Abstract
Essential roles for gonadotropins in gonadal development and reproduction are well established. Over the past decade, however, the expression of luteinizing hormone receptor (LHR) has also been reported in the brain of various mammals and birds. Although suggestive, it has not yet been determined whether this expression pattern supports a novel function for gonadotropins. Here, we demonstrate a CNS-mediated role of gonadotropins in a reproductive behavior: the courtship songs of the South African clawed frog, Xenopus laevis. Male advertisement calling in this species depends on a nongonadal action of gonadotropin. To determine whether this effect is due to action on the CNS, we administered gonadotropin intracerebroventricularly (ICV) or systemically to intact or castrated males with or without concomitant androgen replacement. In intact and androgen-replaced gonadectomized males, gonadotropin significantly increased calling within 1 h after ICV injection. The effective dosage via ICV injections was less than one hundredth of the effective systemic dose. In situ hybridization with a cloned fragment of Xenopus LHR revealed strong expression in ventral forebrain areas important for vocal control. Further, gonadotropin treatment of brain in vitro up-regulates immunoreactivity for the LHR downstream target, egr-1, specifically in these vocal forebrain areas. Up-regulation occurs even when synaptic transmission is suppressed by incubation in Ca2+ free/high magnesium saline. These results demonstrate a neural role for gonadotropin in the control of calling behavior, potentially mediated via LHRs in forebrain vocal nuclei. Gonadotropin may play a novel integrative role in modulating both reproductive physiology and behavior.
Keywords: amphibian, luteinizing hormone receptor, neural action, neuromodulator
Gonadotropins [luteinizing hormone (LH) in particular] play important roles in reproductive physiology across vertebrates. These heterodimeric glycoprotein hormones are typically released from the anterior pituitary in response to gonadotropin releasing hormone (GnRH), although some [e.g., human CG (hCG)] are produced in the placenta (1). LH and hCG stimulate the production of gonadal steroids, and exert their effects through binding to the same seven transmembrane domain G protein-coupled receptor (LHR, (1)). Until recently, LHR expression was believed to be confined to the gonads where it is required for fertility (for a review, see ref. 2). However, recent findings of LHR expression in neural tissues in birds (3) and mammals (4), including humans (5–7), suggest potentially important functions for gonadotropins via direct action in the brain (8).
To date, neural expression of LHRs in various mammalian and avian species has been documented in the hypothalamus, hippocampus, brainstem, cortex, choroid plexus, and pituitary (4). Based on these receptor distributions, several functions for LHRs have been hypothesized, including regulation of GnRH expressing cells (9–11), sensory information processing (12), modulation of hippocampal activity (8, 13) and pathology (14), and neurosteroidogenesis (12). However, with the exception of the role of LHRs in regulating GnRH, direct in vivo tests for the function of gonadotropins within the CNS are few and include effects on the sleep–wake cycle, activity, and stereotypic behavior in female rodents (15, 16). These complex behavioral phenotypes, together with the wide distribution of LHRs in the brain, make it difficult to pinpoint a specific role for neuronal LHRs in behavior.
Advertisement (AD) calling of anuran amphibians provides an excellent model system for elucidating a CNS action of gonadotropin because of a previously reported nongonadal action of gonadotropin on calling (17) and well characterized neuroendocrine circuits (18–20). Calling plays a central role in courtship (21), and male songs are generated by a defined neural circuit that includes the ventral striatum (VST) and preoptic area, brainstem nuclei, dorsal tegmental area of medulla and neurons in cranial nucleus IX-X that innervate the larynx (22–24). Neurons in these nuclei express androgen receptor, and androgen is required for calling (18, 25). In the South African clawed frog, Xenopus laevis, in particular, peripheral administration of hCG evokes male songs and increases levels of circulating androgens. However, androgen treatment in castrated males does not produce levels of calling equivalent to those in intact males, suggesting that androgens alone are not sufficient for vocal production (17). When hCG was also given to castrated, androgen replaced males, calling increased to levels comparable with those of intact males, suggesting that gonadotropin can influence vocal behavior by acting on nongonadal tissues.
Here, we show that gonadotropin can act on the brain to influence a reproductive behavior, and that this CNS action could be mediated by LHRs expressed in the defined-neural circuit controlling this behavior. We investigated the effect of central and peripheral gonadotropin administration on AD calling in male frogs. Dose-response relations for central and peripheral gonadotropins on calling behavior were determined in gonadally intact, gonadectomized, and gonadectomized and androgen-replaced male frogs. To elucidate underlying neural mechanisms, we identified CNS sites for gonadotropin action by in situ hybridization with a cloned fragment of the Xenopus LHR mRNA. By using a downstream target of LHR, the immediate early gene egr-1, we investigated the functionality of LHRs expressed in brain regions important for vocal control.
Results
The Effects of Central and Peripheral Gonadotropin on Calling Behavior.
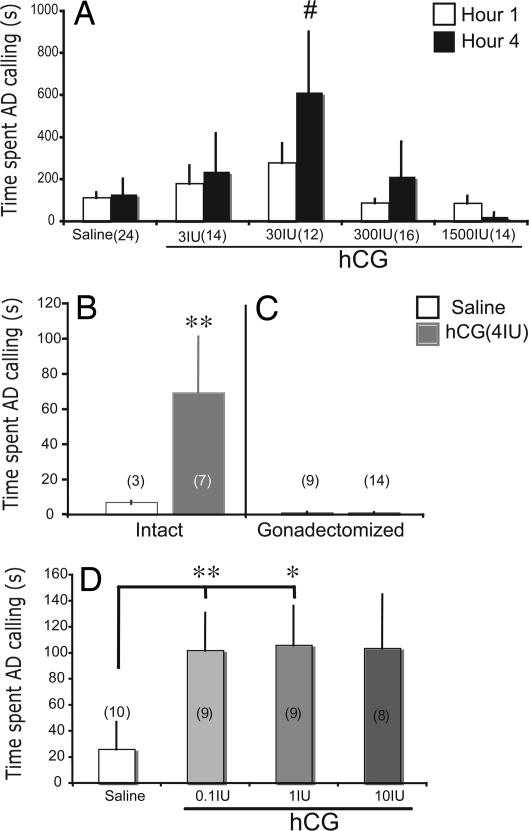
To establish a dose-response relation between hCG and calling behavior, we tested vocal behavior of androgen-replaced, castrated males after peripheral injections of 3, 30, 300, or 1,500 units of hCG. When injected into the dorsal lymph sac, hCG increased levels of AD calling in androgen-treated, castrated male frogs exposed to a female in a dose and time dependent manner (Fig. 1A). In comparison with the saline condition, systemic hCG treatment elicited calling at a dose of 30 units, but not 3, 300, or 1500 units (Dunn's Multiple comparison: 30 units vs. saline, P < 0.05 for both test 1and 2; refer to Materials and Methods for detailed experimental design). The dose-response relation resembles an inverted-U shape with a peak at the 30 units dose. This effect was time dependent. By four hours after injection, hCG increased AD calling (Dunn's: P < 0.05 for both tests 1 and 2; Fig. 1A); the effect was not yet present at the first hour postinjection. This result is consistent with the previous observation of slow incorporation of hCG into the circulation via the dorsal lymph sac (26). Both androgens [testosterone (T) and dihydrotestosterone (DHT)] supported calling equally well in castrated, hCG-treated males (Wilcoxon rank sum: P > 0.66).
Fig. 1.
Behavioral responses to hCG administered systemically or ICV show that gonadotropin can influence calling behavior via its direct action in the brain. (A) Each bar represents mean ± SEM for the combination of two tests at each dosage of hCG administered to androgen-replaced, gonadectomized frogs. In both tests, compared with the saline conditions, 30 units of systemically injected hCG increases advertisement calling (AD, y axes) at the fourth but not the first hour, after injection (#, Dunn's, P < 0.05 in both tests 1 and 2). Because the same effects of hCG were produced by both T and DHT treatment, data were combined. (B) ICV injections of hCG in gonadally intact males produce increases in calling in comparison with males with saline injections. (C) ICV injections of hCG in castrated males yield low amounts of calling equivalent to those produced by saline injections. (D) ICV injections of hCG in various dosages into DHT-treated, castrated males produce significant increases in calling at 0.1- and 1-unit concentrations. Each group is represented by a mean ± SEM, with sample sizes indicated in parentheses. ∗, P < 0.05; ∗∗, P<0.01 (two-tailed) following Dunn's Multiple comparison for nonparametric tests, in comparison with the corresponding saline condition.
To test the hypothesis that gonadotropins act directly on the CNS to modulate calling behavior, we treated intact or castrated males (with or without androgen replacement) with various dosages of hCG via intracerebroventricular (ICV) injection. In intact males, 4 units of hCG increased calling within 1 h after ICV injection (Wilcoxon: P = 0.0227; Fig. 1B). This effect of hCG on calling is androgen-dependent; the same dosage of hCG given to castrated males via ICV injection does not increase calling (Wilcoxon: P > 0.36; Fig. 1C). Further, hCG increases calling in DHT-treated castrated males (Fig. 1D) at ICV doses as low as 0.1 units (1.0 units was also effective, but not 10 units) within 1 h after injection (Kruskal–Wallis = 11.3858, P = 0.0098, 0.1 units vs. saline; Dunn's: P < 0.01, 1 units vs. saline; Dunn's: P < 0.05, 10 units vs. saline; Dunn's: P = NS). Over this dose range there is no sign of the inverted U-shape function seen after systemic injection (Fig. 1A). The effective dose for hCG via ICV is less than one one-hundredth of the effective dose given systemically. Taken together, these results show that hCG can modulate calling behavior in a dose dependent manner, that this effect is androgen-dependent, and that the effect can be produced via direct administration to the CNS at a much lower dose and a shorter latency than that produced by systemic administration.
Xenopus LHR mRNA Cloning and Sequence Similarity to LHRs of Other Species.
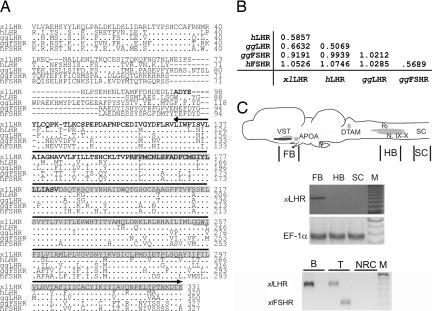
To test the hypothesis that the CNS action of gonadotropin on calling is mediated through its cognate receptors in the brain, we cloned a putative X. laevis LHR mRNA fragment (≈900 bp) with primers generated using a partial sequence of X. laevis LHR mRNA (GenBank CAB62284.1) and using regions of the X. tropicalis genome highly homologous to LHR sequences of other species. Across species, LHRs have a large extracellular domain containing a high affinity hormone-binding site, a highly conserved seven transmembrane domain, and a small intracellular domain mediating signal transduction (Fig. 2A). The XlLHR sequence includes the extracellular domain, which confers specificity. This cloned gene fragment shows 67% identity over a 351-aa sequence to chicken, and 69% identity over a 334-aa sequence to human LHRs. The distance matrix (Fig. 2B) and a phylogenetic tree constructed by using the neighbor joining method (ref. 27 and data not shown) indicate the cloned X. laevis gene clusters with LHRs rather than follicle stimulating hormone receptors (FSHRs).
Fig. 2.
Cloned X. laevis LHR shows similarity with LHRs of other species, and xlLHR is present in the forebrain of X. laevis. (A) Protein sequence of cloned X. laevis LHR (top row; grey box indicates previously reported sequence, CAB 62284) aligned to the LHR and FSHR sequences of human (AAB19917 and P23945) and chick (NP_990267 and P79763). Dots indicate identities, dashes indicate gaps, and upper line indicates seven transmembrane domain. Two probes used to show the specificity of in situ hybridization are indicated by the bold (probe A) and underlined (probe B) sequence. (B) A distance matrix between XlLHR and human LHR and FSHR, chick LHR (ggLHR) and FSHR (ggFSHR) shows that XlLHR is closer to LHRs than FSHRs. (C Top) A schematic saggital drawing of Xenopus brain oriented with anterior to the left, illustrating nuclei implicated in controlling vocal production. The line indicates the level of horizontal sections illustrated in Fig. 5. (Middle) RT-PCRs showing corresponding gene fragments with cDNAs from tissues indicated in the top brain schematic drawing. SC, spinal cord; M, Marker. EF-1α mRNA was amplified as a positive control. The dark bands in M indicate 600 bp. (Bottom) RT-PCRs showing LHRs (probe B) are expressed in both brain and testis, whereas FSHRs are expressed only in the testis. B, brain; T, testis; NRC, testis RNA with no RT. The dark band in M shows 350-bp size.
Xenopus Brain Regions Implicated in Vocal Behavior Express LHR mRNA.
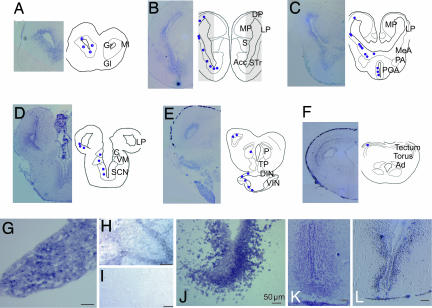
Amplification of the XlLHR mRNA from cDNAs of different brain regions using RT-PCR revealed LHR expression in forebrain (FB in Fig. 2C), not in hindbrain areas encompassing the vocal motor nucleus (“N. IX-X”) nor in the spinal cord (HB and SC in Fig. 2C). Using in situ hybridization, we examined LHR expression in the CNS. An antisense RNA probe from the entire fragment of the cloned XlLHR was hybridized to sections of CNS, pituitary, and testis (Fig. 3). Along with the expression in the testis and pituitary (Fig. 3 G and H, positive controls), brain regions such as olfactory nuclei, striatum, amygdala, lateral pallium, preoptic area (POA), thalamus, and hypothalamic regions show XlLHR mRNA (Fig. 3 A–D and F). Most importantly, strong expression of LHR mRNA was observed in the ventral striatum (VST), amygdala, and anterior POA (Fig. 3 B and C, and high magnification in J and K), ventral forebrain areas implicated in vocal control in amphibians (21–23). Areas involved in neuroendocrine regulation, such as the median eminence (Fig. 3E), also express XlLHR mRNA. Notable brain regions without expression of XlLHR mRNA include septum, medial pallium [the anuran hippocampal homolog, (28)], and several brainstem regions. As specificity controls, two additional antisense RNA probes against nonoverlapping fragments of XlLHR (Fig. 2A and Materials and Methods) were hybridized, and revealed the same pattern of expression of LHR mRNA in the brain as shown in Fig. 3. No signal was detected when sense probes or no probe were used, nor when tissue was pretreated with RNase A (Fig. 3I).
Fig. 3.
LHR mRNA expression in the Xenopus brain detected by in situ hybridization. Photomicrographs of transverse sections through Xenopus brain from anterior (A) through posterior (F). (A–F) Left columns of the sections show LHR mRNA expression as a purple precipitate, whereas Right columns show schematic drawings of brain with purple dots illustrating the positive hybridization signal. High magnification photomicrographs of the striatum (J), POA (K), and ventral hypothalamus (L) show LHR mRNA expression. Strong LHR mRNA hybridization signal was shown in pituitary (G), and Leydig cells in testis (H). RNase pretreatment eliminates hybridization (I). (Scale bar: 50 μm.) Nomenclature is as in refs. 57 and 58. For abbreviations, see abbreviations list.
To determine whether FSHRs (the other major gonadotropin receptor) are also expressed in the brain, we attempted amplification by RT-PCR of a xlFSHR mRNA fragment (GenBank AJ249846) using cDNAs from X. laevis male brains. Although a fragment of LHR mRNA was present in both brain and testis (Fig. 2C Bottom), we did not detect FSHR mRNA from Xenopus brains (two males). As a positive control, both FSHR and LHR mRNA were present in testis. As in all other vertebrate species examined to date (29–32), FSHRs are not expressed in the brain of X. laevis; the neural action of hCG in modulating calling cannot be due to binding to FSHRs.
hCG Treatment in Vitro Up-Regulates EGR-1 Immunoreactive Cells in Vocal Brain Areas.
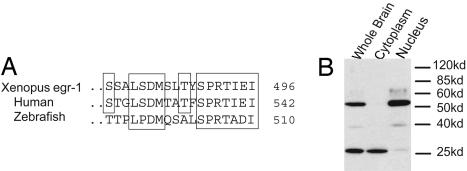
To probe XlLHR activity in the brain, we investigated changes in the immunoreactivity of its downstream target, the immediate early gene egr-1 (33), in response to hCG treatment in vitro. First, we compared sequences and performed immunoblotting to test the specificity of EGR-1 antibody recognition in Xenopus neural tissue. Sequence comparison of the C-terminal regions of human EGR-1 protein against which the EGR-1 antibody (34) was raised shows substantial identity to amino acid sequences of Xenopus EGR-1 (Fig. 4A). Western blot analysis revealed that this EGR-1 antibody shows specific binding to 2 bands in Xenopus brain, the larger (≈55 kDa) of which is the only major band present in nuclear extract (Fig. 4B). Thus, nuclear staining observed in immunocytochemistry can be attributed to the antibody binding to the 55-kDa protein, which is the expected size of Xenopus EGR-1.
Fig. 4.
The EGR-1 antibody shows specific affinity for EGR-1 in Xenopus brains. (A) Comparisons of the C terminus regions of Egr-1 show similarity between Xenopus, human (gi49258078), and zebrafish (gi18858601) EGR-1 aa sequences. (B) Immunoblotting with an EGR-1 antibody shows the predicted size product in the nuclear extract.
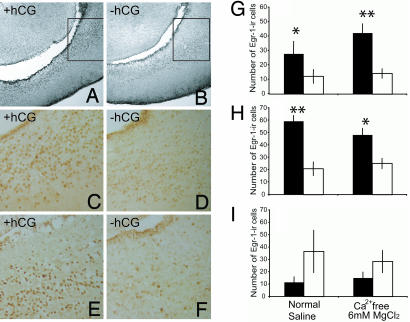
When treated in vitro with 1 unit/ml of hCG in physiological saline for one hour, the number of EGR-1 immunoreactive cells in the VST and anterior POA (APOA) increased compared with the saline treatment (VST: t test, P = 0.034; APOA: t test, P = 0.0036; Fig. 5C and D and G and H Left). This up-regulation in response to gonadotropin was not observed in the medial pallium (Fig. 5I Left), where no expression of XlLHR mRNA was found. This difference in response suggests specificity of EGR-1 activation in the VST and APOA in response to hCG treatment. We further investigated whether the up-regulation of EGR-1 in these brain areas was due to direct activation of XlLHR by hCG treatment or due instead to secondary induction of EGR-1 protein resulting from synaptic transmission. We incubated Xenopus brains in vitro in Ca2+ free/6 mM MgCl2+ containing saline to block synaptic transmission (35, 36) with or without 1 unit/ml of hCG. The number of strongly immunoreactive EGR-1 positive cells in the VST and APOA is higher in hCG-treated than in saline-treated controls (VST: Wilcoxon Rank sum, P = 0.0049; APOA: Wilcoxon, P = 0.029; Fig. 5 A, B, E, and F, and G and H Right). Again, this effect was not observed in the medial pallium under the same saline condition (Fig. 5I Right). In summary, our results show that gonadotropin can up-regulate the downstream transcription factor egr-1 in the VST and APOA. Further, this effect is not observed in a brain region with no LHR mRNAs and is independent of synaptic activation. Taken together, these data suggest a direct activation of XlLHR-expressing cells by hCG treatment and a potential function of XlLHR in the neural circuitry involved in courtship song production.
Fig. 5.
Gonadotropin treatment up-regulates EGR-1 immunoreactivity in the ventral striatum and APOA. (A–F) Photomicrographs in the horizontal plane of Egr-1 immunoreactive cells in the ventral striatum with (A, C, and E) or without (B, D, and F) hCG treatment at low (×10, A and B) or high (×20, C–F) magnifications. In either normal physiological saline (C and D) or Ca2+ free/6 mM MgCl2+ saline (A, B, E, and F), the number of EGR-1 immunoreactive cells is higher after hCG treatment (black bars, mean ± SEM) compared with saline alone (white bars, mean ± SEM) in the ventral striatum (G) and APOA (H), but not medial pallium (I). ∗, P < 0.05; and ∗∗, P < 0.01 (two-tailed).
Discussion
Here, we show that gonadotropin can act directly on the brain of male frogs to stimulate AD calling. AD calls in male anuran amphibians are used to attract mates and to repel other males. In several anuran species, including Xenopus, castration abolishes, but treatment with exogenous androgen does not fully restore calling, suggesting that androgen is necessary but not sufficient to produce the behavior (21, 37, 38). Pituitary factors, including gonadotropins, have been proposed to fill this gap (17, 39). Gonadotropins given systemically to androgen-replaced castrated frogs markedly increase calling above the levels exhibited by castrated frogs with androgen treatment alone (17). These previous findings suggested a locus of action of gonadotropin on calling outside of the testes. Behavioral results from systemic treatment in this study support these observations, and further reveal an inverted U-shape like dose-response relation between gonadotropin and calling. More importantly, we show that gonadotropins administered into the brain can directly affect the CNS to influence calling in an androgen-dependent manner. Effective levels of gonadotropin given via ICV are less than one hundredth of the levels required in systemic administration, again suggesting that the action of gonadotropin is mediated via the CNS.
Distribution and Functionality of LHRs in Comparison with Other Species.
One requirement for the direct central action of gonadotropin is that its receptor is expressed in appropriate neuronal populations. We show here that the amphibian brain expresses LHR mRNA. In particular, among the areas with most dense expressions of LHRs are the ventral striatum (VST), amygdala and APOA, areas implicated in vocal control in anurans, providing a potential mechanism for the CNS action of gonadotropin on calling behavior. In general, there is substantial overlap of expression patterns of LHR in anuran brain with those of other species. Dense expression of LHR mRNA in ventral hypothalamus is also found in birds (3) and rodents (4), whereas expression in olfactory nuclei has been also seen in rats (12). An unexpected finding is that the medial pallium, a hippocampal homolog in anurans, does not express LHR, whereas the mammalian hippocampus shows the highest levels of expression of LHR (4). Although the functional significance of this species difference is not understood, expression of LHRs in hypothalamic areas, highly conserved among vertebrates, is likely to participate in the well established role of providing negative feedback to GnRH cell populations (10). Support for this hypothesis comes from the presence of GnRH-expressing cells in the POA and median eminence in the Xenopus brain, where LHRs are also expressed (40).
Using the immediate early gene egr-1, one of the LHR-mediated downstream targets, we demonstrate that cells in VST and APOA respond to hCG treatment even under conditions known to block synaptic transmission, suggesting direct activation of LHR by hCG. The functionality of neural LHRs in VST and APOA in our study indicates that the CNS action of gonadotropin on calling could be mediated via direct activation of LHRs in these regions. Functional LHR in mammalian CNS has been demonstrated in studies ranging from specific receptor binding activities of [I125]hCG (1) and immunoprecipitation of the mature LHR species (12), to electrophysiological (41, 42) and morphological (43) changes. Taken together, data from a variety of species suggest that LHRs in the brain are active and functional. Our data suggest further that they function in the control of a reproductive behavior, courtship song.
Potential Mechanisms of CNS Gonadotropin Action in Modulating Calling.
Gonadotropins in the CNS might function like neuromodulators, known to be important for reproductive behavior across vertebrates (19). In particular, the important roles of arginine vasotocin (20, 44) and prostaglandin E2 and F2 (45, 46) in vocal production in anuran amphibians and fishes are well established. Strong expression of LHR mRNA in the brain regions in the vocal circuit, activation of cells in these areas by the ligand, and the role of gonadotropin in behavior are consistent with the characteristic features of neuromodulators. Alternatively, indirect actions by gonadotropin, such as facilitating androgen action and increases in local concentration of androgen by facilitating neurosteroid synthesis, are consistent with LH function in the gonads. The role of neurosteroids in mediating androgen-mediated behavior has been observed in birds during gonadal regression periods (47). The expression of both androgen receptor (25) and LHRs in VST and POA suggests a potential locus of interaction between androgen and gonadotropin, possibly underlying the observed synergy between actions of androgen and gonadotropins (17). In amphibian brains, steroidogenic enzyme P450scc expressing cells were found in ventral infundibulum and POA (48) and 17β hydroxysteroid dehydrogenase immunoreactive fibers in amygdala (49), where LHRs are also expressed. Further studies are necessary to elucidate how neural LHR influences calling behavior in this species and in others.
In summary, we have shown that gonadotropin can modulate calling behavior in amphibians via its action on cognate receptors expressed in the brain and can act directly on ventral forebrain areas implicated in vocal control. From an evolutionary perspective, our results illustrate an important example of recent observations that the same molecule serves multiple functions via its action on cognate receptors distributed in the periphery and the CNS. CNS action of several peptides and proteins, such as leptin (50), insulin, insulin-like growth hormone (51), growth hormone (52), prolactin (53), and oxytocin (54), play important roles in cognition, emotion, stress, aggression, anxiety, eating, and homeostasis. The important role of neural LHRs in behavior demonstrated in anuran amphibians provides a model system for elucidating functions of neural LHRs in other species, and illustrates how a protein produced in the periphery can act on the CNS to mediate behavior.
Materials and Methods
Animals.
Sexually mature male X. laevis were purchased from Xenopus I (Dexter, MI), and housed in polycarbonate tanks in either 8 (groups of five) or 2 liters (individually) containing temperature regulated, carbon-filtered water treated with Novaqua (Novlek, Inc.). They were fed with frog chow pellets and cage water was changed twice a week. Animal care protocols followed National Institutes of Health guidelines and were approved by the International Animal Care and Use Committe (AC-AAAA5759) of Columbia University.
Gonadectomy and Steroid Treatment.
Adult male frogs were gonadectomized following a published protocol (17), and implanted s.c. with 5-mg pellets of either testosterone (T, Sigma) or dihydrotestosterone (DHT, Sigma), which have previously been shown to elevate plasma androgen levels to 54 and 33 ng/ml on average. The presence of heavy nuptial pads, an androgen-dependent morphological characteristic, indicated successful androgen treatment. Frogs were treated for either 4 or 6 weeks and housed individually before behavioral experiments.
Behavioral Experiments After Systemic and ICV Injections.
For the behavioral experiments with systemic injections, subgroups of T and DHT-treated castrated frogs were injected into the dorsal lymph sac with one of the following: 0, 3, 30, 300, or 1,500 units of hCG (Sigma) dissolved in 300 μl of saline, and immediately placed into a testing tank (76.86-m3 aquarium filled 2/3 with filtered water) containing a sexually unreceptive female frog, a condition reliably known to elicit AD calling (38). Each frog was randomly assigned to a dosage group and tested twice with an interval of two weeks. Calling was recorded by using a hydrophone (High Tech Inc., MS) and collected by using Sound Analysis Pro (http://ofer.sci.ccny.cuny.edu/html/body_sound_analysis.html), and analyzed by using Signal (Engineering Design) by using male AD call specific temporal and spectral characteristics (i.e., requiring at least 50% of continuous trill composed of inter-click interval <40ms with a ratio of spectral frequencies at 2 and 1.2 kHz <.9) to calculate the amount of AD calling during the first and fourth hours after injection. No order effect of treatment on calling was observed (Wilcoxon test: P > 0.85 and P > 0.55, for the first and fourth hour respectively), and we performed statistical analyses in each behavioral test separately.
For the behavioral test after ICV injections, animals were either intact, gonadectomized, or gonadectomized and androgen-replaced with a 5-mg pellet of DHT. After being anesthetized in 0.1% MS222 (Tricaine methane sulfonate), a small opening through the skull over the right lateral ventricle in each frog was created surgically, and closed by using gelfoam, suture and Vetbond (3M). After 1 day of recovery, frogs were anesthetized by submersion in 0.01% of MS222 and NaHCO3 until movement ceased. The lateral ventricle was visualized through the opening, and 1 μl containing either 0, 0.1, 1, or 10 units of hCG in saline containing 1.62 nM Hoechst was injected by using a glass micropipette connected to a micropump at 0.1 μl/s flow rate. Immediately after injection, the opening was closed with gelfoam and Vetbond. Frogs were allowed to recover (<3 min on average) and subsequently placed into a tank containing an unreceptive female. Calling behavior was monitored for one hour by using the same protocol as with systemic injections, and the amount of AD calling was determined by using Goldwave v.5.10. Each ICV injection was verified by visualizing Hoechst staining around the ventricles. Animals that took longer than 3 min to recover from the infusion procedure, and those that did not show Hoechst staining surrounding the two lateral ventricles, were excluded from the study.
Cloning a X. laevis LHR Fragment.
An LHR fragment was PCR amplified from cDNA reverse transcribed from testis mRNA, by using primers against the published LHR sequence fragment and primers corresponding to a region of the X. tropicalis genome that showed high homology to multiple LHR sequences. The fragment was cloned into TOPO2.1 vector (Invitrogen) following the manufacturer's instructions, and sequenced by Genewiz, Inc. This sequence was then merged with the available X. laevis LHR fragment sequence (CAB62284.1). The sequence of the cloned gene was aligned to other LHRs and FSHRs by using ClustalW (Fig. 2A). Primer sequences for LHR mRNA were as follows: GCAGACTACGAGTACCTGTGCCAGCCCAAG and CTACGGAGGCGATGAGCAGTAGATAAATCC.
Detecting LHR in Xenopus Brain Using RT-PCR.
The brain and spinal cord of adult male Xenopus were dissected out and frozen at −80°C after removal of the meninges. Fresh frozen tissue was cryosectioned transversely, sections were pooled every 1,200 μm, and RNA was extracted by using TRIzol (Invitrogen), followed by Dnase I (Invitrogen) treatment. Twenty-micrometer-thick sections delimiting the most anterior and posterior area of the brain represented in each pool were mounted on a slide for Nissl staining. These sections served as landmarks for identifying nuclei contained in each pool of tissues used for RNA extraction. The presence of LHR mRNA was assayed by RT-PCR. Amplifications of RNA without reverse transcriptase and fragments of elongation factor 1α (EF-1α) served as controls. Primer sequences for EF-1α mRNA were as follows: TGGTGTTGGTGAATTTGAAGCTGGTATCT and ATGCAGTCAAGAGCTTCCAGCAGGGTAG; and primer sequences for Xenopus FSHR mRNA were as follows: GTTAGACCGGAAAGTGCGATT and CTACTGACACCGACAATTGGAAGTAG.
In Situ Hybridization.
In situ hybridization of cRNA was carried out as described in Pérez et al. (25). Templates for digoxygenin-labeled RNA probes were PCR amplified from plasmid DNA, and probes were generated by using the Maxiscript kit (Ambion). Hybridization with fixed 20-μm sections of brain, spinal cord, pituitary, and testis was carried out by using 200 ng of probe overnight at 50°C. After stringent washes, digoxygenin-labeled probe was detected by using an alkaline phosphatase-labeled anti-digoxygenin antibody (Roche) followed by detection with BM purple (Roche). For the negative controls, hybridization with a sense strand of LHR RNA, RNase pretreatment, pretreatment with unlabeled antisense LHR RNA probe, and hybridization with no probe were used. For specificity controls, antisense RNAs of two nonoverlapping fragments of LHR sequences were used, and their hybridization patterns were compared throughout the Xenopus CNS (see Fig. 2A for the sequences for specificity control probes A and B). Primer sequences for LHR probe A and probe B are as follows: probe A, GCAGACTACGAGTACCTGTGCCAGCCCAAG and CTACGGAGGCGATGAGCAGTAGATAAATCC; probe B, GGGGGCTGGATATTTTCACTTGTGATAGC and GCCAACTTTGTATCTTTGTTTGTCGG.
Western Blotting.
For whole brain extract, protein was obtained by homogenizing a male Xenopus brain in Laemmli buffer (2% SDS/10% glycerol/0.01% bromophenol blue/10% 2-mercaptoethanol/60 mM Tris, pH 6.8) followed by sonication. Nuclear and cytoplasmic extracts were prepared by homogenizing a male Xenopus brain in buffer (10 mM KCl/0.1 mM EDTA/1 mM DTT/10 mM Hepes/0.4% IGEPAL/protease inhibitor mix (Sigma), pH 7.7). After brief centrifugation, the supernatant (cytosol) was collected, and the pellet (nuclei) was homogenized as above. After quantification using a noninterfering protein assay (Pierce), equal amounts of protein were separated on a 10% SDS/PAGE gel, transferred to nitrocellulose membrane followed by Western blotting procedure with a 1:200 dilution of antibody against the carboxyl-terminus of EGR-1 (sc-189; Santa Cruz) and 1:2,000 dilution of an HRP labeled anti-rabbit antibody (General Electric) followed by detection using a chemiluminescent HRP substrate (Pierce).
hCG in Vitro Treatment and Egr-1 Immunocytochemistry.
Brains of sexually mature Xenopus males (n = 22) were extracted in physiological saline [96 mM NaCl/20 mM NaHCO3/2 mM CaCl2/2 mM KCl/0.5 mM MgCl2/10 mM Hepes/11 mM glucose, pH 7.8 (55)] and subsequently treated with 1 unit hCG/ml saline or equal volume of saline for 1 h. In subset of brains, the same treatment was carried out in saline with 0 mM Ca2+ and 6 mM MgCl2+, a well established method for blocking synaptic transmission (35, 36). After the treatment, brains were fixed in 4% paraformaldehyde overnight, cryoprotected in 30% sucrose, and cryosectioned at 20 μm at the horizontal plane. Immunocytochemistry with the EGR-1 antibody (1:1,000) used for Western blotting was conducted following a previously published protocol (34, 56) with omission of the biotin blocking step.
Image Acquisition and Quantification of Staining.
All microscopy and image acquisition was performed by using a Leica DMR microscope fitted with an RT-slider SPOT camera (Molecular Diagnostics). Composite photomicrographs (Fig. 3 A–F) of in situ hybridization were created by using Photoshop (Adobe) by combining multiple overlapping images and blurring the edges. Contrast of all of the images shown for the in situ hybridization (Fig. 3) was adjusted. For quantification of immunostaining, images were acquired by using the same image settings (e.g., exposure time, transmitted light level, etc.) for all brains and regions at the horizontal plane. For each region, images of four sections (60 μm apart) of both sides were taken of each brain. Quantification of labeled nuclei was performed following a previously published procedure (56) by using particle quantification function in ImageJ.
Statistics.
When data distribution violated Gaussian assumptions, we performed Kruskal–Wallis tests followed by Dunn's multiple comparison procedure for nonparametric tests, and Wilcoxon Rank sum tests for two-group comparisons. When the assumptions of normal distribution and equal variances between groups were not violated, we performed Student's t tests. Statistical significance for all of the tests was set at the α level of 0.05 (two-tailed).
Acknowledgments
We thank C. Barnard for help with gonadectomy and animal care; C. Vignal for EGR-1 immunocytochemistry protocol; and W. Wilczynski, R. Kaushik, and E. Leininger, and three anonymous reviewers for critical readings of the previous version of this manuscript. This research was supported by National Institutes of Health Grant NS23684.
Abbreviations
- Ad
nucleus anterodorsalis tegmenti
- Acc
nucleus accumbens
- C
central thalamic nucleus
- DIN
dorsal infundibulum
- DP
dorsal pallium
- Gl
glomerular layer of the OB
- Gr
granule cell layer of the OB
- LP
lateral pallium
- Ml
mitral cell layer of the OB
- MeA
medial amygdala nucleus
- MP
medial pallium
- POA
preoptic area
- P
posterior thalamic nucleus
- S
septum
- SCN
suprachiasmatic nucleus
- Str
striatum
- Torus
torus semicircularis
- TP
posterior tuberculum
- VIN
ventral infundibulum
- VM
ventromedial thalamic nucleus.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. EF191189).
References
- 1.Ascoli M, Fanelli F, Segaloff DL. Endocr Rev. 2002;23:141–174. doi: 10.1210/edrv.23.2.0462. [DOI] [PubMed] [Google Scholar]
- 2.Huhtaniemi I, Zhang FP, Kero J, Hamalainen T, Poutanen M. Mol Cell Endocrinol. 2002;187:49–56. doi: 10.1016/s0303-7207(01)00698-0. [DOI] [PubMed] [Google Scholar]
- 3.You S, Kim H, Hsu CC, El Halawani ME, Foster DN. Biol Reprod. 2000;62:108–116. doi: 10.1095/biolreprod62.1.108. [DOI] [PubMed] [Google Scholar]
- 4.Lei ZM, Rao CV, Kornyei JL, Licht P, Hiatt ES. Endocrinology. 1993;132:2262–2270. doi: 10.1210/endo.132.5.8477671. [DOI] [PubMed] [Google Scholar]
- 5.Rao CV, Zhou XL, Lei ZM. Biol Reprod. 2004;71:579–587. doi: 10.1095/biolreprod.104.027300. [DOI] [PubMed] [Google Scholar]
- 6.Eblen A, Bao S, Lei ZM, Nakajima ST, Rao CV. J Clin Endocrinol Metab. 2001;86:2643–2648. doi: 10.1210/jcem.86.6.7533. [DOI] [PubMed] [Google Scholar]
- 7.Carlson HE, Kane P, Lei ZM, Li X, Rao CV. J Clin Endocrinol Metab. 2004;89:4119–4123. doi: 10.1210/jc.2003-031882. [DOI] [PubMed] [Google Scholar]
- 8.Lei ZM, Rao CV. Semin Reprod Med. 2001;19:103–109. doi: 10.1055/s-2001-13917. [DOI] [PubMed] [Google Scholar]
- 9.Hu L, Wada K, Mores N, Krsmanovic LZ, Catt KJ. J Biol Chem. 2006;281:25231–25240. doi: 10.1074/jbc.M603768200. [DOI] [PubMed] [Google Scholar]
- 10.Mores N, Krsmanovic LZ, Catt KJ. Endocrinology. 1996;137:5731–5734. doi: 10.1210/endo.137.12.8940408. [DOI] [PubMed] [Google Scholar]
- 11.Li X, Lei ZM, Rao CV. Endocrinology. 1996;137:899–904. doi: 10.1210/endo.137.3.8603601. [DOI] [PubMed] [Google Scholar]
- 12.Apaja PM, Harju KT, Aatsinki JT, Petaja-Repo UE, Rajaniemi HJ. J Biol Chem. 2004;279:1899–1906. doi: 10.1074/jbc.M311395200. [DOI] [PubMed] [Google Scholar]
- 13.Zhang W, Lei ZM, Rao CV. Life Sci. 1999;65:2083–2098. doi: 10.1016/s0024-3205(99)00474-9. [DOI] [PubMed] [Google Scholar]
- 14.Casadesus G, Webber KM, Atwood CS, Pappolla MA, Perry G, Bowen RL, Smith MA. Biochim Biophys Acta. 2006;1762:447–452. doi: 10.1016/j.bbadis.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 15.Lukacs H, Hiatt ES, Lei ZM, Rao CV. Horm Behav. 1995;29:42–58. doi: 10.1006/hbeh.1995.1004. [DOI] [PubMed] [Google Scholar]
- 16.Toth P, Lukacs H, Hiatt ES, Reid KH, Iyer V, Rao CV. Brain Res. 1994;654:181–190. doi: 10.1016/0006-8993(94)90478-2. [DOI] [PubMed] [Google Scholar]
- 17.Wetzel DM, Kelley DB. Horm Behav. 1983;17:388–404. doi: 10.1016/0018-506x(83)90048-x. [DOI] [PubMed] [Google Scholar]
- 18.Kelley DB. Science. 1980;207:553–555. doi: 10.1126/science.7352269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moore FL, Boyd SK, Kelley DB. Horm Behav. 2005;48:373–383. doi: 10.1016/j.yhbeh.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 20.Wilczynski W, Lynch KS, O'Bryant EL. Horm Behav. 2005;48:440–450. doi: 10.1016/j.yhbeh.2005.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmidt RS. Behaviour. 1966;26:251–285. doi: 10.1163/156853965x00228. [DOI] [PubMed] [Google Scholar]
- 22.Wetzel DM, Haerter UL, Kelley DB. J Comp Physiol A. 1985;157:749–761. doi: 10.1007/BF01350072. [DOI] [PubMed] [Google Scholar]
- 23.Brahic CJ, Kelley DB. J Comp Neurol. 2003;464:115–130. doi: 10.1002/cne.10772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Emerson SB, Boyd SK. Brain Behav Evol. 1999;53:187–197. doi: 10.1159/000006594. [DOI] [PubMed] [Google Scholar]
- 25.Pérez J, Cohen MA, Kelley DB. J Neurobiol. 1996;30:556–568. doi: 10.1002/(SICI)1097-4695(199608)30:4<556::AID-NEU10>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 26.Wu KH, Tobias ML, Kelley DB. Neuroendocrinology. 2001;74:22–32. doi: 10.1159/000054667. [DOI] [PubMed] [Google Scholar]
- 27.Saitou N, Nei M. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 28.Roth G, Westhoff G. Eur J Morphol. 1999;37:166–171. doi: 10.1076/ejom.37.2.166.4759. [DOI] [PubMed] [Google Scholar]
- 29.Simoni M, Gromoll J, Nieschlag E. Endocr Rev. 1997;18:739–773. doi: 10.1210/edrv.18.6.0320. [DOI] [PubMed] [Google Scholar]
- 30.Maugars G, Schmitz M. Gen Comp Endocrinol. 2006;149:108–117. doi: 10.1016/j.ygcen.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 31.Bluhm AP, Toledo RA, Mesquita FM, Pimenta MT, Fernandes FM, Ribela MT, Lazari MF. Gen Comp Endocrinol. 2004;137:300–311. doi: 10.1016/j.ygcen.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 32.You S, Bridgham JT, Foster DN, Johnson AL. Biol Reprod. 1996;55:1055–1062. doi: 10.1095/biolreprod55.5.1055. [DOI] [PubMed] [Google Scholar]
- 33.Russell DL, Doyle KM, Gonzales-Robayna I, Pipaon C, Richards JS. Mol Endocrinol. 2003;17:520–533. doi: 10.1210/me.2002-0066. [DOI] [PubMed] [Google Scholar]
- 34.Mello CV, Ribeiro S. J Comp Neurol. 1998;393:426–438. doi: 10.1002/(sici)1096-9861(19980420)393:4<426::aid-cne3>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 35.Petrak L, Harris K, Kirov S. J Comp Neurol. 2005;484:183–190. doi: 10.1002/cne.20468. [DOI] [PubMed] [Google Scholar]
- 36.Lin C, Lin P, Chen Y, Lin P, Chen I, Lu K, Chang Y, Tsai M. Exp Neurol. 2005;194:384–392. doi: 10.1016/j.expneurol.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 37.Dodd JM. In: Marshall's Physiology of Reproduction. Parkes AS, editor. Vol 1. London: Longmans Green; 1960. pp. 417–582. [Google Scholar]
- 38.Kelley DB, Pfaff DW. Horm Behav. 1976;7:159–182. doi: 10.1016/0018-506x(76)90045-3. [DOI] [PubMed] [Google Scholar]
- 39.Chang CY, Witschi E. Proc Soc Exp Biol Med. 1956;93:140–144. doi: 10.3181/00379727-93-22688. [DOI] [PubMed] [Google Scholar]
- 40.Rastogi RK, Meyer DL, Pinelli C, Fiorentino M, D'Aniello B. Gen Comp Endocrinol. 1998;112:330–345. doi: 10.1006/gcen.1998.7144. [DOI] [PubMed] [Google Scholar]
- 41.Sanghera M, Harris MC, Morgan RA. Brain Res. 1978;140:63–74. doi: 10.1016/0006-8993(78)90238-x. [DOI] [PubMed] [Google Scholar]
- 42.Terasawa E, Whitmoyer DI, Sawyer CH. Am J Physiol. 1969;217:1119–1126. doi: 10.1152/ajplegacy.1969.217.4.1119. [DOI] [PubMed] [Google Scholar]
- 43.Al-Hader AA, Lei ZM, Rao CV. Biol Reprod. 1997;56:1071–1076. doi: 10.1095/biolreprod56.5.1071. [DOI] [PubMed] [Google Scholar]
- 44.Goodson JL, Bass AH. Brain Res Brain Res Rev. 2001;35:246–265. doi: 10.1016/s0165-0173(01)00043-1. [DOI] [PubMed] [Google Scholar]
- 45.Weintraub AS, Kelley DB, Bockman RS. Horm Behav. 1985;19:386–399. doi: 10.1016/0018-506x(85)90036-4. [DOI] [PubMed] [Google Scholar]
- 46.Schmidt RS. Horm Behav. 1993;27:82–91. doi: 10.1006/hbeh.1993.1006. [DOI] [PubMed] [Google Scholar]
- 47.Schlinger BA, London SE. J Exp Zoolog A Comp Exp Biol. 2006;305A:743–748. doi: 10.1002/jez.a.303. [DOI] [PubMed] [Google Scholar]
- 48.Takase M, Ukena K, Yamazaki T, Kominami S, Tsutsui K. Endocrinology. 1999;140:1936–1944. doi: 10.1210/endo.140.4.6641. [DOI] [PubMed] [Google Scholar]
- 49.Mensah-Nyagan AM, Feuilloley M, Do-Rego JL, Marcual A, Lange C, Tonon MC, Pelletier G, Vaudry H. Proc Natl Acad Sci USA. 1996;93:1423–1428. doi: 10.1073/pnas.93.4.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maness LM, Banks WA, Kastin AJ. Brain Res. 2000;873:165–167. doi: 10.1016/s0006-8993(00)02520-8. [DOI] [PubMed] [Google Scholar]
- 51.Lupien SB, Bluhm EJ, Ishii DN. J Neurosci Res. 2003;74:512–523. doi: 10.1002/jnr.10791. [DOI] [PubMed] [Google Scholar]
- 52.Pan W, Yu Y, Cain CM, Nyberg F, Couraud PO, Kastin AJ. Endocrinology. 2005;146:4898–4904. doi: 10.1210/en.2005-0587. [DOI] [PubMed] [Google Scholar]
- 53.Landgraf R, Neumann ID. Front Neuroendocrinol. 2004;25:150–176. doi: 10.1016/j.yfrne.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 54.Bosch OJ, Meddle SL, Beiderbeck DI, Douglas AJ, Neumann ID. J Neurosci. 2005;25:6807–6815. doi: 10.1523/JNEUROSCI.1342-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zornik E, Kelley DB. J Comp. Neurol. 501:303–315. doi: 10.1002/cne.21145. [DOI] [PubMed] [Google Scholar]
- 56.Vignal C, Andru J, Mathevon N. Eur J Neurosci. 2005;22:949–955. doi: 10.1111/j.1460-9568.2005.04254.x. [DOI] [PubMed] [Google Scholar]
- 57.Brox A, Puelles L, Ferreiro B, Medina L. J Comp Neurol. 2004;474:562–577. doi: 10.1002/cne.20152. [DOI] [PubMed] [Google Scholar]
- 58.Marin O, Gonzalez A, Smeets WJ. J Comp Neurol. 1997;380:23–50. [PubMed] [Google Scholar]