Abstract
Infantile neuronal ceroid lipofuscinosis (INCL) is a pediatric neurodegenerative disease caused by mutations in the human CLN1 gene. CLN1 encodes palmitoyl–protein thioesterase 1 (PPT1), suggesting an important role for the regulation of palmitoylation in normal neuronal function. To further elucidate Ppt1 function, we performed a gain-of-function modifier screen in Drosophila using a collection of enhancer–promoter transgenic lines to suppress or enhance the degeneration produced by overexpression of Ppt1 in the adult visual system. Modifier genes identified in our screen connect Ppt1 function to synaptic vesicle cycling, endo-lysosomal trafficking, synaptic development, and activity-dependent remodeling of the synapse. Furthermore, several homologs of the modifying genes are known to be regulated by palmitoylation in other systems and may be in vivo substrates for Ppt1. Our results complement recent work on mouse Ppt1−/− cells that shows a reduction in synaptic vesicle pools in primary neuronal cultures and defects in endosomal trafficking in human fibroblasts. The pathways and processes implicated by our modifier loci shed light on the normal cellular function of Ppt1. A greater understanding of Ppt1 function in these cellular processes will provide valuable insight into the molecular etiology of the neuronal dysfunction underlying the disease.
THE neuronal ceroid lipofuscinoses (NCLs) are a set of predominantly recessive pediatric neurological diseases that cause neurodegeneration in the retina, cortex, and cerebellum, leading to symptoms that include loss of vision, motor dysfunction, intellectual decline, and seizures (Wisniewski et al. 2001). The NCLs have a worldwide occurrence of 0.1–7/100,000 births (Wisniewski 2005). A subset of a wider group of disorders, each NCL subtype is classified by its characteristic lysosomal inclusion pathology and age of onset. Genetic analysis of the NCLs has identified eight loci, CLN1–6, CLN8, and CTSD, each with a different age of onset (Wisniewski 2005; Siintola et al. 2006). Most patient cells display the signature inclusions and an accumulation of autofluorescent lipopigment although only neurons undergo degeneration (Kida et al. 2001; Wisniewski 2005). Infantile onset NCL (INCL), produced by loss of palmitoyl–protein thioesterase 1 (PPT1) function, is the earliest and most severe form of NCL with symptoms appearing as early as 6 months of age and culminating with death between 24 months and 53 years of age (Wisniewski 2005). Although primarily associated with INCL, mutations in Ppt1 can also give rise to late-infantile, juvenile, and adult-onset NCL (Mitchison et al. 1998; van Diggelen et al. 2001). This variation in age of onset is likely related to residual enzyme activity produced by the specific mutation(s) a patient carries (Wisniewski 2005). INCL is characterized at the cellular level by the presence of granular osmiophilic deposits (GRODs), the lysosomal accumulation of saposins A and D, and an almost complete loss of cortical neurons at the time of patient death (Wisniewski et al. 2001).
An open question in the understanding of NCLs and other lysosomal storage disorders is the identification of the underlying molecular cause of the disease phenotype. In particular, is the inclusion pathology the primary insult to the cell or do specific disease pathways, whether or not they lead to inclusions, underlie the physical symptoms and eventual neuronal cell death (reviewed in Futerman and van Meer 2004)? Recent work on several lysosomal storage disorders has shown that the storage phenotype may be secondary to more significant changes in cell physiology produced by the loss of a particular protein. This loss of protein function may have a primary effect on specific cellular processes or signal transduction mechanisms, leading directly or through secondary effects to the observed disease pathology (reviewed in Futerman and van Meer 2004). Work on NCLs, including INCL, in mice, sheep, and humans has described the amount and regional location of cellular storage material in affected brains and compared it to the progressive cellular pathology seen in each subtype (Bible et al. 2004; Mitchison et al. 2004). These analyses suggest no clear connection between the amount of storage material and the observed neurodegeneration in these systems, leading to the hypothesis that it is the disruption of certain cellular processes or signaling pathways in the patient's neuronal cells that produces dysfunction and degeneration (Oswald et al. 2005). Identifying the earliest cellular insults in INCL patient neurons requires the characterization of the normal cellular function of PPT1 so that a more targeted approach to understanding the disease process can be undertaken.
The identification of human PPT1 as the gene mutated in INCL indicates that there is a significant role for the regulation of palmitoylation in normal neuronal function (Vesa et al. 1995). Palmitoylation, a dynamic post-translational modification, is critical for the cellular localization and modulation of many signaling proteins (Milligan et al. 1995; Smotrys and Linder 2004). Consistent with an important role in neurons, several proteins integral to the development and regulation of the synapse are known to be palmitoylated (El-Husseini and Bredt 2002). As expected from a lysosomal enzyme, histochemical and biochemical analysis of PPT1 has demonstrated localization within the endo-lysosomal compartment in neuronal and non-neuronal cell lines (Camp and Hoffman 1993; Hellensten et al. 1996; Verkruyse and Hoffmann 1996; Cho and Dawson 2000; Virmani et al. 2005). In cultured neuronal cells, PPT1 has also been found associated with lipid raft membrane microdomains and synaptosomes, suggesting a neuron-specific function for the protein that may be important in the development of the disease (Lehtovirta et al. 2001; Ahtiainen et al. 2003; Goswami et al. 2005). PPT1's importance for neuronal function is underscored by the expression of Ppt1 mRNA and protein in the retina and brain in a regulated pattern that coincides with major developmental changes in the nervous system (Isosomppi et al. 1999; Suopanki et al. 1999a,b; Zhang et al. 1999). Finally, a model of excito-toxicity in the rat brain confirmed a presynaptic localization for the protein and suggests that PPT1 may be neuro-protective during an excito-toxic event (Suopanki et al. 2002).
Recently, progress has been made toward understanding the primary cellular defects underlying INCL disease pathology. Expression of PPT1 has been shown to modulate apoptotic cell death mediated by the activation of the Ras–AKT pathway and neutral sphinogmyelinase 2 in several different neural-derived cell lines (Cho and Dawson 2000; Goswami et al. 2005). Work in cultured Ppt1−/− mouse neuronal cells demonstrated a reduction in synaptic vesicle pool size in the absence of large amounts of storage material, suggesting that synaptic abnormalities may contribute to the early progression of INCL (Virmani et al. 2005). Changes in endo-lysosomal function have also been associated with the accumulation of saposin A and D, the main protein component of INCL storage material, in cultured Ppt1−/− fibroblasts (Ahtiainen et al. 2006). Finally, Ppt1 was identified in a cell-based screen as a modifier of amyloid precursor protein shedding, a process that is regulated in part by endocytosis (Schobel et al. 2006). A picture is now emerging on the basis of these results whereby Ppt1 may impact the efficient synaptic function of neurons by regulating specific signaling pathways and modulating endocytosis.
The analysis of the Drosophila homologs of the human cathepsin D and PPT1 genes suggests that the fruit fly is a valuable model system for studying lysosomal storage disorders and the NCLs in particular. Mutations in the fly homolog of the lysosomal aspartyl protease cathepsin D produce the characteristic autoflourescent storage material and GRODs associated with human cases of congenital NCL as well as a low level of age-dependent neurodegeneration in the adult Drosophila brain (Myllykangas et al. 2005). Ppt1− flies are viable, have a reduced life span, and show a central nervous system (CNS)-specific accumulation of autoflourescent storage material. The cytoplasmic inclusions, however, are different in morphology in contrast to the granular osmiophilic deposits typical of INCL patients (Hickey et al. 2006). These two mutants suggest an evolutionary conservation of function in the fly that make it a powerful model for understanding the molecular etiology of human NCL phenotypes (Myllykangas et al. 2005; Hickey et al. 2006). Previously, we showed that the targeted overexpression of Ppt1 in the developing Drosophila visual system leads to the loss of cells, including neurons, through apoptotic cell death both early in eye development and after ommatidial differentiation has finished, yielding black ommatidial spots (Korey and MacDonald 2003). Taken together, the fly models of Ppt1 function indicate the importance of the proper regulation of enzyme activity for normal cellular function.
The development of both loss-of-function and gain-of-function models for Ppt1 in Drosophila is critical for the future examination of the cellular basis of neuronal dysfunction and degeneration in INCL patients. An advantage of Drosophila and other small eukaryotic model systems lies in the ability to perform large-scale second-site genetic modifier screens (Muquit and Feany 2002; Phillips et al. 2006). This approach allows for the unbiased identification of specific genes and cellular pathways that are relevant to protein function and disease progression. To identify in vivo substrates and signaling pathways that are regulated by Ppt1 activity, we have here performed an F1 dominant gain-of-function modifier screen using our Ppt1 overexpression model and a collection of enhancer–promoter lines (Rorth et al. 1998). The modifiers identified in this screen further support a role for Ppt1 in the regulation of synaptic vesicle endocytosis. Our results also suggest that Ppt1 modulates the activity of several pathways known to play a role in synaptic development either directly through its depalmitoylation activity or indirectly through effects on general endocytic mechanisms.
MATERIALS AND METHODS
Drosophila stocks and fly husbandry:
Flies were raised on standard media and all crosses were carried out at 25°. The enhancer–promoter (EP) stocks and other stocks were obtained from both the Szeged and Bloomington Stock Centers. The enhancer–yellow (EY) stocks were obtained from the Bloomington Stock Center. The UAS:Ppt1 transgenic lines were previously described in Korey and MacDonald (2003). All other lines used in this study were obtained from Bloomington except for the following: UAS:mysβPS (Beumer et al. 1999), fasIIeb112 (Grenningloh et al. 1991), UAS:fasII (Holmes and Heilig 1999), fafBX4, and UAS:faf (Huang et al. 1995), endoAΔ4 and UAS:endoA (Verstreken et al. 2003), UAS:dFos (Eresh et al. 1997), UAS:sax (Yu et al. 2004), UAS:syt1 (Littleton et al. 1999), and UAS:stonedA and UAS:stonedB (Estes et al. 2003).
Screen genetics:
EP and EY genetic modifiers of Ppt1-induced degeneration were identified by their ability to modify GMR:Gal4, UAS:Ppt18.1/+; UAS:Ppt12.1/+ adult flies. All screen crosses were done blinded, with neither the EP or EY line number nor with the molecular location of the insertion known to the screeners. The EP/EY lines were unblinded after the secondary screen. Nonspecific modification due to the effects on the Gal4/upstream activating sequence (UAS) system were eliminated by their ability to also modify two unrelated phenotypes produced by UAS:TauV337M/+; GMR:Gal4/+ adults (Whitmann et al. 2001; Shulman and Feany 2003) and GMR:Gal4/+ adults at 29° (Fernandez-Funez et al. 2000). The UAS:TauV337M line expresses a mutant Val337 → Met (V337M) human Tau protein that is associated with early onset, familial dementia (Wittmann et al. 2001).
Scanning electron microscopy:
Newly eclosed adults of the specified genotypes were collected and aged for several days in a yeast-free food vial. These flies were then subjected to a series of ethanol dehydration steps. Initially placed in 25% ethanol, they were moved to 50% ethanol after a 12-hr incubation time. This process continued through the following dilutions: 50, 75, 95, and 2× 100% ethanol. They remained 100% ethanol until critical point drying and sputter coating for scanning electron microscopy at the Northeastern Electron Microscopy facility.
In situ hybridization:
EP/EY lines that were unable to be confirmed using UAS lines were further analyzed using in situ hybridization. Each line was crossed to Gal4 driver lines and the resulting trans-heterozygote embryos or larvae were processed for in situ hybridization as previously described (Van Vactor and Kopczynski 1999). cDNA clones of modifier genes were used for production of sense and antisense probes. The clones were identified with the Berkeley Drosophila Genome Project database and obtained from the Drosophila Genomic Research Center.
Ppt1 enzyme assay:
Ppt1 enzyme activity levels were assayed as described in Hickey et al. (2006). The heads of single flies of the correct genotype were dissected and placed in individual wells of a 96-well plate on ice. Each well was then filled with 20 μl of ddH20 and 10 μl of substrate (0.375 mg/ml 4MU-6S-palm-β-Glc substrate (Moscerdam Substrates); 0.2 m Na phosphate/0.1 m citrate, pH 4.0; 15 mm DTT; 0.09% BSA; and 5 units/ml β-glucosidase). The individual heads were homogenized in the substrate buffer using a 96-well plate homogenizer (Burkard Scientific, UK). The homogenate was incubated for 2 hr at 30°. The reaction was stopped by adding 100 μl of stop buffer (0.5 m NaHCO3/Na2CO3, pH 10.7, 0.025% Triton X-100) and then the amount of fluorescence at 460 nm was read on a Perkin-Elmer (Norwalk, CT) HTS7000 BioAssay Reader. All samples were done in triplicate.
RESULTS
Ppt1 dominant modifier screen:
Previously we have shown that overexpressed Drosophila Ppt1 has a degenerative effect on the developing adult visual system (Korey and MacDonald 2003). This effect is dose dependent and requires a catalytically active Ppt1 enzyme. The overexpression phenotype was generated with the Gal4/UAS system that permits the expression of a gene in a specific tissue or during a particular developmental stage (Brand and Perrimon 1993). We found that GMR:Gal4-directed expression of a UAS:Ppt1 transgene in the developing eye led to the loss of cells, including neurons, through apoptotic cell death both early in eye development and also after ommatidial differentiation had finished, yielding black ommatidial spots (Korey and MacDonald 2003). Finally, the Ppt1 overexpression phenotype is suppressed by coexpression of a UAS:Ppt1-RNAi transgene, indicating that the phenotype is specific to Ppt1 function (Hickey et al. 2006; data not shown).
This degenerative phenotype provides a useful assay to identify other genetic loci that may modify this phenotype and thus elucidate the cellular role of Ppt1. We performed an F1 dominant gain-of-function modifier screen using a subset of the EP and EY collections available from the Bloomington and Szeged Drosophila stock centers (Rorth et al. 1998). EP/EY lines have a UAS-containing transposable P-element randomly inserted within the genome. If the element is within the 5′ untranslated region or close to the transcriptional start site of an open reading frame, it can direct the expression of this gene in a Gal4-dependent fashion. We combined our previous GMR:Gal4; UAS:Ppt1 phenotype with the EP/EY lines to identify a collection of genes that could modify the Ppt1-induced degeneration when coexpressed.
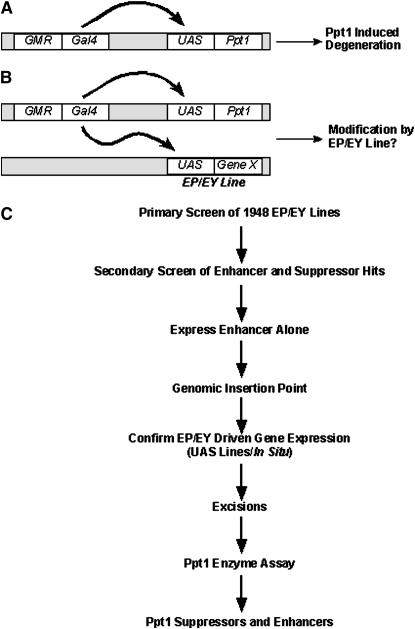
We screened 1948 EP/EY lines using the GMR:Gal4; UAS:Ppt1 overexpression system (Figure 1, A and B). Progeny from crosses between GMR:Gal4; UAS:Ppt1 and each EP/EY line were compared to control flies expressing Ppt1 alone to determine if the unknown gene being coexpressed enhanced or suppressed the degenerative effect. Suppressors showed a restoration of the outer ommatidial surface of the eye and an increased surface area, while enhancers further reduced the size of the eye with a concomitant loss of ommatidial structure on the eye surface.
Figure 1.—
Gain-of-function modifier screen. (A) Ppt1 was overexpressed using the Gal4/UAS system. Transcription factors binding to the GMR promoter initiate transcription of Gal4 that in turn binds to the upstream activating sequence, allowing the synthesis of Ppt1. (B) When EP/EY lines were crossed to flies diagrammed in A, Gal4 turned on a random gene driven by the EP/EY transgene as well as Ppt1. Progeny in the F1 were screened to determine if the unknown gene being coexpressed with Ppt1 enhances or suppresses the degeneration produced by Ppt1 activity. (C) A schematic of the secondary screening procedures used to finalize the genetic modifiers of Ppt1 listed in Table 1.
After the primary genetic screen, putative enhancers and suppressors were put through a series of elimination criteria to produce a final list of modifiers (Figure 1C). First, all identified modifier lines went through a secondary screen to confirm the repeatability of the enhancer or suppressor effect. Those that did not yield the result obtained in the primary screen were eliminated. Confirmed enhancers were then crossed to GMR:Gal4 alone to determine if the lines produced eye defects on their own. A rough eye indicated that the EP/EY line enhancement might be due to a nonspecific additive effect of the expression of both proteins rather than an enhancement specific to Ppt1 function. Thus these lines were also eliminated.
The genomic insertion point of the transgene was then checked using the resources available at NCBI to determine if the EP/EY element was inserted in the correct position for producing expression of the associated open reading frame. Those that were inserted within the gene but past the start codon, and therefore were likely to produce a truncated form of the protein, were eliminated. Transgene insertions >1000 bp away from an open reading frame and those in a region with no identifiable open reading frames were also eliminated. Modifier lines advanced to further screening if the EP/EY element was inserted within 500 bp of the gene, before the start codon, and in the correct orientation for gene expression.
Some of the modifiers could affect the Gal4/UAS system employed in the screen rather than Ppt1's cellular activity. To account for this possibility, we crossed all of the remaining EP/EY lines to two unrelated Gal4-induced rough-eye phenotypes: GMR:Gal4 alone and GMR:Gal4; UAS:TauV337M. Due to high levels of Gal4 expression, GMR:Gal4 flies develop a rough eye when grown at 29° (Fernandez-Funez et al. 2000). GMR-driven UAS:TauV337M is a previously published model for tauopathies in Drosophila (Shulman and Feany 2003). If a candidate Ppt1 modifier produced genetic modification in the progeny of both of these crosses that was similar to that which we observed in our original screen, we assumed that the EP/EY line might have a generalized effect on the driver system, and these lines were eliminated as well.
To control for the effects of genetic background, excisions of the EP/EY element were produced for each modifier line. Excision lines were crossed to the original GMR:Gal4; UAS:Ppt1 line to determine if the modifying effect was retained. Those lines that retained the suppressor or enhancer effect in the absence of the EP/EY insertion likely do so because of genetic alterations other than the presence of the transgene and were eliminated from consideration. Finally, the remaining EP/EY modifier genes were further characterized to determine whether the associated gene was being expressed. For a subset of lines (7/20), we were able to confirm expression with available published UAS lines that are known to express a specific gene. The expression of the remaining EP/EY lines (13/20) was confirmed by driving the associated locus with a tissue-specific Gal4 line and visualizing mRNA production by in situ hybridization.
Finally, we used a fluorogenic assay designed for the diagnosis of INCL in humans to confirm that the suppression or enhancement observed in these modifier lines was not due to significantly decreased or increased levels of Ppt1 (van Diggelen et al. 1999). Since the expression of Ppt1 and the EP/EY modifier lines was under the control of GMR:Gal4 in the developing adult visual system, we used a modified version of the assay in which single adult heads of the appropriate genotypes were dissected and homogenized in the presence of the 4-methylumbelliferyl-6-thiopalmitoyl-β-d-glucoside substrate (Hickey et al. 2006). While the assay does not directly assay for total Ppt1 protein concentration, enzyme activity provides a reliable proxy for the determination of major changes in enzyme function. We found that none of the 20 modifier lines significantly changed the levels of Ppt1 activity compared to the control GMR:Gal4; UAS:Ppt1 line used in the screen (data not shown). Of the original 1948 lines, 10 enhancers and 10 suppressors of the Ppt1 eye phenotype survived our stringent prioritization criteria. These are described in Table 1 with the accompanying functional information and verification procedures.
TABLE 1.
Genetic modifiers of Ppt1-induced degeneration
| Gene | Mammalian homolog | Line | Insertion | Modificationa | Verificationb | Loss-of-function allelesc |
|---|---|---|---|---|---|---|
| Endocytosis/trafficking | ||||||
| Endophilin A | Endophilin A | EP(3)464 | 91D4 | Enh | UAS | endoAΔ4/NE |
| CG14709 | Multidrug resistance-associated protein | EP(3)430 | 86E11 | Enh | In situ | NA |
| Blue cheese | ALFY | EY(3)2503 | 26A6 | Enh | In situ | NA |
| CG5991 | Phosphatidylserine decarboxylase | EY(3)3559 | 95D5 | Enh | In situ | NA |
| CG32138 | Formin-like 2 | EY(3)3931 | 70D1 | Enh | In situ | NA |
| Ubiquitination | ||||||
| Fat facets | Ubiquitin protease 9 | EY(3)2018 | 100E1 | Enh | UAS | fafBX4/NE |
| UbcE2H | Ubc-E2H | EP(X)1303 | 7D6 | Sup | In situ | NA |
| CG7023 | Ubiquitin protease 12-like | EY(3)249 | 94C4-6 | Sup | In situ | NA |
| Cell adhesion | ||||||
| Myospheroid | β-integrin | EP(X)1033 | 7D3-5 | Enh | UAS | mys1/NE |
| Fasciclin 2 | NCAM2 | EP(X)1462 | 4B1-3 | Sup | UAS | fasIIeb112/NE |
| Miniature | Novel | EP(X)406 | 10D8 | Sup | In situ | m1/NE |
| Signaling pathways | ||||||
| Misshapen | Ste20-related kinase | EP(3)549 | 62E6-7 | Enh | UAS | msn102/NE |
| Kayak | FosB | EY(3)283 | 99C2 | Sup | UAS | kaysro-1/NE |
| Saxophone | TGF-β receptor type I | EY(2)4377 | 43E18 | Sup | UAS | sax5/NE |
| Mesr4 | Novel | EP(2)386 | 54C3-7 | Sup | In situ | NA |
| Fs(1)N | Novel | EP(X)1336 | 1F01-02 | Sup | In situ | fs(1)N1/E |
| Miscellaneous | ||||||
| Hsc70-3 | BiP chaperone | EP(X)1507 | 10E3-4 | Sup | In situ | NA |
| CG18177 | N-acetyltransferase | EP(3)3301 | 67C5 | Enh | In situ | NA |
| CG3654 | Novel | EY(3)142 | 67B9 | Enh | In situ | NA |
| CG5859 | Integrator complex subunit 8 | EP(2)2090 | 53D13 | Sup | In situ | NA |
Enh, enhancer; Sup, suppressor.
UAS, published line; In situ, In situ hybridization.
Loss-of-function alleles used for each gene; NE, no effect; E, enhance; NA, no specific alleles available.
Endocytosis and endosomal trafficking:
A major class of modifiers of our Ppt1-induced degenerative phenotype is associated with endocytic mechanisms. EP(3)464-induced expression of endophilin A (endoA) was found to be an enhancer of the Ppt1 phenotype (Figure 2E). EndoA, the fly homolog of the SH3-domain-containing protein endophilin, plays a role in synaptic vesicle endocytosis and recycling. We confirmed the enhancement by overexpression of endoA using a UAS-endoA transgene (Table 1).
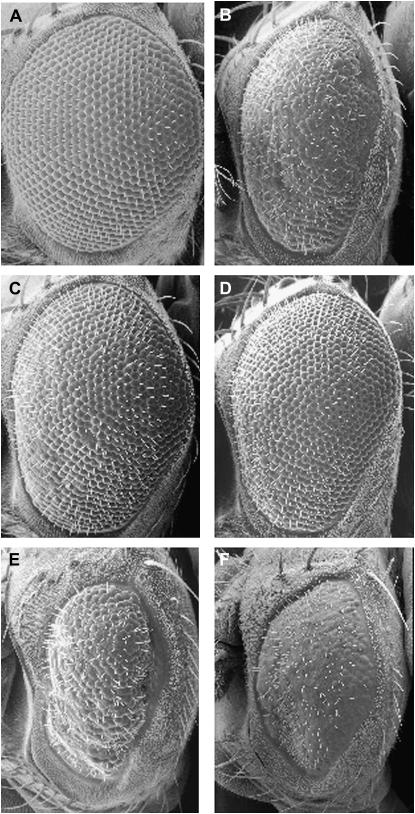
Figure 2.—
Enhancers and suppressors of Ppt1-induced degeneration scanning electron images of adult eyes (×200). (A–F) SEM images showing the external surface of control and identified Ppt1 modifiers. (A) UAS:Ppt18.1/CyO. (B) GMR:Gal4, UAS:Ppt18.1/+; UAS:Ppt12.1/+. (C) EP(X)1462/+; GMR:Gal4, UAS:Ppt18.1/+; UAS:Ppt12.1/+. (D) GMR:Gal4, UAS: Ppt18.1/+; UAS:Ppt12.1/EY(3)283. (E) GMR:Gal4, UAS:Ppt18.1/+; UAS:Ppt12.1/EP(3)464. (F) GMR:Gal4, UAS:Ppt18.1/+; UAS:Ppt12.1/EP(3)549.
The enhancer EY(3)2503 is predicted to drive the expression of blue cheese (bchs). Bchs is localized to CNS neurons/axons, and loss-of-function mutants in Drosophila show progressive neural degeneration (Finley et al. 2003). The presence of BEACH and FYVE domains in Bchs suggests a role for Bchs in endo-lysosomal maturation and trafficking in neurons (Finley et al. 2003). We confirmed the overexpression of bchs by EY(3)2503 by mRNA in situ hybridization (Table 1).
Hsc70-3, when driven by EP(X)1507, was found to be a suppressor. Hsc70-3 is the fly homolog of BiP, a chaperone for secretory and membrane proteins in endoplasmic reticulum. This protein is upregulated in Aplysia models of long-term memory and may be required for the efficient trafficking of new proteins in support of activity-dependent synaptic changes (Kuhl et al. 1992).
We also identified two genes involved with lipid metabolism that may impact endocytic mechanisms. EY(3)3559, an enhancer of the Ppt1 eye phenotype, is predicted to direct the expression of CG5991, a gene that encodes the enzyme phosphatidylserine decarboxylase, which converts phosphatidylserine to phosphatidylethanolamine in the mitochondrial inner membrane. Overexpression of CG14709, a protein with homology to ATP-binding cassette/multi-drug-resistance-associated proteins, by EP(3)430 was identified as an enhancer. This family of integral membrane proteins is involved in the transport of various substrates across the lipid bilayer.
Since several modifiers from our screen, particularly those associated with neuronal function, implicated Ppt1 in endocytic processes, we obtained published UAS lines that expressed proteins known to play a role in synaptic vesicle endocytosis. For example, synaptotagmin is a calcium sensor for synaptic vesicle fusion and facilitates endocytosis (Yoshihara and Montana 2004) while stoned A and B are atypical adaptor proteins that bind to and facilitate synaptotagmin recycling (Fergestad et al. 1999; Phillips et al. 2000). We examined UAS lines for synaptotagmin (syt1), stoned A (stnA), and stoned B (stnB) for their ability to modify the Ppt1 phenotype when coexpressed using GMR-Gal4. We found that both syt1 and stnA suppressed eye degeneration (Figure 3, B and C) while stnB had no effect as compared to expression of Ppt1 alone (data not shown). In contrast to genes involved in endocytosis, we found that a UAS line that expresses the ATPase Hsc70-4, a protein involved in synaptic vesicle exocytosis (Bronk et al. 2001), had no modifying effect (data not shown).
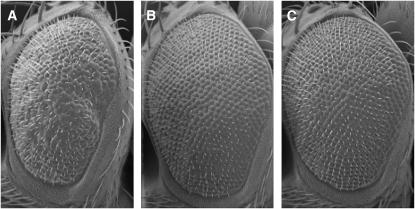
Figure 3.—
Ppt1-induced degeneration is modified by genes involved in endocytosis scanning electron images of adult eyes (×200). (A–C) SEM images showing the external surface of control and identified Ppt1 modifiers. (A) GMR:Gal4,UAS:Ppt18.1/+; UAS:Ppt12.1/+. (B) UAS:syt1/+; GMR:Gal4,UAS:Ppt18.1/+; UAS:Ppt12.1/+. (C) GMR:Gal4,UAS:Ppt18.1/UAS:stnA; UAS:dPpt12.1/+.
Ubiquitination:
In addition, we identified two proteases and one conjugating enzyme associated with the process of protein ubiquitination. The enhancer EY(3)2018 drives the expression of the gene fat facets (faf), the fly homolog of ubiquitin-specific protease 9. In Drosophila, faf is important for eye differentiation and synaptic development at the larval neuromuscular junction (Fischer and Overstreet 2002). We confirmed the enhancement produced by EY(3)2108 using a UAS:faf transgene (Table 1). The other predicted protease found to be a suppressor when driven by EY(3)249 is CG7023, the fly homolog of human ubiquitin-specific protease 12-like and mouse Ubh1. Finally, we identified EP(X)1303 as a suppressor. This line is predicted to drive the expression of the fly homolog of the ubiquitin-conjugating enzyme, E2H.
Cell adhesion:
Our modifier collection also revealed several genes that control cell adhesion. The β-integrin homolog myospheroid (mys), driven by EP(X)1033, was identified as an enhancer of Ppt1-induced degeneration. In Drosophila, mys is involved in several cell adhesion-dependent processes during development, including axon guidance and activity-dependent synaptic development (Hoang and Chiba 1998; Beumer et al. 1999; Rohrbough et al. 2000). We confirmed mys-specific modification of Ppt1 degeneration using a UAS:mys transgene (Table 1; data not shown).
Expression of the neural cell adhesion molecule fasciclin II (fasII) by EP(X)1462 was found to be suppressor (Figure 2C). FasII function is critical for axon path finding, synaptogenesis, and synaptic plasticity in the fly. We confirmed suppression of the eye phenotype using a UAS:fasII transgene (Table 1; data not shown). In addition, EP(X)406-driven expression of the novel gene miniature was a suppressor of the Ppt1 phenotype. We confirmed overexpression of miniature by in situ hybridization (Table 1).
Signaling:
Components of known intracellular signaling pathways were also recovered in our modifier screen. EP(3)549, an enhancer of Ppt1, expresses the Ste-20-related serine/threonine kinase misshapen (msn) (Figure 2F). We confirmed the enhancement by overexpression of misshapen using a UAS:msn transgene (Table 1; data not shown). Recent work has identified msn as a participant in the regulation of dorsal closure through the activation of Jun kinase (JNK) signaling and retinal axon guidance (Dan et al. 2001).
Kayak, driven by EY(3)283, was identified as a suppressor of Ppt1-induced degeneration (Figure 2D). Also known as dFos, kayak encodes a transcription factor that is part of the AP1 transcriptional activation complex with dJun (Goberdhan and Wilson 1998). AP1-stimulated transcription is activated by JNK and is involved in both embryonic dorsal closure and synaptic plasticity (Goberdhan and Wilson 1998; Sanyal et al. 2002). The suppression of the eye phenotype was confirmed using a UAS:dfos transgene (Table 1; data not shown).
Saxophone (sax), the fly homolog of a TGF-β type I receptor, was also identified as a suppressor. EY(2)4377-driven expression of sax was confirmed using a UAS:sax transgene (Table 1; data not shown). TGF-β signaling is known to play a role in multiple processes during Drosophila development, including the formation of the neuromuscular junction (Marques 2005).
EY(3)3931, an enhancer of the Ppt1 eye phenotype, expresses CG32138. CG32138, the fly homolog of the formin-like 2 protein, contains a formin homology-2, formin homology-3, and GTPase-binding domains. Formin-domain-containing proteins function in actin fiber formation and play a role in a diverse array of cellular processes, including stress fiber formation, cytokinesis, cell motility, and endocytosis (Faix and Grosse 2006).
EP(X)1336-driven expression of the novel protein fs(1)Nasrat [fs(1)N] suppressed the Ppt1 eye phenotype and was confirmed by in situ hybridization. We also found that the loss-of-function allele, fs(1)N1, produces a dominant enhancement of the Ppt1 degeneration (Table 1; data not shown). Finally, we identified EP(2)386 as an enhancer of the Ppt1 eye phenotype. EP(2)386 drives the expression of mesr4, a transcription factor identified in a RAS1 genetic modifier screen, and expression was confirmed by in situ hybridization (Huang and Rubin, 2000).
Miscellaneous modifiers:
We identified CG18177, driven by EP(3)3301, as an enhancer of Ppt1-induced degeneration. CG18177 encodes a novel protein with predicted n-acetyltransferase activity. The enhancer EY(3)142 drives the expression of CG3654, a novel putative transcription factor. CG5859, the fly homolog of integrator complex subunit 8, suppresses Ppt1-induced degeneration when expressed under the control of EP(2)2090.
DISCUSSION
We have presented an F1 gain-of-function genetic modifier screen that has identified 10 enhancers and 10 suppressors of degeneration induced by the expression of Ppt1 in the developing adult visual system. The use of a random collection of EP and EY lines spanning the X, second, and third chromosomes allowed for an unbiased identification of a characterized set of Drosophila Ppt1 modifier loci. These begin to place Ppt1 function in its cellular context and provide candidate in vivo substrates for the enzyme. Recent work on several different systems has demonstrated an involvement of Ppt1 in cellular processes associated with endocytosis and endo-lysosomal trafficking. The polarity and shape of neurons require efficient endosomal processes to maintain cellular structure, transport cellular metabolites, and allow rapid communication between the axon/dendrites and the cell soma (Nixon and Cataldo 1995; Nixon 2005). Changes in these pathways might lead to the symptoms associated with INCL due to the importance of the endosomal system for neuronal function (Nixon and Cataldo 1995; Nixon 2005). Furthermore, neurons also make use of rapid exo- and endocytic mechanisms to precisely control synaptic transmission between pre- and postsynaptic regions.
The initial identification of endoA as an enhancer of Ppt1-induced degeneration led us to consider a role for Ppt1 in endocytosis. EndoA, a cytoplasmic protein essential for synaptic vesicle endocytosis, contains an SH3 domain that binds to other endocytic proteins such as synaptojanin and dynamin and a lysophosphatidic acid acyl transferase domain that plays a role in altering membrane curvature (Reutens and Begley 2002). The identification of both EndoA homologs and Ppt1 in a cell-based cDNA screen for genes that altered the surface shedding of amyloid precursor protein (APP) suggests a connection between their functions (Schobel et al. 2006). The expression of the mammalian homologs endophilin-A1 and endophilin-A3 was shown to activate APP shedding in this assay through the inhibition of endocytosis (Schobel et al. 2006). These findings indicate that the overexpression of Ppt1 in the Drosophila retina may lead to degeneration by negatively impacting synaptic vesicle and general endocytosis. Similar to endoA, our other modifiers implicate Ppt1 function both in the regulation of synaptic vesicle endocytosis and in the modulation of signaling pathways important for neuronal function through the regulation of endocytosis (Figure 4A).
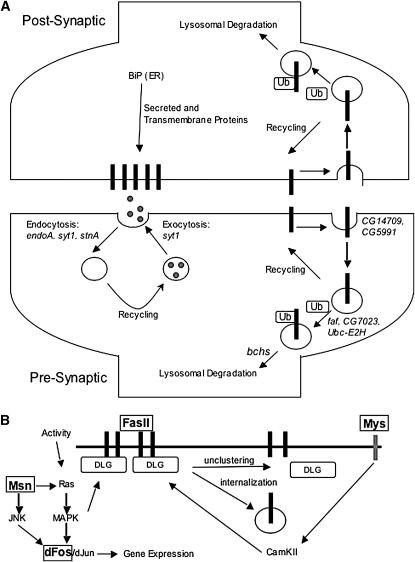
Figure 4.—
A model of Ppt1-related cellular processes. (A) An illustration of pre- and postsynaptic compartments showing synaptic vesicle cycling and endocytic pathways. The modifiers identified in the screen are placed in the model on the basis of the hypothesis that Ppt1 is connected to these activities. (B) A representation of pathways known to play a role in neuromuscular junction development and activity-dependent remodeling of the synapse. The genes identified as modifiers in our screen are boxed and in boldface type.
Synaptic vesicle cycling:
In addition to the identification of endophilin in the primary screen, the demonstration that the expression of both syt1 and stnA suppressed the Ppt1 eye phenotype indicates a connection between Ppt1 function and synaptic vesicle endocytosis. As is observed in cultured Ppt1−/− neurons from mice, both syt1 and stn mutants in Drosophila showed decreased synaptic vesicle density at the larval neuromuscular junction (Fergestad et al. 1999). Work on the fly has demonstrated that Stn proteins not only bind to Syt1, but also are necessary for its maintenance at the synapse (Fergestad et al. 1999; Phillips et al. 2000). Furthermore, overexpression of syt1 in a stn mutant background rescues the lethality and endocytosis defects associated with loss of stn (Phillips et al. 2000). The suppression of the Ppt1-induced degeneration by expression of syt1 or stnA links Ppt1 expression with the inhibition of synaptic vesicle endocytosis. Palmitoylation of human Syt1 has been shown to be critical for the sorting of the protein to presynaptic vesicle pools, and mutant Syt1 proteins that are not palmitoylated show an increase in general cell surface expression and a reduction in endocytosis (Kang et al. 2004). Although Syt1 in Drosophila has not been shown to be palmitoylated, the genetic interaction that we observe with Ppt1 indicates that Syt1 may be an in vivo substrate of Ppt1.
Signaling and endocytosis:
Our screen identified several genes that have been shown to play a role in endo-lysosomal trafficking or whose cellular functions suggest that they are likely to play a role in this process. These modifiers further support a role for Ppt1 in endocytic mechanisms that was originally indicated by defects observed in Ppt1−/− fibroblasts (Ahtiainen et al. 2006). We identified two deubiquitinating enzymes and an ubiquitin-conjugating enzyme suggesting a connection to ubiquitination, a signal that serves as a molecular tag for internalization of surface proteins and maturation of late endosomes into multi-vesicular bodies for the proteolysis of cellular proteins (Seto et al. 2002). Another modifier, the conserved BEACH- and FYVE-domain-containing protein Blue Cheese, is thought to play a role in trafficking through the endosomal pathway (Finley et al. 2003). Our identification of the enhancer CG32138, a formin-like protein, may also indicate a potential connection to endocytosis in light of recent work that has linked several formin-domain-containing proteins to endosome transport along actin fibers (Faix and Grosse 2006). Finally, both phosphatidylserine decarboxylase and CG14709 modulate lipids that are known to play an important role in regulating membrane curvature, vesicle trafficking, and cell signaling (van Meer and Sprong 2004; Vance and Steenbergen 2005).
A large body of work has demonstrated how cellular signaling is intimately connected to endocytic mechanisms (Seto et al. 2002). Endocytosis not only is a downregulator of cell surface receptors, but also is important for the activation of signaling, shaping morphogen gradients, and the proper spatial and temporal localization of signaling molecules (Seto et al. 2002). These functions of endocytic trafficking are on display in all cell types, including neurons where it controls the development and activity-dependent modulation of synapses. The identification of the TGF-β type I receptor sax as a suppressor in our screen suggests a connection between Ppt1 function and a pathway whose modulation by endocytosis is critical for the development and maintenance of the neuromuscular junction in Drosophila (Sweeney and Davis 2002; Dermaut et al. 2005; Marques 2005).
The control of cell adhesion at the neuromuscular junction is important for synapse growth during development and activity-dependent synapse remodeling. Many pathways that control these processes in Drosophila converge on the neural cell adhesion molecule (NCAM) homolog fasciclin II (fasII), the main integrator of these signals at the synapse (Figure 4B). The levels and localization of Fas II at the synapse help to define the degree of synapse stability such that a reduction in FasII adhesion permits the expansion of the synapse (Schuster et al. 1996a,b). Remodeling of the synapse is also controlled by an activity-dependent downregulation of FasII, possibly through an endocytic mechanism (Hoeffer et al. 2003). Studies in Aplysia revealed that during long-term facilitation, synapse elaboration is achieved through a MAPK-dependent endocytosis of the Aplysia NCAM (Bailey et al. 1992, 1997). In Drosophila, work from several labs has shown that activation of the Ras–MAPK pathway produces a similar downregulation of FasII, suggesting an evolutionary conservation of this synaptic plasticity mechanism (Koh et al. 2002; Hoeffer et al. 2003). Furthermore, MAPK activation causes the transcriptional upregulation of dFos which, with dJun, is part of the AP1 transcriptional activator complex that is involved in the activity-dependent activation of genes involved in long-term plasticity (Sanyal et al. 2002; Hoeffer et al. 2003). Finally, the endoplasmic reticulum chaperone protein BiP is upregulated during long-term facilitation in Aplysia (Kuhl et al. 1992). It has been proposed that increased expression of this protein is important for the rapid trafficking of the secreted and membrane proteins required for synaptic remodeling (Kuhl et al. 1992; Rubio and Wenthold 1999).
We have demonstrated that fasII, myospheroid (β-integrin), kayak (dFos), and Hsc70-3 (BiP) all modify the degeneration produced by Ppt1 expression. Furthermore, both misshapen and mesr4 were identified in a screen for modifiers of Ras1 function (Huang and Rubin 2000). In addition to the regulation of Ras signaling, the Ste20-related kinase Msn also activates JNK signaling during dorsal closure (Dan et al. 2001). Similar to the genetic interaction that we observe with sax, the identities of these modifiers strongly indicate that the overexpression of Ppt1 appears to have a targeted effect on a subset of pathways critical for the formation and remodeling of the synapse. In future work, it will be important to extend this work and examine the connection among Ppt1, trafficking, and signaling by directly assessing loss- and gain-of-function Ppt1 lines for defects in endocytosis using several well-characterized tissues such as the larval neuromuscular junction (NMJ) and garland cells. Since published work has shown that neural degeneration does not directly correlate with the amount of storage material, modification of the Ppt1 loss-of-function inclusion phenotype in the fly may not be relevant to the disease pathology (Oswald et al. 2005). Thus, the results of future NMJ and endocytosis analyses will provide crucial phenotypic resources for the further characterization of the relationships between our modifier loci and Ppt1 function.
Ppt1 substrates:
It is possible that some of the effects that we see are due to the misregulation of palmitoylation on specific substrate proteins. For example, the vertebrate NCAM homologs of fasII are known to be palmitoylated, providing a second membrane anchor for the protein that is important for NCAM lipid-raft localization (Little et al. 1998). Mutant NCAM proteins that cannot be palmitoylated fail to localize correctly, and consequently their signaling activity is reduced (Niethammer et al. 2002). In light of the localization of Ppt1 to lipid rafts, it is possible FasII may also be a Ppt1 substrate.
Ppt1 was originally identified by its ability to depalmitoylate H-Ras (Camp and Hofmann 1993). While the palmitoylation state of the Drosophila homolog is unknown, the fly homolog does have a C-terminal cysteine residue that could serve as a site of palmitoylation. Since our modifier data suggest an interaction with the Ras–MAPK pathway, some of the genetic interactions that we observe may be due to misregulation of Ras palmitoylation. Work on palmitoylation in several systems has shown that there is no tight consensus sequence that indicates that a particular cysteine will be palmitoylated (Smotrys and Linder 2004). Until recently, this has meant that the palmitoylation state of proteins has been determined on an individual basis. The development of a new biochemical modification technique for palmitoylated proteins has, for the first time, permitted the identification of modified proteins on a global scale in yeast (Roth et al. 2006). In Drosophila, very little is known either about specific palmitoylated proteins or about the role of palmitoylation during development. To identify in vivo substrates of Ppt1, it will be important to characterize specific candidates, such as Syt1, FasII, and Ras, and to undertake proteome-wide studies of protein palmitoylation in the fly.
Is INCL a synaptopathy?:
Many neurodegenerative disorders display cellular dysfunction before the death of specific populations of neuronal cells, particularly in processes related to endocytosis (Bossy-Wetzel et al. 2004; Nixon 2005). For example, in Huntington's disease, changes in dendritic spine morphology and number precede cell death (Li et al. 2003) and symptoms can precede overt cell loss (Mizuno et al. 2000). These observations, as well as studies in Huntington's disease mouse models, suggest that changes in synaptic function, including synaptic vesicle cycling and endocytosis, may explain the early progression of the disease (Li et al. 2003; Smith et al. 2005). Changes in endosomal function are also one of the earliest pathological markers of Alzheimer's disease (Nixon 2005). Finally, these observations extend to lysosomal storage disorders such as Niemann-Pick Type C and juvenile-onset NCL (JNCL), where recent work has documented changes in endo-lysosomal trafficking and function (Fossale et al. 2004; Nixon 2005; Cao et al. 2006).
Consistent with the importance of these changes for the pathogenesis of JNCL, membrane-trafficking defects are observed in a cell culture model before the accumulation of the characteristic storage material (Fossale et al. 2004). Our results, combined with those of others working on Ppt1 models, support the extension of this observation to INCL. Our modifiers suggest the hypothesis that early trafficking changes in patients—both synaptic vesicle and signaling proteins involved in synaptic structure—may lead to a progressive dysfunction of neurons, producing disease symptoms and ultimately widespread cell death. As with all large-scale screens, the modifiers that we have identified must be further validated in Drosophila Ppt1 mutants and higher eukaryotic systems such as the INCL mouse, as well as in INCL patient cells. The pathways and processes implicated by our results will be valuable points of entry in the future development of therapeutics that aim to ameliorate the consequences of PPT1 deficiency, avoiding the neurodegeneration typical of later stages of INCL.
Acknowledgments
We thank Robert Glaser and Heather Chotkowski for their technical help in setting up the Ppt1 enzyme assay, Sue Cotman and Marcy MacDonald for critical comments on the manuscript, and William Fowle for assistance with scanning electron microscopy. In addition to the Bloomington Stock Center, the Szeged Stock Center, and the Drosophila Genome Research Center, we also thank the following individuals for their generous gifts of Drosophila lines: Hugo Bellen, Kendall Broadie, Mel Feany, Janice Fischer, Scott Goode, Troy Littleton, Karen Palter, Mani Ramaswami, Subhabrata Sanyal, Jessica Treisman, and Kristi Wharton. This work was supported by funds from a Biology Department Research Grant (C.A.K.), a College of Charleston Summer Undergraduate Research Fellowship (C.A.K. and A.C.S.), a Support of Mentors and their Students in the Neurosciences grant from the National Science Foundation (DUE-0426266) (C.A.K. and H.B.), and National Institutes of Health grants P20-RR16461 (C.A.K.) and R15-HD052362 (C.A.K.)
References
- Ahtiainen, L., O. P. Van Diggelen, A. Jalanko and O. Kopra, 2003. Palmitoyl protein thioesterase 1 is targeted to the axons in neurons. J. Comp. Neurol. 455: 368–377. [DOI] [PubMed] [Google Scholar]
- Ahtiainen, L., K. Luiro, M. Kauppi, J. Tyynela, O. Kopra et al., 2006. Palmitoyl protein thioesterase 1 (PPT1) deficiency causes endocytic defects connected to abnormal saposin processing. Exp. Cell Res. 312: 1540–1553. [DOI] [PubMed] [Google Scholar]
- Bailey, C. H., M. Chen, F. Keller and E. R. Kandel, 1992. Serotonin-mediated endocytosis of apCAM: an early step of learning-related synaptic growth in Aplysia. Science 256: 645–649. [DOI] [PubMed] [Google Scholar]
- Bailey, C. H., B. K. Kaang, M. Chen, K. C. Martin, C. S. Lim et al., 1997. Mutation in the phosphorylation sites of MAP kinase blocks learning-related internalization of apCAM in Aplysia sensory neurons. Neuron 18: 913–924. [DOI] [PubMed] [Google Scholar]
- Beumer, K. J., J. Rohrbough, A. Prokop and K. Broadie, 1999. A role for PS integrins in morphological growth and synaptic function at the postembryonic neuromuscular junction of Drosophila. Development 126: 5833–5846. [DOI] [PubMed] [Google Scholar]
- Bible, E., P. Gupta, S. L. Hofmann and J. D. Cooper, 2004. Regional and cellular neuropathology in the palmitoyl protein thioesterase-1 null mutant mouse model of infantile neuronal ceroid lipofuscinosis. Neurobiol. Dis. 16: 346–359. [DOI] [PubMed] [Google Scholar]
- Bossy-Wetzel, E., R. Schwarzenbacher and S. A. Lipton, 2004. Molecular pathways to neurodegeneration. Nat. Med. 10: S2–S9. [DOI] [PubMed] [Google Scholar]
- Brand, A. H., and N. Perrimon, 1993. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118: 104–115. [DOI] [PubMed] [Google Scholar]
- Bronk, P., J. J. Wenniger, K. Dawson-Scully, X. Guo, S. Hong et al., 2001. Drosophila Hsc70–4 is critical for neurotransmitter exocytosis in vivo. Neuron 30: 475–488. [DOI] [PubMed] [Google Scholar]
- Camp, L. A., and S. L. Hofmann, 1993. Purification and properties of a palmitoyl-protein thioesterase that cleaves palmitate from H-Ras. J. Biol. Chem. 268: 22566–22574. [PubMed] [Google Scholar]
- Cao, Y., J. A. Espinola, E. Fossale, A. C. Massey, A. M. Cuervo et al., 2006. Autophagy is disrupted in a knock-in mouse model of juvenile neuronal ceroid lipofuscinosis. J. Biol. Chem. 281: 20483–20493. [DOI] [PubMed] [Google Scholar]
- Cho, S., and G. Dawson, 2000. Palmitoyl protein thioesterase 1 protects against apoptosis mediated by Ras-Akt-caspase pathway in neuroblastoma cells. J. Neurochem. 74: 1478–1488. [DOI] [PubMed] [Google Scholar]
- Dan, I., N. M. Watanabe and A. Kusumi, 2001. The Ste20 group kinases as regulators of MAP kinase cascades. Trends Cell Biol. 11: 220–230. [DOI] [PubMed] [Google Scholar]
- Dermaut, B., K. K. Norga, A. Kania, P. Verstreken, H. Pan et al., 2005. Aberrant lysosomal carbohydrate storage accompanies endocytic defects and neurodegeneration in Drosophila benchwarmer. J. Cell Biol. 170: 127–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Husseini, A. E., and D. S. Bredt, 2002. Protein palmitoylation: a regulator of neuronal development and function. Nat. Rev. Neurosci. 3: 791–802. [DOI] [PubMed] [Google Scholar]
- Eresh, S., J. Riese, D. B. Jackson, D. Bohmann and M. Bienz, 1997. A CREB-binding site as a target for decapentaplegic signalling during Drosophila endoderm induction. EMBO J. 16: 2014–2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes, P.S., T. C. Jackson, D. T. Stimson, S. Sanyal, L. E. Kelly et al., 2003. Functional dissection of a eukaryotic dicistronic gene: transgenic stonedB, but not stonedA, restores normal synaptic properties to Drosophila stoned mutants. Genetics 165: 185–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faix, J., and R. Grosse, 2006. Staying in shape with formins. Dev. Cell 10: 693–706. [DOI] [PubMed] [Google Scholar]
- Fergestad, T., W. S. Davis and K. Broadie, 1999. The stoned proteins regulate synaptic vesicle recycling in the presynaptic terminal. J. Neurosci. 19: 5847–5860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Funez, P., M. L. Nino-Rosales, B. de Gouyon, W. C. She, J. M. Luchak et al., 2000. Identification of genes that modify ataxin-1-induced neurodegeneration. Nature 408: 101–106. [DOI] [PubMed] [Google Scholar]
- Finley, K. D., P. T. Edeen, R. C. Cumming, M. D. Mardahl-Dumesnil, B. J. Taylor et al., 2003. blue cheese mutations define a novel, conserved gene involved in progressive neural degeneration. J. Neurosci. 23: 1254–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer, J. A., and E. Overstreet, 2002. Fat facets does a highwire act at the synapse. BioEssays 24: 13–16. [DOI] [PubMed] [Google Scholar]
- Fossale, E., P. Wolf, J. A. Espinola, T. Lubicz-Nawrocka, A. M. Teed et al., 2004. Membrane trafficking and mitochondrial abnormalities precede subunit c deposition in a cerebellar cell model of juvenile neuronal ceroid lipofuscinosis. BMC Neurosci. 5: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futerman, A. H., and G. van Meer, 2004. The cell biology of lysosomal storage disorders. Nat. Rev. Mol. Cell Biol. 5: 554–565. [DOI] [PubMed] [Google Scholar]
- Goberdhan, D. C., and C. Wilson, 1998. JNK, cytoskeletal regulator and stress response kinase? A Drosophila perspective. BioEssays 20: 1009–1019. [DOI] [PubMed] [Google Scholar]
- Goswami, R., M. Ahmed, J. Kilkus, T. Han, S. A. Dawson et al., 2005. Differential regulation of ceramide in lipid-rich microdomains (rafts): antagonistic role of palmitoyl:protein thioesterase and neutral sphingomyelinase 2. J. Neurosci. Res. 81: 208–217. [DOI] [PubMed] [Google Scholar]
- Grenningloh, G., E. J. Rehm and C. S. Goodman, 1991. Genetic analysis of growth cone guidance in Drosophila: fasciclin II functions as a neuronal recognition molecule. Cell 67: 45–57. [DOI] [PubMed] [Google Scholar]
- Hellensten, E., J. Vesa, V. M. Olkkonen, A. Jalanko and L. Peltonen, 1996. Human palmitoyl protein thioesterase: evidence for lysosomal targeting of the enzyme and disturbed cellular routing in infantile neuronal ceroid lipofuscinosis. EMBO J. 15: 5240–5245. [PMC free article] [PubMed] [Google Scholar]
- Hickey, A. J., H. L. Chotkowski, N. Singh, J. G. Ault, C. A. Korey et al., 2006. Palmitoyl-protein thioesterase 1 deficiency in Drosophila melanogaster causes accumulation of abnormal storage material and reduced life span. Genetics 172: 2379–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeffer, C. A., S. Sanyal and M. Ramaswami, 2003. Acute induction of conserved synaptic signaling pathways in Drosophila melanogaster. J. Neurosci. 23: 6362–6372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang, B., and A. Chiba, 1998. Genetic analysis on the role of integrin during axon guidance in Drosophila. J. Neurosci. 18: 7847–7855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes, A. L., and J.S. Heilig, 1999. Fasciclin II and Beaten path modulate intercellular adhesion in Drosophila larval visual organ development. Development 126: 261–272. [DOI] [PubMed] [Google Scholar]
- Huang, A. M., and G. M. Rubin, 2000. A misexpression screen identifies genes that can modulate RAS1 pathway signaling in Drosophila melanogaster. Genetics 156: 1219–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, Y., R. T. Baker and J. A. Fischer-Vize, 1995. Control of cell fate by a deubiquitinating enzyme encoded by the fat facets gene. Science 270: 1828–1831. [DOI] [PubMed] [Google Scholar]
- Isosomppi, J., O. Heinonen, J. O. Hiltunen, N. D. Greene, J. Vesa et al., 1999. Developmental expression of palmitoyl protein thioesterase in normal mice. Brain Res. Dev. Brain Res. 118: 1–11. [DOI] [PubMed] [Google Scholar]
- Kang, R., R. Swayze, M. F. Lise, K. Gerrow, A. Mullard et al., 2004. Presynaptic trafficking of synaptotagmin I is regulated by protein palmitoylation. J. Biol. Chem. 279: 50524–50536. [DOI] [PubMed] [Google Scholar]
- Kida, E., A. A. Golabek and K. E. Wisniewski, 2001. Cellular pathology and pathogenic aspects of neuronal ceroid lipofuscinoses. Adv. Genet. 45: 35–68. [DOI] [PubMed] [Google Scholar]
- Koh, Y. H., C. Ruiz-Canada, M. Gorczyca and V. Budnik, 2002. The Ras1-mitogen-activated protein kinase signal transduction pathway regulates synaptic plasticity through fasciclin II-mediated cell adhesion. J. Neurosci. 22: 2496–2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korey, C. A., and M. E. MacDonald, 2003. An over-expression system for characterizing Ppt1 function in Drosophila. BMC Neurosci. 4: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl, D., T. E. Kennedy, A. Barzilai and E. R. Kandel, 1992. Long-term sensitization training in Aplysia leads to an increase in the expression of BiP, the major protein chaperon of the ER. J. Cell Biol. 119: 1069–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehtovirta, M., A. Kytalla, E-L. Eskelinen, M. Hess, O. Heinonen et al., 2001. Palmitoyl protein thioesterase (PPT) localizes into synaptosomes and synaptic vesicles in neurons: implications for infantile neuronal ceroid lipofuscinosis (INCL). Hum. Mol. Genet. 10: 69–75. [DOI] [PubMed] [Google Scholar]
- Li, J. Y., M. Plomann and P. Brundin, 2003. Huntington's disease: A synaptopathy? Trends Mol. Med. 9: 414–420. [DOI] [PubMed] [Google Scholar]
- Little, E. B., G. M. Edelman and B. A. Cunningham, 1998. Palmitoylation of the cytoplasmic domain of the neural cell adhesion molecule N-CAM serves as an anchor to cellular membranes. Cell Adhes. Commun. 6: 415–430. [DOI] [PubMed] [Google Scholar]
- Littleton, J. T., T. L. Serano, G. M. Rubin, B. Ganetzky and E. R. Chapman, 1999. Synaptic function modulated by changes in the ratio of synaptotagmin I and IV. Nature 400: 757–760. [DOI] [PubMed] [Google Scholar]
- Marques, G., 2005. Morphogens and synaptogenesis in Drosophila. J. Neurobiol. 64: 417–434. [DOI] [PubMed] [Google Scholar]
- Milligan, G., M. Parenti and A. I. Magee, 1995. The dynamic role of palmitoylation in signal transduction. Trends Biochem. Sci. 20: 181–187. [DOI] [PubMed] [Google Scholar]
- Mitchison, H. M., S. L. Hofmann, C. H. Becerra, P. B. Munroe, B. D. Lake et al., 1998. Mutations in the palmitoyl-protein thioesterase gene (PPT; CLN1) causing juvenile neuronal ceroid lipofuscinosis with granular osmiophilic deposits. Hum. Mol. Genet. 7: 291–297. [DOI] [PubMed] [Google Scholar]
- Mitchison, H. M., M. J. Lim and J. D. Cooper, 2004. Selectivity and types of cell death in the neuronal ceroid lipofuscinoses. Brain Pathol. 14: 86–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno, H., H. Shibayama, F. Tanaka, M. Doyu, G. Sobue et al., 2000. An autopsy case with clinically and molecular genetically diagnosed Huntington's disease with only minimal non-specific neuropathological findings. Clin. Neuropathol. 19: 94–103. [PubMed] [Google Scholar]
- Muquit, M. M. K., and M. B. Feany, 2002. Modelling neurodegenerative diseases in Drosophila: A fruitful approach? Nat. Rev. Neurosci. 3: 237–243. [DOI] [PubMed] [Google Scholar]
- Myllykangas, L., J. Tyynela, A. Page-McCaw, G. M. Rubin, M. J. Haltia et al., 2005. Cathepsin D-deficient Drosophila recapitulate the key features of neuronal ceroid lipofuscinoses. Neurobiol. Dis. 19: 194–199. [DOI] [PubMed] [Google Scholar]
- Niethammer, P., M. Delling, V. Sytnyk, A. Dityatev, K. Fukami et al., 2002. Cosignaling of NCAM via lipid rafts and the FGF receptor is required for neuritogenesis. J. Cell Biol. 157: 521–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon, R. A., 2005. Endosome function and dysfunction in Alzheimer's disease and other neurodegenerative diseases. Neurobiol. Aging 26: 373–382. [DOI] [PubMed] [Google Scholar]
- Nixon, R. A., and A. M. Cataldo, 1995. The endosomal-lysosomal system of neurons: new roles. Trends Neurosci. 18: 489–496. [DOI] [PubMed] [Google Scholar]
- Oswald, M. J., D. N. Palmer, G. W. Kay, S. J. Shemilt, P. Rezaie et al., 2005. Glial activation spreads from specific cerebral foci and precedes neurodegeneration in presymptomatic ovine neuronal ceroid lipofuscinosis (CLN6). Neurobiol. Dis. 20: 49–63. [DOI] [PubMed] [Google Scholar]
- Phillips, A. M., M. Smith, M. Ramaswami and L. E. Kelly, 2000. The products of the Drosophila stoned locus interact with synaptic vesicles via synaptotagmin. J. Neurosci. 20: 8254–8261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips, S. N., N. Muzaffar, S. Codlin, C. A. Korey, P. E. M. Taschner et al., 2006. Characterizing pathogenic processes in Batten disease: use of small eukaryotic model systems. Biochim. Biophys. Acta 1762: 906–919. [DOI] [PubMed] [Google Scholar]
- Reutens, A. T., and C. G. Begley, 2002. Endophilin-1: a multifunctional protein. Int. J. Biochem. Cell Biol. 34: 1173–1177. [DOI] [PubMed] [Google Scholar]
- Rohrbough, J., M. S. Grotewiel, R. L. Davis and K. Broadie, 2000. Integrin-mediated regulation of synaptic morphology, transmission, and plasticity. J. Neurosci. 20: 6868–6878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorth, P., K. Szabo, A. Bailey, T. Laverty, J. Rehm et al., 1998. Systematic gain-of-function genetics in Drosophila. Development 25: 1049–1057. [DOI] [PubMed] [Google Scholar]
- Roth, A. F., J. Wan, A. O. Bailey, B. Sun, J. A. Kuchar et al., 2006. Global analysis of protein palmitoylation in yeast. Cell 125: 1003–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio, M. E., and R. J. Wenthold, 1999. Differential distribution of intracellular glutamate receptors in dendrites. J. Neurosci. 19: 5549–5562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanyal, S., D. J. Sandstrom, C. A. Hoeffer and M. Ramaswami, 2002. AP-1 functions upstream of CREB to control synaptic plasticity in Drosophila. Nature 416: 870–874. [DOI] [PubMed] [Google Scholar]
- Schobel, S., S. Neumann, B. Seed and S. F. Lichtenthaler, 2006. Expression cloning screen for modifiers of amyloid precursor protein shedding. Int. J. Dev. Neurosci. 24: 141–148. [DOI] [PubMed] [Google Scholar]
- Schuster, C. M., G. W. Davis, R. D. Fetter and C. S. Goodman, 1996. a Genetic dissection of structural and functional components of synaptic plasticity. I. Fasciclin II controls synaptic stabilization and growth. Neuron 17: 641–654. [DOI] [PubMed] [Google Scholar]
- Schuster, C. M., G. W. Davis, R. D. Fetter and C. S. Goodman, 1996. b Genetic dissection of structural and functional components of synaptic plasticity. II. Fasciclin II controls presynaptic structural plasticity. Neuron 17: 655–667. [DOI] [PubMed] [Google Scholar]
- Seto, E. S., H. J. Bellen and T. E. Lloyd, 2002. When cell biology meets development: endocytic regulation of signaling pathways. Genes Dev. 16: 1314–1336. [DOI] [PubMed] [Google Scholar]
- Shulman, J. M., and M. B. Feany, 2003. Genetic modifiers of tauopathy in Drosophila. Genetics 165: 1233–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siintola, E., S. Partanen, P. Stromme, A. Haapanen, M. Haltia et al., 2006. Cathepsin D deficiency underlies congenital human neuronal ceroid-lipofuscinosis. Brain 129: 1438–1445. [DOI] [PubMed] [Google Scholar]
- Smith, R., P. Brundin and J. Y. Li, 2005. Synaptic dysfunction in Huntington's disease: a new perspective. Cell. Mol. Life Sci. 62: 1901–1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smotrys, J. E., and M. E. Linder, 2004. Palmitoylation of intracellular signaling proteins: regulation and function. Annu. Rev. Biochem. 73: 559–587. [DOI] [PubMed] [Google Scholar]
- Suopanki, J., J. Tyynela, M. Baumann and M. Haltia, 1999. a The expression of palmitoyl-protein thioesterase is developmentally regulated in neural tissues but not in nonneural tissues. Mol. Genet. Metab. 66: 290–293. [DOI] [PubMed] [Google Scholar]
- Suopanki, J., J. Tyynela, M. Baumann and M. Haltia, 1999. b Palmitoyl-protein thioesterase, an enzyme implicated in neurodegeneration, is localized in neurons and is developmentally regulated in rat brain. Neurosci. Lett. 265: 53–56. [DOI] [PubMed] [Google Scholar]
- Suopanki, J., M. Lintunen, H. Lahtinen, M. Haltia, P. Panula et al., 2002. Status epilepticus induces changes in the expression and localization of endogenous palmitoyl-protein thioesterase 1. Neurobiol. Dis. 10: 247. [DOI] [PubMed] [Google Scholar]
- Sweeney, S. T., and G. W. Davis, 2002. Unrestricted synaptic growth in spinster—a late endosomal protein implicated in TGF-beta-mediated synaptic growth regulation. Neuron 36: 403–416. [DOI] [PubMed] [Google Scholar]
- Vance, J. E., and R. Steenbergen, 2005. Metabolism and functions of phosphatidylserine. Prog. Lipid Res. 44: 207–234. [DOI] [PubMed] [Google Scholar]
- van Diggelen, O. P., J. L. Keulemans, B. Winchester, I. L. Hofman, S. L. Vanhanen et al., 1999. A rapid fluorogenic palmitoyl-protein thioesterase assay: pre- and postnatal diagnosis of INCL. Mol. Genet. Metab. 66: 240–244. [DOI] [PubMed] [Google Scholar]
- van Diggelen, O. P., S. Thobois, C. Tilikete, M. T. Zabot, J. L. Keulemans et al., 2001. Adult neuronal ceroid lipofuscinosis with palmitoyl-protein thioesterase deficiency: first adult-onset patients of a childhood disease. Ann. Neurol. 50: 269–272. [DOI] [PubMed] [Google Scholar]
- Van Meer, G., and H. Sprong, 2004. Membrane lipids and vesicular traffic. Curr. Opin. Cell Biol. 16: 373–378. [DOI] [PubMed] [Google Scholar]
- Van Vactor, D., and C. Kopczynski, 1999. Anatomical techniques for analysis of nervous system development in the Drosophila embryo, pp. 490–513 in A Comparative Methods Approach to the Study of Oocytes and Embryos, edited by J. Richter. Oxford University Press, New York.
- Verkruyse, L. A., and S. L. Hofmann, 1996. Lysosomal targeting of palmitoyl-protein thioesterase. J. Biol. Chem. 271: 15831–15836. [DOI] [PubMed] [Google Scholar]
- Verstreken, P., T. W. Koh, K. L. Schulze, R. G. Zhai, P. R. Hiesinger et al., 2003. Synaptojanin is recruited by endophilin to promote synaptic vesicle uncoating. Neuron 40: 733–748. [DOI] [PubMed] [Google Scholar]
- Vesa, J., E. Hellsten, L. A. Verkruyse, L. A. Camp, J. Rapola et al., 1995. Mutations in the palmitoyl-protein thioesterase gene causing infantile neuronal ceroid lipofuscinosis. Nature 376: 584–587. [DOI] [PubMed] [Google Scholar]
- Virmani, T., P. Gupta, X. Liu, E. T. Kavalali and S. L. Hofmann, 2005. Progressively reduced synaptic vesicle pool size in cultured neurons derived from neuronal ceroid lipofuscinosis-1 knockout mice. Neurobiol. Dis. 20: 314–323. [DOI] [PubMed] [Google Scholar]
- Wisniewski, K. E., 2005. Neuronal ceroid-lipofuscinoses. Gene reviews at http://www.genetests.org.
- Wisniewski, K. E., E. Kida, A. A. Golabek, W. Kaczmarski, F. Connell et al., 2001. Neuronal ceroid lipofuscinoses: classification and diagnosis. Adv. Genet. 45: 1–33. [DOI] [PubMed] [Google Scholar]
- Wittmann, C. W., M. F. Wszolek, J. M. Shulman, P. M. Salvaterra, J. Lewis et al., 2001 2001. Tauopathy in Drosophila: neurodegeneration without neurofibrillary tangles. Science 293: 711–714. [DOI] [PubMed] [Google Scholar]
- Yoshihara, M., and E. S. Montana, 2004. The synaptotagmins: calcium sensors for vesicular trafficking. Neuroscientist 10: 566–574. [DOI] [PubMed] [Google Scholar]
- Yu, K., K. H. Kang, P. Heine, U. Pyati, S. Srinivasan et al., 2004. Cysteine repeat domains and adjacent sequences determine distinct bone morphogenetic protein modulatory activities of the Drosophila Sog protein. Genetics 166: 1323–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Z., A. K. Mandal, N. Wang, C. L. Keck, D. B. Zimonjic et al., 1999. Palmitoyl-protein thioesterase gene expression in the developing mouse brain and retina: implications for early loss of vision in infantile neuronal ceroid lipofuscinosis. Gene 231: 203. [DOI] [PubMed] [Google Scholar]