Abstract
Estrogen receptor α (ERα) plays a pivotal role in the regulation of food intake and energy expenditure by estrogens. Although it is well documented that a disruption of ERα signaling in ERα knockout (ERKO) mice leads to an obese phenotype, the sites of estrogen action and mechanisms underlying this phenomenon are still largely unknown. In the present study, we exploited RNA interference mediated by adeno-associated viral vectors to achieve focused silencing of ERα in the ventromedial nucleus of the hypothalamus, a key center of energy homeostasis. After suppression of ERα expression in this nucleus, female mice and rats developed a phenotype characteristic for metabolic syndrome and marked by obesity, hyperphagia, impaired tolerance to glucose, and reduced energy expenditure. This phenotype persisted despite normal ERα levels elsewhere in the brain. Although an increase in food intake preceded weight gain, our data suggest that a leading factor of obesity in this model is likely a decline in energy expenditure with all three major constituents being affected, including voluntary activity, basal metabolic rate, and diet-induced thermogenesis. Together, these findings indicate that ERα in the ventromedial nucleus of the hypothalamus neurons plays an essential role in the control of energy balance and the maintenance of normal body weight.
Keywords: adeno-associated virus, body weight, energy metabolism, obesity, RNA interference
Estrogen receptor α (ERα) is the main mediator of estrogen effects on energy homeostasis. ERα knockout (ERKO) mice with targeted deletion of this receptor develop several hallmark features often associated with obesity including increased visceral adiposity, elevated insulin levels, and impaired glucose tolerance (1). Although the nature of events leading to this phenotype is unclear, hyperphagia does not seem to be the cause because food consumption was not altered in ERKO mice. Instead, several observations suggest that the weight gain in this model is due to a decrease in energy expenditure, which given the normal food intake would result in a state of positive energy balance (1, 2). In this respect, this condition partially resembles an obesity syndrome following lesions of the ventromedial nucleus of hypothalamus (VMN), which is also marked by a significant weight gain due to an accumulation of visceral fat, impaired glucose homeostasis, and reduced energy expenditure (3, 4). Although VMN-lesioned animals initially become hyperphagic as well, when tube-pair-fed with control animals to ensure equal food consumption, lesioned rats still gained more weight and accumulated more fat, albeit to a lesser extent than rats fed ad libitum (3, 5). There are additional lines of evidence that are consistent with a functional role of VMN ERα in the regulation of body weight. VMN has a high density of estrogen-binding sites (6), and the neurons in this nucleus express ERα at high levels (7). Although VMN lesioning or ovariectomy both lead to increased food intake and body weight, the effect does not appear to additive. Furthermore, after ovariectomy, VMN-lesioned animals are less responsive to the catabolic effects of estrogen treatment (8).
Although the above observations suggest that ERα expressed in VMN neurons is an important player in the central control of body weight by estrogens, direct evidence is lacking. Experiments with estradiol microinjections used to map the function of distinct hypothalamic nuclei have produced conflicting results, likely because of differences in needle placement and diffusion of the hormone beyond targeted areas (9, 10). Deletion of ERα in ERKO mice is global and is further confounded by potential adverse effects on the development of neural networks caused by the lack of ERα signaling during embryogenesis. We therefore elected to exploit viral vector-mediated RNA interference to reduce ERα expression selectively in the VMN of adult females. We recently reported that in this model VMN-specific ERα silencing produced a phenotype reminiscent of ERKO mice marked by suppression of female sexual behaviors and concomitant augmentation of rejection toward males (11). In addition, compared with control animals these mice displayed a progressive increase in body weight. To further investigate this phenomenon, we designed a follow-up study using ovariectomized (OVXed) and gonad-intact female mice and rats. After ERα knockdown in the VMN, the animals developed several features characteristic for metabolic syndrome such as weight gain, increased visceral adiposity, hyperphagia, hyperglycemia, and impaired energy expenditure. Together, our findings demonstrate an important role for VMN ERα in the maintenance of normal female body weight.
Results
Obese Phenotype in Mice After ERα Silencing in the VMN.
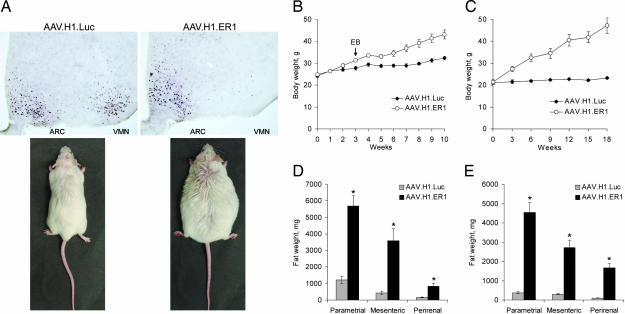
In the first set of experiments, Swiss–Webster mice were OVXed and 3 days later injected bilaterally into the VMN with adeno-associated viral (AAV) vectors designed to silence ERα (AAV.H1.ER1) or luciferase (AAV.H1.Luc). The latter was used to control for any potential nonspecific adverse effects of surgery or toxicity of encoded products [small hairpin RNA (shRNA) and EGFP]. Consistent with our previous study (11), AAV.H1.ER1 efficiently reduced ERα expression in the VMN but not in the surrounding ERα-positive regions, such as the arcuate nucleus (ARC; Fig. 1A Upper) or the medial preoptic area (data not shown).
Fig. 1.
Increased body weight and adiposity in mice after ERα knockdown in the VMN. (A) Representative images of ERα immunostaining in the hypothalamic regions (Upper) of OVXed mice (Lower) 14 weeks after injections with either AAV.H1.Luc (Left) or AAV.H1.ER1 (Right). Note reduced ERα expression in the VMN but not in the ARC in AAV.H1.ER1-treated mice. (B) Body weight of OVXed mice injected with the indicated vectors and housed in running wheel activity cages. At week 3 all of the animals were treated with 21-day time-release EB pellets (indicated by an arrow). The difference between the groups became significant (P < 0.01) as early as at week 3. (C) A similar increase in body weight was also observed in gonad-intact females (P < 0.001). (D and E) Accumulation of adipose tissue in several fat depot areas in OVXed (D) and gonad-intact (E) animals treated with AAV.H1.ER1 (∗, P < 0.001). Data are means ± SEM.
A striking consequence of ERα silencing in the VMN was a significant weight gain displayed by AAV.H1.ER1-treated females compared with AAV.H1.Luc-injected mice (Fig. 1A Lower). Consistent with the effect of ovariectomy on body weight, animals in both groups started to gain weight within days after surgery (Fig. 1B). Nonetheless, in the mice treated with AAV.H1.ER1, this change was more profound, which was evident as early as 3 weeks after vector injection. To address the sensitivity to estrogen, at week 3 all of the animals received s.c. pellets designed to continuously release 17β-estradiol-3-benzoate (EB; 5 mg) over a period of 3 weeks. As shown in Fig. 1B, whereas body weight increase in control mice halted for a few weeks and then resumed, this effect was blunted in AAV.H1.ER1-treated females, indicating that they were less responsive to estrogen. Consistent with these results, the gonad-intact females treated with AAV.H1.ER1 also showed a progressive weight gain starting 3 weeks after surgery (Fig. 1C), although the difference between the two groups was more pronounced due to constant weight of gonad-intact control females. Obesity observed in both experiments was associated with an increase in visceral adiposity (Fig. 1 D and E) but not an accumulation of s.c. fat (data not shown). Together these results indicate that ERα expression in the VMN neurons of adult female mice is essential for maintaining normal body weight.
Hyperphagia and Hyperglycemia.
No difference in food intake was observed between the two groups of OVXed mice within the first week after surgery (Fig. 2A). During the second week, all of the animals started to increase their food intake, which likely reflects a hyperphagic effect of ovariectomy. Nonetheless, the animals treated with AAV.H1.ER1 consumed ≈15% more food compared with control mice, and this difference remained relatively constant throughout the study. In addition, females from either group appeared to be responsive to estrogen because they decreased food consumption to a similar degree after implantation of EB pellets; however, food intake in AAV.H1.ER1-treated mice never reduced to the level of control animals (Fig. 2A). Consistent with this finding, hyperphagia after ERα silencing was also observed in gonad-intact females (Fig. 2B).
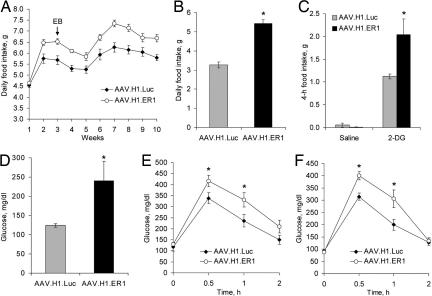
Fig. 2.
Hyperphagia and impaired glucose tolerance. (A) Food intake of OVXed animals described in Fig. 1B. Notice that whereas estrogen administration (marked by an arrow) reduced food intake in AAV.H1.ER1-treated animals (P < 0.01), they remained hyperphagic compared with control mice throughout the experiment (P < 0.01). (B) An increase in daily food intake was also observed in gonad-intact females treated with AAV.H1.ER1 (∗, P < 0.05). (C) 2-DG-induced hyperphagia. Nonfasted OVXed animals were injected with saline or 2-DG (250 mg/kg, i.p.), and food intake was measured for 4 h during the light phase. AAV.H1.ER1-treated mice demonstrated a stronger hyperphagic response (∗, P < 0.01) compared with AAV.H1.ER1-treated animals. (D) Hyperglycemia in OVXed mice injected with AAV.H1.ER1 under normal feeding condition (∗, P < 0.05). (E) Glucose tolerance test in OVXed mice. Fasting glucose concentrations were similar between the groups. However, after a glucose challenge blood glucose reached higher levels in AAV.H1.ERa-treated mice (∗, P < 0.05). (F) Glucose tolerance test in gonad-intact females produced similar results (∗, P < 0.05). Data represent means ± SEM.
VMN neurons are essential for the integrated hormonal and behavioral responses to hypoglycemia (12, 13). To address the role of ERα in this process, we assessed food intake immediately after a systemic injection with 2-deoxy-d-glucose (2-DG), a nonmetabolizable glucose derivative that induces hyperphagia by inhibiting glucose utilization (14). In OVXed AAV.H1.Luc-treated animals, 2-DG significantly increased food intake over 4 h compared with saline (Fig. 2C). Still, in AAV.H1.ER1-treated mice, 2-DG induced a stronger response, and the animals consumed approximately twice as much food over the same period compared with AAV.H1.Luc-treated animals. Although we cannot conclude from this experiment whether glucosensing function per se was affected in the neurons of the VMN, our finding point to ERα as an important player in hypoglycemic counterregulation mediated by VMN.
To provide further insight into alterations in glucose homeostasis, we measured blood glucose levels under normal feeding conditions. As shown in Fig. 2D, glucose concentration was elevated in OVXed AAV.H1.ER1-injected mice compared with control animals. Furthermore, after glucose challenge, AAV.H1.ER1-treated mice developed a more significant and sustained increase in blood glucose, whereas fasting baseline glucose levels were comparable for the two groups (Fig. 2E). Similar results were also observed in gonad-intact females (Fig. 2F).
Reduced Energy Expenditure.
The primary cause of obesity in ERKO mice is believed to be a decline in energy expenditure (1). To determine whether such mechanism is implicated in our model as well, we first examined voluntary physical activity of OVXed females in running wheel cages. As can be seen in Fig. 3A, in control mice time-release EB implants effective for 3weeks produced a 2.5-fold increase in motor activity, which then returned to baseline levels after the termination of EB treatment. In contrast, in AAV.H1.ER1-treated animals estrogen did not have a significant effect, and their running wheel activity persisted at relatively steady low levels. Consistent with this finding, gonad-intact females injected with AAV.H1.ER1 demonstrated reduced horizontal and vertical activity in an open field test (Fig. 3 B and C).
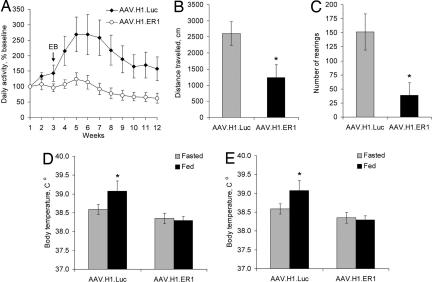
Fig. 3.
Decreased physical activity and diet-induced thermogenesis. (A) Voluntary locomotor activity. OVXed mice from the experiments described in Figs. 1B and 2A were continuously monitored in running-wheel activity cages for 12 weeks. AAV.H1.ER1-injected mice had reduced levels of activity and were resistant to EB treatment. (B and C) Open-field test. Gonad-intact female mice injected with AAV.H1.ER1 demonstrated lower horizontal (B) and vertical (C) activity levels compared with control animals (∗, P < 0.05) during the last 20 min of a 30-min test. (D) Diet-induced thermogenesis. Consumption of food for 1 h after a 24-h fast raised body temperature in control mice (∗, P < 0.05) but not in AAV.H1.ER1-injected mice. (E) An impaired thermogenic response to feeding was also seen in gonad-intact females. Data are means ± SEM.
We also assessed changes in diet-induced thermogenesis as another component of daily energy expenditure. Feeding after a 24-h fast increased core body temperature after 1 h in both OVXed (Fig. 3D) and gonad-intact (Fig. 3E) AAV.H1.Luc-treated mice but not in the animals injected with AAV.H1.ER1.
Metabolic Syndrome in Rats.
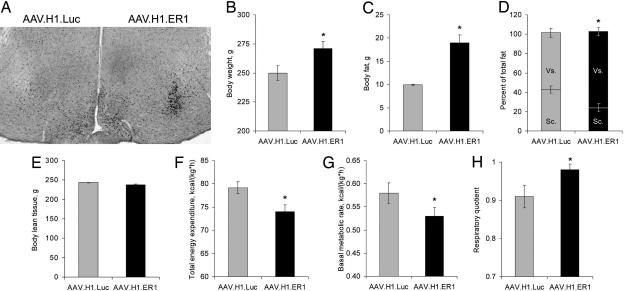
To ensure that our findings reflect a generic phenomenon and are not limited to mouse species, we performed a set of experiments in rats. For this study we used the same AAV.H1.ER1 vector because it harbors an shRNA target sequence (ggcatggagcatctctaca) that matches both murine and rat ERα mRNAs. As shown in Fig. 4A, infusion of AAV.H1.ER1 significantly reduced ERα immunoreactivity in the VMN, whereas the control vector AAV.H1.Luc had no effect. The subsequent experiments were performed in gonad-intact female rats injected bilaterally with either vector. In line with our previous findings, ERα silencing in rats produced a phenotype marked by increased body weight (Fig. 4B) and total adiposity (Fig. 4C) due to fat accumulation in visceral but not s.c. fat depots (Fig. 4D). The mass of the lean tissue was not affected (Fig. 4E). Although we did not measure food intake in this study, both total energy expenditure (Fig. 4F) and basal metabolic rate (Fig. 4G) were reduced in AAV.H1.ER1-treated rats. In addition, they had an elevated respiratory quotient (the ratio of carbon dioxide production to oxygen consumption) suggesting a decrease in the efficiency of lipid utilization (Fig. 4H).
Fig. 4.
Obese phenotype in rats. (A) A representative photomicrograph of ERα immunoreactivity in the rat hypothalamus 3 weeks after unilateral injections with the indicated vectors. (B–H) Effects of bilateral vector infusions into the VMN of gonad-intact female rats on body weight (B), total adiposity (C), visceral to s.c. fat ratio (D), lean tissue mass (E), total energy expenditure (F), basal metabolic rate (G), and respiratory quotient (H). Data are means ± SEM (∗, P < 0.05).
Discussion
Despite numerous studies on the role of the VMN in body weight regulation, the underlying mechanisms are still a subject of controversy (3). Early experiments suggested a primary role of hyperphagia in the development of obesity after VMN destruction. Nonetheless, subsequent studies with pair-fed animals established that weight gain was a consequence of a metabolic disorder associated with reduced energy expenditure, whereas an increase in food intake was secondary and was simply necessary to maintain a higher body weight. There is also evidence that VMN lesions produce both a primary metabolic impairment as well as an independent alteration in feeding behavior (3). VMN neurons thus appear to be able to generate integrated responses to changes in energy balance by affecting energy intake and expenditure.
Given the similarities between phenotypes produced by ERα knockdown in the VMN and VMN-specific lesions, we performed a thorough analysis of the transduced regions to exclude artifacts due to tissue damage. Consistent with our previous findings (11), in the present study we did not observe any neuronal death after injection with either vector, and the transduced neurons retained normal gross morphology and expressed EGFP reporter as well as a number of endogenous markers including ERβ (data not shown). Although we cannot exclude a neuronal dysfunction caused by an off-target effect of shRNA, this scenario is unlikely given that the selected ERα target sequence does not match any other murine mRNA in GenBank database.
Here we present several lines of evidence that support the significance of ERα in the modulation of food intake and energy expenditure by the VMN neurons. After ERα silencing in the VMN, the mice displayed a higher increase in food consumption as early as 2 weeks after surgery (Fig. 2A). Notably, these animals were still responsive to the hypophagic effect of estradiol, albeit to a lesser degree than control mice. A likely explanation for this finding is estrogen action in other brain regions involved in the regulation of food intake, such as the ARC, which appear to have normal ERα levels in our model (Fig. 1A). Despite the increase in food consumption (Fig. 2A), body weight did not yet differ significantly between the two groups at early time points (Fig. 1B), indicating that hyperphagia preceded obesity in this experiment. Furthermore, between weeks 6 and 10, food intake in AAV.H1.ER1-treated mice remained relatively constant (Fig. 2A), whereas body weight continued to rise during this period (Fig. 1B). This divergence between food intake and weight gain over time is indicative of a greater caloric efficiency and suggests a decline in energy expenditure. Although these experiments do not reveal a relative contribution of either factor, a leading cause of obesity in these mice appears to be impaired energy expenditure which was affected at several levels. First, OVXed AAV.H1.ER1-treated mice displayed reduced voluntary physical activity compared with controls (Fig. 3A). Although at late time points this effect could be secondary to obesity, during the first few weeks hypoactivity in AAV.H1.ER1-treated mice (Fig. 3A) was evident before a profound weight gain (Fig. 1B). In addition, these animals demonstrated impaired thermogenic response to feeding. In rats, ERα silencing produced a decrease in basal metabolic rate (Fig. 4G) and a concomitant increase in respiratory quotient (Fig. 4H). The latter reflects a reduction in the efficiency of fatty acids oxidation, a finding consistent with excess body fat in these animals (Fig. 4C). Critical to our data interpretation is an observation that increased adiposity in both rats and mice was due to fat accumulation in visceral but not s.c. depots (Fig. 4D and data not shown). Although obesity per se can be caused by excess fat in either area, it is visceral adiposity rather than the amount of s.c. fat that most strongly correlates with several hallmark features of the metabolic syndrome in humans, including glucose intolerance, hypertension, and resistance to insulin (15).
It is important to recognize that the animals in our model developed a more manifest phenotype compared with ERKO mice. We can resolve this apparent discrepancy by highlighting the fact that ERKO animals express a truncated form of ERα as a result of rearrangements in the ERα locus during genetic manipulations. The presence of this chimeric receptor is believed to mediate several estrogen effects in the brain of these animals, including up-regulation of the progesterone receptor immunoreactivity in the VMN (16). In contrast, RNA interference allowed a more significant suppression of ERα function because no progesterone receptor immunoreactivity could be detected in the VMN of AAV.H1-ER1-treated mice after EB administration (11). In addition, we cannot exclude a possible role of ERα in regions other than the VMN in our model or a compensatory response to impaired ERα signaling during development in ERKO females.
We were surprised to discover that physiological changes caused by ERα silencing occurred in OVXed mice even in the absence of estrogen treatment (Figs. 1–3). Because the animals were fed with phytoestrogen-free diet, we can rule out the influence of estrogen-like compounds in food. Although trace amounts of estrogenic substances from other sources in the environment, such as polycarbonate cages or corn cob bedding, could contribute to the observed effects, we favor two other possible scenarios. First, ERα could elicit some of the downstream effects in a ligand-independent manner. Although data supporting the importance of this mechanism in vivo are scarce, several observations in cultured cells established the significance of this pathway in dynamic regulation of transcription by ERα (17–19). In the absence of estrogen, ERα can modulate expression of selected genes by recruiting both transcriptional repressors and activators as well as by remodeling chromatin structure (17, 20). Notably, some of these events appear to depend on interaction of unliganded ERα with DNA sequences that are distinct from classical consensus sites recognized by estrogen-bound ERα (17).
An alternative explanation is that the effects observed in OVXed females are mediated by 17α-estradiol. Although this optical isomer of the hormone has long been considered far less active than 17β-estradiol, a recent study established that the two isomers have comparable affinities to ERα and are able to activate estrogen-responsive reporter expression to a similar extent (21). Furthermore, in contrast to 17β-estradiol, 17α-estradiol is present at significantly higher concentrations in the adult female brain, including the hypothalamus, and these levels increase even further after ovariectomy (21). According to this line of reasoning, it is conceivable that in OVXed animals 17α-estradiol continues to activate ERα signaling providing a partial compensation, whereas silencing of the receptor itself leads to a full manifestation of deficient phenotype.
In summary, our findings established that suppression of ERα levels in the VMN of adult female animals triggered the development of metabolic syndrome marked by a profound increase in body weight, excess visceral fat, hyperphagia, glucose intolerance, reduced physical activity, impaired thermogenic response to feeding, and low basal metabolic rate. These observations extent our current knowledge and further support the significance of VMN ERα in neural networks that regulate energy homeostasis by modulating a balance between energy intake and energy expenditure.
Materials and Methods
Mice.
A mouse model of VMN-specific ERα knockdown has been described (11). Briefly, C57BL/6J or Swiss–Webster female mice (12–30 weeks old) were injected bilaterally into the VMN with recombinant AAV vectors encoding for luciferase-specific (AAV.H1.Luc) or ERα-specific (AAV.H1.ER1) shRNA. Both vectors also express EGFP as a reporter to visualize transduced neurons. Swiss–Webster mice were OVXed 3 days before surgery, whereas C57BL/6J mice remained gonad-intact throughout the study. Total of 2 × 109 genomic particles (2 μl in PBS) were stereotactically injected bilaterally into the VMN (anteroposterior −0.9, mediolateral ± 0.7, dorsoventral −6.0) over 10 min by using a microinfusion pump (World Precision Instruments, Sarasota, FL). The next day after vector injection Swiss–Webster animals were placed individually into plastic cages equipped with a running wheel (25-cm diameter; Mini Mitter, Bend, OR). Wheel revolutions were continuously recorded by a magnetic switch to monitor animal voluntary locomotor activity for the duration of the study. C57BL/6J mice were single-housed in regular plastic cages. All animals were maintained on a reverse 12-h light/12-h dark cycle and were fed with phytoestrogen-free diet (AIN76A; Ralston Purina, St. Louis, MO) to eliminate exposure to estrogen-like compounds normally present in a chow.
Rats.
Adult female (220–225 g) Long–Evans rats (Harlan, Indianapolis, IN) were individually housed in Plexiglas cages and maintained on a 12-h light/12-h dark cycle in a temperature-controlled facility. Water and food were provided ad libitum unless otherwise noted. Stereotactic injections of viral vectors into the VMN were performed by using the following coordinates: anteroposterior ±1.0, mediolateral ± 3.1, dorsoventral −8.2. Vector dose and infusion parameters were the same as those described for mice.
Open Field Test.
Mice were tested for 30 min in an open field apparatus (27.9 × 27.9 cm; Med Associates, St. Albans, VT) under dim red light during the dark phase of the cycle. Distance traveled (horizontal activity) and number of rearings (vertical activity) were recorded for each mouse. Because the level of activity during the first few minutes in an open field primarily reflects exploratory behavior and anxiety rather than overall motor activity, only the data collected during the last 20 min were used.
Body Weight, Food Intake, and 2-DG Test.
Body weight and food intake were measured once a week at the end of the light phase immediately before lights out. To study 2-DG-induced hyperphagia, nonfasted animals were injected with 2-DG (250 mg/kg in saline, i.p.) during the light phase, and consumption of preweighed food was measured over 4 h. Water was available ad libitum.
Glucose Tolerance Test.
The test was performed during the dark phase of the cycle after a 24-h fast in clean cages. Blood samples were collected from the tail vein before and 30, 60, and 120 min after glucose challenge (2 g/kg in saline, i.p.). Blood glucose concentration was measured immediately by using Glucometer Elite (Bayer, Leverkusen, Germany).
Body Fat Determination.
In mice, body fat was measured at the end of each experiment. Parametrial, mesenteric, and perirenal fat pads were dissected and weighed. In rats, body fat was estimated by using two methods. During an ongoing experiment, total lean tissue, fat tissue, and water were assessed by NMR (EchoMedical Systems, Houston, TX). At the end of the study, distribution of s.c. and visceral fat was analyzed by ether extraction method essentially as described (22).
Indirect Calorimetry.
Energy expenditure and respiratory quotient in rats were determined by using sealed air-tight cages equipped with two equal-flow indirect calorimetry systems to continuously measure oxygen consumption and carbon dioxide production. The animals were allowed to acclimate for at least 72 h or until their food and water intake normalized to exclude any interference caused by stress or the new environment.
Histology.
All animals used in the present study were killed at the end of each experiment, and their brains were analyzed by immunohistochemistry using reagents and protocols as described (11, 23). The sections were stained for EGFP to visualize transduced brain regions as well as ERα to evaluate the efficiency of knockdown. Similar to our previous study (11), approximately half of the animals were eliminated at this point because of inadequate vector distribution caused primarily by needle misplacement during stereotactic surgery. Only the mice which had efficient ERα silencing restricted to the VMN on both sides of the brain were used in subsequent data analysis.
Acknowledgments
We thank Mihaela Stavarache for excellent technical assistance. This work was supported by National Institutes of Health Grants MH62147 and MH67775 (to S.O.).
Abbreviations
- ERα
estrogen receptor α
- ERKO
ERα knockout
- AAV
adeno-associated virus
- VMN
ventromedial nucleus of hypothalamus
- OVXed
ovariectomized
- ARC
arcuate nucleus
- EB
17β-estradiol-3-benzoate
- 2-DG
2-deoxy-d-glucose
- shRNA
small hairpin RNA.
Footnotes
The authors declare no conflict of interest.
References
- 1.Heine PA, Taylor JA, Iwamoto GA, Lubahn DB, Cooke PS. Proc Natl Acad Sci USA. 2000;97:12729–12734. doi: 10.1073/pnas.97.23.12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ogawa S, Chan J, Gustafsson JA, Korach KS, Pfaff DW. Endocrinology. 2003;144:230–239. doi: 10.1210/en.2002-220519. [DOI] [PubMed] [Google Scholar]
- 3.King BM. Physiol Behav. 2006;87:221–244. doi: 10.1016/j.physbeh.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 4.Vilberg TR, Keesey RE. Am J Physiol. 1984;247:R183–R188. doi: 10.1152/ajpregu.1984.247.1.R183. [DOI] [PubMed] [Google Scholar]
- 5.Han PW. Am J Physiol. 1968;215:1343–1350. doi: 10.1152/ajplegacy.1968.215.6.1343. [DOI] [PubMed] [Google Scholar]
- 6.Pfaff D, Keiner M. J Comp Neurol. 1973;151:121–158. doi: 10.1002/cne.901510204. [DOI] [PubMed] [Google Scholar]
- 7.Li HY, Blaustein JD, De Vries GJ, Wade GN. Brain Res. 1993;631:304–312. doi: 10.1016/0006-8993(93)91549-8. [DOI] [PubMed] [Google Scholar]
- 8.Beatty WW, O'Briant DA, Vilberg TR. Pharmacol Biochem Behav. 1975;3:539–544. doi: 10.1016/0091-3057(75)90169-0. [DOI] [PubMed] [Google Scholar]
- 9.Wade GN, Zucker I. J Comp Physiol Psychol. 1970;72:328–336. doi: 10.1037/h0029461. [DOI] [PubMed] [Google Scholar]
- 10.Butera PC, Beikirch RJ. Brain Res. 1989;491:266–273. doi: 10.1016/0006-8993(89)90062-0. [DOI] [PubMed] [Google Scholar]
- 11.Musatov S, Chen W, Pfaff DW, Kaplitt MG, Ogawa S. Proc Natl Acad Sci USA. 2006;103:10456–10460. doi: 10.1073/pnas.0603045103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Borg MA, Sherwin RS, Borg WP, Tamborlane WV, Shulman GI. J Clin Invest. 1997;99:361–365. doi: 10.1172/JCI119165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sanders NM, Dunn-Meynell AA, Levin BE. Diabetes. 2004;53:1230–1236. doi: 10.2337/diabetes.53.5.1230. [DOI] [PubMed] [Google Scholar]
- 14.Smith GP, Epstein AN. Am J Physiol. 1969;217:1083–1087. doi: 10.1152/ajplegacy.1969.217.4.1083. [DOI] [PubMed] [Google Scholar]
- 15.Wajchenberg BL. Endocr Rev. 2000;21:697–738. doi: 10.1210/edrv.21.6.0415. [DOI] [PubMed] [Google Scholar]
- 16.Shughrue PJ, Askew GR, Dellovade TL, Merchenthaler I. Endocrinology. 2002;143:1643–1650. doi: 10.1210/endo.143.5.8772. [DOI] [PubMed] [Google Scholar]
- 17.Alotaibi H, Yaman EC, Demirpence E, Tazebay UH. Biochem Biophys Res Commun. 2006;345:1487–1496. doi: 10.1016/j.bbrc.2006.05.049. [DOI] [PubMed] [Google Scholar]
- 18.Newton CJ, Buric R, Trapp T, Brockmeier S, Pagotto U, Stalla GK. J Steroid Biochem Mol Biol. 1994;48:481–486. doi: 10.1016/0960-0760(94)90197-x. [DOI] [PubMed] [Google Scholar]
- 19.Weigel NL, Zhang Y. J Mol Med. 1998;76:469–479. doi: 10.1007/s001090050241. [DOI] [PubMed] [Google Scholar]
- 20.Metivier R, Penot G, Carmouche RP, Hubner MR, Reid G, Denger S, Manu D, Brand H, Kos M, Benes V, Gannon F. EMBO J. 2004;23:3653–3666. doi: 10.1038/sj.emboj.7600377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Toran-Allerand CD, Tinnikov AA, Singh RJ, Nethrapalli IS. Endocrinology. 2005;146:3843–3850. doi: 10.1210/en.2004-1616. [DOI] [PubMed] [Google Scholar]
- 22.Clegg DJ, Brown LM, Woods SC, Benoit SC. Diabetes. 2006;55:978–987. doi: 10.2337/diabetes.55.04.06.db05-1339. [DOI] [PubMed] [Google Scholar]
- 23.Nomura M, Akama KT, Alves SE, Korach KS, Gustafsson JA, Pfaff DW, Ogawa S. Neuroscience. 2005;130:445–456. doi: 10.1016/j.neuroscience.2004.09.028. [DOI] [PubMed] [Google Scholar]