylor OR. J Chem Ecol. 1996;22:605–615. doi: 10.1007/BF02033573. [DOI] [PubMed] [Google Scholar]
- 34.Neckameyer WS. Dev Biol. 1996;176:209–219. doi: 10.1006/dbio.1996.0128. [DOI] [PubMed] [Google Scholar]
- 35.Harris JW, Woodring J. Physiol Entomol. 1999;24:285–291. [Google Scholar]
- 36.McClung C, Hirsh J. Curr Biol. 1998;8:109–112. doi: 10.1016/s0960-9822(98)70041-7. [DOI] [PubMed] [Google Scholar]
- 37.Porzgen P, Park SK, Hirsh J, Sonders MS, Amara SG. Mol Pharmacol. 2001;59:83–95. doi: 10.1124/mol.59.1.83. [DOI] [PubMed] [Google Scholar]
- 38.Riemensperger T, Voller T, Stock P, Buchner E, Fiala A. Curr Biol. 2005;15:1953–1960. doi: 10.1016/j.cub.2005.09.042. [DOI] [PubMed] [Google Scholar]
- 39.Unoki S, Matsumoto Y, Mizunami M. Eur J Neurosci. 2005;22:1409–1416. doi: 10.1111/j.1460-9568.2005.04318.x. [DOI] [PubMed] [Google Scholar]
- 40.Vergoz V. Toulouse, France: Paul Sabatier Univ; 2004. Master's thesis. [Google Scholar]
- 41.Perk CG, Mercer AR. J Neurophysiol. 2006;95:1147–1157. doi: 10.1152/jn.01220.2004. [DOI] [PubMed] [Google Scholar]
- 42.Mercer AR, Erber J. J Comp Physiol. 1983;151:469–476. [Google Scholar]
- 43.Kehren V, Baumann A. Arch Insect Biochem Physiol. 2005;59:118–131. doi: 10.1002/arch.20062. [DOI] [PubMed] [Google Scholar]
- 44.Dombroski TCD, Simones Z, Bitondi M. Apidologie. 2003;34:281–289. [Google Scholar]
- 45.Harano K, Sasaki K, Nagao T. Naturwissenschaften. 2005;92:310–313. doi: 10.1007/s00114-005-0631-3. [DOI] [PubMed] [Google Scholar]
- 46.Bustin SA. J Mol Endocrinol. 2000;25:169–193. doi: 10.1677/jme.0.0250169. [DOI] [PubMed] [Google Scholar]
- 47.Bloch G, Toma DP, Robinson GE. J Biol Rhythms. 2001;16:444–456. doi: 10.1177/074873001129002123. [DOI] [PubMed] [Google Scholar]