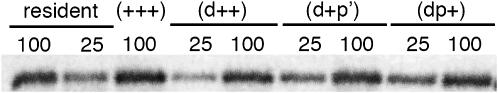Abstract
Ref(2)P has been described as one of the Drosophila proteins that interacts with the sigma virus cycle. We generated alleles to identify critical residues involved in the restrictive (inhibiting viral multiplication) or permissive (allowing viral multiplication) character of Ref(2)P. We demonstrate that permissive alleles increase the ability of the sigma virus to infect Drosophila when compared to null alleles and we confirm that restrictive alleles decrease this capacity. Moreover, we have created alleles unfunctional in viral cycling while functional for Ref(2)P fly functions. This type of allele had never been observed before and shows that fly- and virus-related activities of Ref(2)P are separable. The viral status of Ref(2)P variants is determined by the amino-terminal PB1 domain polymorphism. In addition, an isolated PB1 domain mimics virus-related functions even if it is similar to a loss of function toward fly-related activities. The evolutionary tree of the Ref(2)P PB1 domain that we could build on the basis of the natural allele sequences is in agreement with an evolution of PB1 domain due to successive transient selection waves.
THE sigma rhabdovirus is a vertically transmitted virus, which endemically infects natural populations of Drosophila melanogaster (for a review, see Fleuriet 1988). The sigma virus provokes pathogenic effects (i.e., lower viability of the eggs), which, interestingly, are strongly enhanced when females are mated with uninfected males issued from a different population (Fleuriet 1994). This observation suggests selection of the host genome, with the aim of controlling virus multiplication in the infected population. One of the genes implicated in this control, although not essential to infection, is the ref(2)P gene (Guillemain and Plus 1954). This gene of unknown function is strongly polymorphic: 14 different proteins were predicted from the 14 sequenced haplotypes (Wayne et al. 1996). Two classes of alleles have been found in natural populations: permissive alleles that permit sigma virus multiplication and restrictive alleles that slow down viral multiplication. The efficiency of a particular restrictive allele in restraining the virus depends on the viral strain (Contamine 1981).
According to the ref(2)P locus, vertical transmission of the sigma virus does not follow the same hereditary transmission rules. For example, the frequency of infected flies in the progeny of homozygous or heterozygous permissive males carrying the sigma virus decreases with the dose of restrictive alleles from its mate. Similar observations hold true in the case of maternal transmission of the virus (Gay and Ozolins 1968). Along generations, such modifications of the hereditary transmission rules would lead to a cure of infected fly populations. However, viruses that have become adapted to multiply in restrictive flies may appear in viral populations (Contamine 1981) and can eventually invade the fly population, as observed in Languedoc populations (Fleuriet and Periquet 1993).
The Ref(2)P protein contains three domains involved in protein–protein interactions: PB1, ZZ, and UBA (Figure 1A). The N-terminal end consists of a PB1 domain (Phox and Bem 1, amino acids 6–88). The ZZ zinc finger and the UBA (ubiquitin associated) domains are located between amino acids 121 and 165 and 554 and 594, respectively. Comparative analysis of polymorphism in a typical restrictive [ref(2)Pp, renamed ref(2)P(dpp′)Pa here] and permissive [ref(2)PO1, renamed ref(2)P(+++)O1] allele has shown that eight sites are involved (Figure 1A; Dru et al. 1993). Only three of them are found within one of the conserved domains, and all three are included in the PB1 domain. These three mutations will be designated d, p, and p′ in this study. The d mutation (for divergence) consists of the replacement of CAGAAT by GGA (Q28N29 by G). It is systematically encountered in the restrictive alleles, but never in the permissive alleles studied (Dru et al. 1993; Wayne et al. 1996). Therefore, this polymorphic site could be essential in defining a restrictive allele. The p and p′ mutations (for punctual, p is I32V and p′ is Q43L) have been found in the three sequenced restrictive alleles (Wayne et al. 1996). Since p and p′ are located close to the d mutation (i.e., in PB1), we hypothesized a possible contribution of the p and p′ mutations to the restrictive character of ref(2)P. In this study, ref(2)P alleles are named according to the PB1-located polymorphic sites. The permissive ref(2)P(+++) allele does not bear the mutations whereas ref(2)P(dpp′) carries all of them. Mutated sites outside the PB1 domain are not studied in detail in this article. Therefore, they will be collectively defined according to the wild fly strain from which alleles were isolated (OM or O1, permissive, and Pa, restrictive). Typical permissive alleles are thus named ref(2)P(+++)OM or ref(2)P(+++)O1 while the typical restrictive allele is ref(2)P(dpp′)Pa.
Figure 1.—
D. melanogaster Ref(2)P proteins used in this study. The PB1 (PFAM00564), ZZ (zinc finger, ZZ type, PFAM00569), and UBA (PFAM00627) domains are indicated by shaded boxes, with their localizations on the amino acid sequence. Solid boxes represent deletions and thick lines represent single amino acid changes. (A) Typical permissive Ref(2)P(+++)O1 and typical restrictive Ref(2)P(dpp)Pa sequences. The modifications found along the restrictive protein, including the dpp′ mutations, are shown below the sequence. (B) Ref(2)P proteins expressed by recombinant transgenes (Dru et al. 1993; Wayne et al. 1996; Marchler-Bauer et al. 2003).
To further explore the role of the three mutations d, p, and p′ on sigma virus multiplication, we have used in vitro recombination and mutagenesis to obtain the eight possible combinations of these mutations (only three of those have been found in natural alleles). Transgenic fly lines were established for the different combinations and studied for their susceptibility to the sigma virus.
To test whether efficient viral cycles occurred, we used the method of sensitivity to CO2. Indeed, for unclear reasons, flies whose thoracic ganglia have been invaded by the sigma virus develop irreversible paralysis upon exposure to pure CO2 (Bussereau 1970; Brun and Plus 1980). This CO2-induced paralysis is also observed when Drosophila is infected by Cocal or New Jersey vesiculoviruses. For these viruses, invasion of the ganglia is clearly linked to the viral titer (Bussereau 1971). In the case of the sigma virus, use of thermosensitive variants showed that completion of the viral replicative cycle is essential in CO2 sensitivity. Blocking late stages of the viral cycle at elevated temperatures suppresses CO2 symptoms. Decreasing temperature afterward allows CO2-induced paralysis to reappear (Contamine 1984).
We show here that the effect of Ref(2)P on viral multiplication is determined by its amino-terminal PB1 domain. Concomitant presence of the d and p mutations is necessary to switch from a permissive to a restrictive allele. Nevertheless, for certain viral strains the p′ mutation is also necessary to confer the restrictive character. A comprehensive analysis of our data allowed the construction of an evolutionary tree based on the PB1 domain, which suggests that the mutations would have appeared in the following order: p′, p, and d.
MATERIALS AND METHODS
Construction of the recombinant alleles of ref(2)P:
The ref(2)P gene consists of three exons. Previous studies (Wayne et al. 1996) have demonstrated that resistance to sigma virus infection can be affected by changes in exon 1. Exon 1 includes the three PB1-located mutations d, p, and p′ studied in detail in this work. A unique SalI restriction site is found between exon 1 and exon 2 in both natural alleles, ref(2)P(dpp′)Pa and ref(2)P(+++)O1 [formerly named ref(2)PP and ref(2)PO1, respectively; Dru et al. 1993]. Therefore the ref(2)P(dpp′)O1 and ref(2)P(+++)Pa recombinant alleles were generated from ref(2)P(dpp′)Pa and ref(2)P(+++)O1 alleles subcloned in the pEMBL vector, through exchange of a SalI–SalI fragment between the two constructs.
To generate the ref(2)P(d++)O1 allele, introduction of the d mutation was performed by PCR amplification using the pEMBL–ref(2)P(+++)O1 construct (Dezelee et al. 1989; Dru et al. 1993) as a template. A unique NruI restriction site is present between the p and p′ mutation sites in natural alleles. The synthetic oligonucleotide 5′-ATTTCGCGACGCAATATGGTGTATCCGGAGGGCATC-3′ (the NruI restriction site is underlined, and the d mutation is in boldface type) was used together with the Puc reverse primer to amplify a DNA fragment containing a NruI restriction site followed by the promoter and the 5′ region of the gene. Following digestion with the NruI restriction enzyme, this 945-bp-long sequence was introduced in place of the original NruI–NruI fragment of pEMBL–ref(2)P(+++)O1, thus creating the pEMBL–ref(2)P(d++)O1 plasmid. Sequencing revealed PCR-generated mutations in the promoter region of the gene. Elimination of those mutations was made possible by exchanging a NruI–SacI fragment between the pEMBL–ref(2)P(+++)O1 and pEMBL–ref(2)P(d++)O1 constructs, a SacI restriction site being located 125 bp upstream from the start codon.
The recombinant alleles ref(2)P(++p′)O1 and ref(2)P(dp+)O1 were generated from ref(2)P(dpp′)O1 and ref(2)P(+++)O1 by exchange of the NruI–NruI fragments. Following a procedure similar to that described above, the ref(2)P(+pp′)O1 allele was constructed from the ref(2)P(++p′)O1 allele through use of the synthetic oligonucleotide 5′-CGATTTCGCGACGCAATACGGTGTAATTCTG-3′. Exchange of the NruI–NruI fragments between ref(2)P(d++)O1 and ref(2)P(++p′)O1 and between ref(2)P(+pp′)O1 and ref(2)P(+++)O1 permitted the construction of the ref(2)P(d+p′)O1 and ref(2)P(+p+)O1 alleles, respectively.
The ref(2)PΔn gene construct is a truncated form of the ref(2)P(dpp′)Pa allele for which the majority of exon 2, intron 2, and a part of exon 3 have been deleted (Wyers et al. 1995). Unique BstXI and NarI restriction sites are found 26 bp downstream from the start codon and 97 bp downstream from the beginning of exon 2, respectively. So as to eliminate intron 1, BstXI–NarI fragments were exchanged between the ref(2)PΔn construct and the cDNA clones of the ref(2)Pn (dpp′ type) and ref(2)PO2 (+++ type) (Dru et al. 1993), thus creating ref(2)P(dpp′)Δ92-599 and ref(2)P(+++)Δ92-599. Numbering of the deleted amino acids is in reference to the sequence of Ref(2)P(+++)O1. For each construct, the chimeric genes were cloned in the PW6 transformation vector (Klemenz et al. 1987), and transformed fly lines were established following standard procedure using the Δ2-3 P element as the source of transposase and an OM genetic background (Rubin and Spradling 1982; Robertson et al. 1988).
D.melanogaster and sigma virus strains:
Oregon Ménétréol (OM) fly line was used as the reference permissive strain (Contamine et al. 1989). The reference restrictive strain carries chromosomes 1 and 3 from the OM fly line and chromosome 2 from the strain Paris. The ref(2)Pod3/Df(2L)E55 flies (Gay and Contamine 1993) displays homozygous loss of function at the ref(2)P locus and are designated ref(2)P−/ref(2)P− in this study.
Viral strains are natural strains and have been previously described (Gay 1978; Contamine 1981). The viral suspensions used were from stocks kept in 10% calf serum at −80°. The stocks were obtained by grinding naturally infected permissive flies with 23 (clone DAa; Contamine 1981) or A3 viruses, 50 flies/ml, and filtering the suspension on 45-μm pore-size filters.
Sigma virus assay:
Two modes of infection were performed: inoculation of viral suspension into the adult fly abdomen and transmission to the progeny by persistently infected mothers. In both cases, viral infection was tested by exposure to pure CO2 at 15° (Bussereau 1970; Brun and Plus 1980) after a 20-day incubation at 25° for artificially inoculated flies or 4 days after emergence for hereditarily infected flies. Flies that became paralyzed were considered as infected. Determination of viral titer, measurement of efficiencies of the infecting unit according to different host genotypes, as well as statistical tests, were performed as described in Contamine (1981).
Expression of ref(2)P:
The quantity of transcripts produced by the different ref(2)P alleles was assayed by a dot-blotting analysis, as previously described (Wyers et al. 1995). Quantification of the Ref(2)P protein was performed as follows. Proteins were extracted from 3- to 4-day-old adult flies by grinding flies in lysis buffer (10 μl/fly of 10 mm Tris–HCl, pH 7.4, 10 mm KCl, 150 μm MgCl2 with a mixture of CLAPA protease inhibitors: 1 μg/ml chymostatin, 1 μg/ml leupeptin, 1 μg/ml antipain, 1 μg/ml pepstatin, 5 μg/ml aprotinin; Boehringer Mannheim, Indianapolis). To eliminate cell debris, samples were centrifuged twice for 30 min at 12,000 × g. Total protein concentration was estimated in the supernatant by the Bradford protein assay (Bio-Rad, Hercules, CA). For immunoprecipitation of Ref(2)P, a volume of sample corresponding to 25 or 100 flies was supplemented with lysis buffer to a final concentration of 3 mg protein/ml. Samples were adjusted to 100 mm NaCl, 5mm MgCl2, 0.1 mm EDTA, 0.1% Triton X-100. Following incubation with 10 μl of F-REF antiserum (Wyers et al. 1993) per milliliter of extract for 2 hr 30 min at 4° on a rotating wheel, 20 μl of ProteinA-Sepharose beads (CL-4B, Pharmacia Biotech, Piscataway, NJ) per milliliter of sample were added. After a second incubation for 1 hr at 4° on a rotating wheel, the pellets were collected by centrifugation at 8000 × g for 5 min. The pellets were washed four times with the incubation buffer and resuspended in 5 μl of 5× Laemmli buffer (Laemmli 1970) before freezing at −20°. Before loading on 10% SDS–PAGE gels, 20 μl of H2O was added to the samples, which were boiled for 8 min. After electrophoresis, proteins were transferred to a nitrocellulose membrane. The Ref(2)P protein was identified by use of polyclonal antibodies MTN (1/1000, produced as described in Wyers et al. 1993 using baculovirus recombinant Ref(2)Pn). Detection was performed with an ECL Western blotting kit (Pharmacia Biotech) in PBS–Tween buffer according to the manufacturer's instructions. Quantification was performed using a Bio-Rad Molecular Imager system. The quantity of Ref(2)P proteins produced by the different transgenes was tested at least three times and compared to that of a resident ref(2)P(+++)OM allele.
RESULTS
The sigma virus uses the ref(2)P permissive allele to improve its multiplication:
The D. melanogaster ref(2)P alleles studied so far have been distributed into two categories: the restrictive alleles that inhibit multiplication of sigma virus and the permissive alleles that allow viral infection. Infection by sigma of both permissive and restrictive flies is possible. Permissive and restrictive ref(2)P alleles are thus defined, for a particular sigma virus strain, by the concentration of virus required to observe infected flies. Previous studies (Contamine et al. 1989; Gay and Contamine 1993) have stated that the frequencies of infection of ref(2)P-deficient mutants and permissive flies are similar, thus implying that the permissive protein is not essential to efficient viral cycles.
To refine knowledge of the effect of ref(2)P alleles on sigma virus infection, we aimed to achieve a thorough study on the behavior of a typical permissive and a typical restrictive allele. For this purpose, we performed crosses to obtain, in similar genetic backgrounds, every possible combination of typical permissive [ref(2)P(+++)OM], restrictive [ref(2)P(dpp′)Pa], and null alleles [ref(2)P−] at the ref(2)P locus. Flies were subsequently inoculated with either viral strain A3, which is very sensitive to the restrictive allele ref(2)P(dpp′)Pa, or strain 23, which is less sensitive (Dru et al. 1993). For each of the fly lines, we measured the concentration of virus necessary to observe infection. All values were normalized to those obtained for the homozygous loss-of-function control flies [ref(2)P−/ref(2)P−].
The results are shown in Figure 2. For both viral strains A3 and 23, the inoculum required for infection in the presence of restrictive alleles is larger (Figure 2, 4th, 5th, 10th, and 11th bar from the left) than it is for the control [i.e., ref(2)P deficiency, 6th and 12th bar from the left]. Increasing the number of copies of the restrictive allele has no detectable effect on sensitivity to the virus (compare 4th and 10th bars to 5th and 11th bars, respectively, Figure 2), but this may be due to the limits of our assay. In addition, strain 23 is systematically less sensitive to restrictive alleles than the A3 virus since the overall quantity of virus required for infection is higher in the case of A3.
Figure 2.—
Dose of A3 or 23 sigma virus necessary to infect flies according to the genotype at the ref(2)P locus. The concentration of the efficient inoculum was determined by the end-point dilution method from a dilution series of each viral stock. Error bars are defined for the limits of the confidence interval at the 5% level of significance. The reference strain was ref(2)P−/ref(2)P−. The size of inoculum necessary to infect these flies was arbitrarily defined as 1.00. The y-axis values are the logarithm of the smallest inoculum able to infect flies (Contamine 1981); thus positive values on the histogram reflect an increase in the minimum quantity of virus necessary for infection while negative values reveal a decreased quantity of virus compared to the control [log(1) = 0]. k and k′ are the minimum inoculum size necessary to infect homozygous permissive flies with A3 and 23 sigma virus, respectively. Values expressed in the number of k or k′ on top of each bar represent the minimum inoculum size necessary to infect flies of the corresponding genotype. The ref(2)P−/ref(2)P− are ref(2)Pod3/Df(2L)E55. When these control flies are compared to hemi-zygotes [ref(2)P(+++)/ref(2)P− or ref(2)P(dpp′)/ref(2)P−], the results are the same regardless of whichever ref(2)P− was used to build hemi-zygote flies [ref(2)Pod3 or Df(2L)E55]. The number of copies of ref(2)P(dpp′) (dashed lines) and ref(2)P(+++) (solid lines) is indicated under the histogram.
The inoculum necessary to infect hemizygous [ref(2)P(+++)/ref(2)P−, second and eighth bars from the left in Figure 2] or homozygous permissive flies [ref(2)P(+++)/ref(2)P(+++), first and seventh bars from the left in Figure 2) is smaller than that required for the control ref(2)P−/ref(2)P−, revealing a difference between a ref(2)P deficiency and the presence of a permissive allele. This result implies that the permissive protein somehow allows better viral cycling in Drosophila cells. Our data show that it is the case for both viral strains. To the best of our knowledge, this is the first description of permissive alleles used by the sigma virus to improve its efficiency of multiplication.
Behavior toward infection by sigma differs in the case of heterozygous flies [ref(2)P(+++)/ref(2)P(dpp′)]. For the A3 virus, flies are restrictive (third bar from the left, Figure 2), while they are permissive when viral strain 23 is inoculated (ninth bar from the left, Figure 2), thus revealing differences concerning the dominant relationships of ref(2)P alleles.
Assaying the fly and viral functions of ref(2)P:
We seeked to identify the critical residues responsible for the restrictive/permissive character of the Ref(2)P protein. This protein contains eight polymorphic sites: three of them belong to the amino-terminal PB1 domain (the d, p, and p′ mutations of restrictive alleles), and the five remaining are located between amino acids 186 and 599 (see Figure 1A). Any of these polymorphic sites may have an effect on the restrictive/permissive character of ref(2)P alleles. To study the mutations independently, we used in vitro recombination and mutagenesis to generate, from the natural restrictive [ref(2)P(dpp′)Pa] and the natural permissive [ref(2)P(+++)O1] alleles, new alleles displaying different combinations of the eight polymorphic sites. We then established transgenic lines carrying a P element allowing expression of the different alleles in the same genetic background [homozygous loss of function of ref(2)P].
The effect of the transgenes was tested through two types of experiments. The first concerns the role of ref(2)P in the host fly. Although the exact function of ref(2)P in Drosophila remains unclear, it has previously been shown that a homozygous loss of function results in male sterility in Su(P)ste homozygous flies (Bichon et al. 2001). Advantage was taken of this observation to develop an assay for fly functionality of ref(2)P. Transgenes whose expression in Su(P)ste male flies restores fertility are thus designated “fly functional,” while those for which males remain sterile are called “fly unfunctional.”
The second type of experiment consists of assaying the role of ref(2)P in viral infection, i.e., determining whether the transgene is permissive, restrictive, or—since we have shown that infection by sigma differs in permissive and deficient alleles (Figure 2)—has no effect on viral cycling. In the latter case, the allele is designated virus unfunctional and is characterized by a behavior similar to that of a null allele when studying the interaction between Ref(2)P and sigma. On the other hand, permissive and restrictive alleles are called “virus functional.” Virus-related functions of Ref(2)P were thus tested by assaying sensitivity to CO2 of flies carrying the transgene and infected by the A3 virus through maternal vertical transmission.
ref(2)P transgenes expressed in a ref(2)P-deficient background recapitulate the effects of resident alleles:
Before attempting to study ref(2)P polymorphism, it appeared necessary to control the typical permissive and restrictive transgenes so that they behave similarly to resident alleles. One of the control experiments, using a transgene expressing the typical permissive allele [ref(2)P(+++)O1], is presented in detail in Table 1. Eleven ref(2)P transgenic lines were studied. For all of them, ref(2)P−/ref(2)P− males carrying the transgene are fertile (Table 1, third column). The ref(2)P(+++)O1 allele is therefore fly functional. As for virus-related functions, flies with transgenes resulted in CO2 symptoms in most cases. This implies efficient viral multiplication in these flies (Table 1, fifth column). Hence, the transgene does not block viral cycling, i.e., is not restrictive.
TABLE 1.
Detailed results obtained with the ref(2)P(+++)O1 transgene for virus and host functions
| Male fertility frequency: | Sigma-infected flies frequency
|
||||||
|---|---|---|---|---|---|---|---|
| Genetic background
|
ref(2)P−/ref(2)P− transgene
|
ref(2)P−/ref(2)P− transgene
|
ref(2)P(dpp′)/ref(2)P− transgene
|
||||
| Line | Chromosome | + | − | + | − | + | − |
| 56 | 3 | 3/3 | 0/5 | 110/114 | 105/112 | 164/172 | 5/174 |
| 63 | 3 | 5/5 | 0/5 | 79/79 | 73/73 | 380/383 | 19/262 |
| 69 | 1 | 4/4 | 0/2 | 105/105 | 88/90 | 93/101 | 3/73 |
| 70 | 3 | 5/5 | 0/5 | 38/38 | 8/8 | 314/325 | 1/109 |
| 77 | 3 | 5/5 | 0/5 | 63/64 | 64/64 | 209/225 | 6/240 |
| 83 | 3 | 5/5 | 0/4 | 62/64 | 73/75 | 15/226 | 28/261 |
| 87 | 1 | 4/5 | 0/5 | 64/67 | 63/67 | 182/214 | 3/279 |
| 88 | 1 | 4/4 | 0/5 | 72/72 | 47/47 | 234/234 | 0/189 |
| 92-1 | 1 | 4/4 | 0/5 | 73/73 | 78/79 | 208/210 | 3/173 |
| 92-5 | 3 | 5/5 | 0/5 | 121/122 | 68/68 | 154/154 | 2/173 |
| 99 | 3 | 5/5 | 0/3 | 89/89 | 69/72 | 146/146 | 1/156 |
As far as we can speculate, a nonrestrictive allele may be either permissive or deficient. We have shown above (see Figure 2) that permissive and null alleles did not display the same effect in viral multiplication. Another type of experiment was considered, with the aim of distinguishing between these two possibilities. Crosses were performed to obtain a hemi-zygous restrictive ref(2)P(dpp′)Pa/ref(2)P− progeny. In such a progeny, the virus is unable to develop enough to induce CO2 sensitivity due to the presence of the restrictive resident allele. Without transgene, these flies are therefore insensitive to CO2 exposure. Upon expression of the transgene, either (1) flies remain insensitive to CO2 exposure due to inhibition of the virus cycle by the resident restrictive allele while no effect of the transgene is detected and therefore the transgene is “virus unfunctional” or (2) CO2 symptoms are observed, and we can conclude that the virus multiplies through genetic interaction with a permissive transgene.
As expected with our control experiment, in 10 of 11 lines, data obtained for the CO2 assay are different with (seventh column, Table 1) or without transgenes (eighth column, Table 1). This result clearly demonstrates, as expected, that the ref(2)P(+++)O1 transgene is indeed permissive. Only one transgenic line (83) appears to be virus unfunctional. This could be due to many reasons, most likely to characteristics of the insertion point of the transgene.
Our second control experiment consisted of establishing that the transgene carrying the genomic sequence of the typical restrictive allele, ref(2)P(dpp′)Pa (data not shown; results summarized in Table 2, line 2), indeed behaves like a restrictive allele in our assay. Our data therefore confirm that our permissive (Table 1 summarized in Table 2, line 1) and restrictive (Table 2, line 2) constructs reproduce the original characteristics of resident alleles. Moreover, transcriptional quantitative and qualitative differences observed with the resident alleles (Contamine et al. 1989) have been reproduced with the transgenes (data not shown).
TABLE 2.
Effect of ref(2)P polymorphism on its sigma-virus-related functions
| Transgene | No. of studied lines | No. of functional lines for sigma virus | Phenotype for sigma virus | |
|---|---|---|---|---|
| Control lines | ref(2)P(+++)O1 | 11 | 10 | Permissive |
| ref(2)P(dpp′)Pa | 6 | 5 | Restrictive | |
| Test of the five non-PB1 mutations | ref(2)P(dpp′)O1 | 2 | 2 | Restrictive |
| ref(2)P(+++)Pa | 14 | 14 | Permissive | |
| Test of the dpp′ mutations | ref(2)P(dp+)O1 | 7 | 5 | Restrictive |
| ref(2)P(+pp′)O1 | 10 | 9 | Permissive | |
| ref(2)P(++p′)O1 | 11 | 11 | Permissive | |
| ref(2)P(+p+)O1 | 15 | 14 | Permissive | |
| ref(2)P(d++)O1 | 13 | 0 | Unfunctional | |
| ref(2)P(d+p′)O1 | 13 | 0 | Unfunctional |
Mutations in the PB1 domain of ref(2)P define restrictivity:
All the ref(2)P transgenes were studied for fly- and virus-related functions, as described above. The results are summarized in Table 2, for which only the lines carrying a fly-functional transgene are shown.
To test the potential collective effect on restrictivity of the five mutations situated outside the PB1 domain, two recombinants were built from the natural permissive ref(2)P(+++)O1 and restrictive ref(2)P(dpp′)Pa alleles. The ref(2)P(dpp′)O1 recombinant carries the three PB1 polymorphic sites from the restrictive allele ref(2)P(dpp′)Pa and the five other sites from the permissive ref(2)P(+++)O1 allele. The ref(2)P(+++)Pa construct is the reciprocal recombinant (Figure 1B). Our data demonstrate that ref(2)P(+++)O1 and ref(2)P(+++)Pa are permissive (Table 2, lines 1 and 4), while ref(2)P(dpp′)Pa and ref(2)P(dpp′)O1 are restrictive (Table 2, lines 2 and 3). Therefore, a ref(2)P allele carries its restrictive/permissive character on its PB1 domain. The five polymorphic sites located outside the PB1 domain are neutral in our experiments. We consequently focused on the d, p, and p′ mutations situated in the PB1 domain.
Viral and host functions of ref(2)P can be separated:
The study of the independent effect of these three mutations on entire ref(2)P alleles was pursued with the construction of transgenes derived from the natural permissive allele ref(2)P(+++)O1 carrying the different combinations of the d, p, and p′ mutations. The results obtained are presented in Table 2, lines 5–10.
Expression of all the recombinant transgenes restored fertility to Su(P)ste ref(2)P-deficient males, demonstrating that the ref(2)P fly function was recovered due to our constructs. A new phenotype arose for two of the recombinant alleles, ref(2)P(d++)O1 and ref(2)P(d+p′)O1 (Table 2, lines 9 and 10). Infection by the sigma virus produced CO2 symptoms in ref(2)P-deficient flies carrying these transgenes, but not in a ref(2)P(dpp′)Pa/ref(2)P− background. These alleles, being neither restrictive nor permissive but retaining fly functionality, are therefore defined as virus unfunctional.
A reduced synthesis of the Ref(2)P protein, due to low expression of the transgene, might explain why ref(2)P(d++) and ref(2)P(d+p′) constructs appeared unfunctional for the virus. We monitored expression of our constructs, at both the RNA and the protein level. The transcripts produced by the transgene were quantified by dot blotting in two independent fly lines for each of the two virus-unfunctional transgenes. The quantity of transcripts produced by a transgene was similar to that observed with a resident allele (data not shown). Immunodetection of the Ref(2)P protein in adult flies (Figure 3) revealed no obvious difference among the different fly lines or with a reference permissive fly line. Therefore, the virus-unfunctional character of ref(2)P(d++) and ref(2)P(d+p′) is unlikely to result from a reduced expression of the transgenes.
Figure 3.—
Immunodetection of Ref(2)P in different transgenic fly lines. After immunoprecipitation of Ref(2)P from 100 or 25 flies, protein samples were loaded on a 10% SDS–PAGE gel and transferred before immunodetection with an anti-Ref(2)P antibody. Fly lines homozygous for ref(2)PO1 transgenes bearing different combinations of dpp′ mutations in a ref(2)P−/ref(2)P− loss-of-function background. Similar levels of protein were observed in each fly line. These levels were not distinguishable from what is observed in a wild-type permissive fly line (OM, resident).
Hence, we can conclude that these two alleles belong to a new category of ref(2)P alleles: they are neither restrictive nor permissive, and they are different from loss-of-function alleles. While fly functionality is maintained, the functions of the virus are impaired. The existence of such virus-unfunctional alleles demonstrates that the viral and host functions of ref(2)P can be separated.
The Q28N29G mutation d induces a loss of permissivity and the additional I32V mutation p defines restrictivity:
Our data suggest that absence of the d mutation is essential to the permissive character of ref(2)P alleles. Indeed, the alleles carrying a +xx site [ref(2)P(+++)O1, ref(2)P(+pp′)O1, ref(2)P(++p′)O1, and ref(2)P(+p+)O1—Table 2, lines 1, 6, 7, and 8, respectively] are all permissive. On the contrary, when the d mutation is present, alleles are either restrictive [ref(2)P(dpp′) and ref(2)P(dp+), Table 2, lines 3 and 5, respectively] or virus unfunctional [ref(2)P(d+p′) and ref(2)P(d++), Table 2, lines 9 and 10, respectively]. The d mutation hence induces a loss of permissivity, but does not suffice to define a restrictive allele.
The second mutation, p, modulates virus-related functions in the presence of the d mutation. While the presence of the d mutation alone or associated with the p′ mutation results in virus-unfunctional alleles [ref(2)P(d+p′) and ref(2)P(d++), Table 2, lines 9 and 10], concomittance of d and p produces restrictive alleles [ref(2)P(dpp′) and ref(2)P(dp+), Table 2, lines 3 and 5]. The p mutation hence defines whether alleles are restrictive or virus unfunctional when the d mutation is present. Taken together, these results demonstrate that the Q28N29G mutation d induces a loss of permissivity, and the additional I32V mutation p defines restrictivity.
The Q43L mutation p′ is essential to restrictivity with viral strain 23:
A potential contribution of the p′ mutation to the restrictive character of ref(2)P was not detected in the preceding experiments with the A3 virus. Thus, we next focused on this mutation. A quantitative analysis of the inoculum necessary to infect adult ref(2)P-deficient flies carrying the different transgenes was performed. Following inoculation of transgenic adult flies, the restrictive alleles ref(2)P(dpp′)Pa and ref(2)P(dpp′)O1 behave similarly in this test (data not shown; P = 0.63). The permissive alleles ref(2)P(+++)Pa and ref(2)P(+++)O1 are also undistinguishable (data not shown; P = 0.69), thus confirming that the mutations located outside the PB1 domain display no effect on the virus-related functions of ref(2)P. Data obtained with the A3 virus are shown in Figure 4A, and Figure 4B presents the results obtained with viral strain 23.
Figure 4.—
Role of the p′ mutation in restrictivity for viral strain A3 (A) and 23 (B) and of the PB1 domain alone on restrictivity for the A3 strain (A). The concentration of the efficient inoculum necessary to infect adult flies and the error bars were determined as in Figure 2. The size of inoculum necessary to infect ref(2)P−/ref(2)P− (i.e., in the absence of any transgene) was arbitrarily defined as 1.00. (A) Role of the p′ mutation and PB1 domain in sensitivity to the A3 virus. The transgenes used are indicated in the figure. (B) Effect of ref(2)P mRNA quantity on sensitivity to the viral strain 23: role of the p′ mutation. On the x-axis, mRNA quantity for one copy of the permissive allele is arbitrarily defined as 1. Additional copies of transgenes in Drosophila do not necessarily result in a proportional increase in mRNA. ▵, ref(2)P(dp+)O1; ○, ref(2)P(dpp′)O1.
With the A3 virus (Figure 4A), the ref(2)P(dp+) allele was clearly restrictive when compared to ref(2)P-deficient (control without transgene defining value 0) or permissive [ref(2)P(+++)] alleles. However, it could be slightly less restrictive than the restrictive control allele ref(2)P(dpp′) although both values are not statistically different (P = 0.13). An effect of the p′ mutation on restrictivity may be observed with the A3 virus, but our assay does not allow us to conclude firmly.
We also tested the effect on restrictivity of the p′ mutation with the virus strain 23 (Figure 4B). In this experiment, we aimed to test the effect of an increased copy number of recombinant alleles. This was possible only with viral strain 23, since it is less sensitive to restrictive alleles than strain A3. Indeed, in the case of A3 inoculated in flies with high expression of restrictive alleles, the concentration of viral inoculum to obtain CO2 symptoms would be too high. Our data obtained for an mRNA level equal to 1 show that, although protein levels are equivalent (see Figure 3, dp+ and resident), the ref(2)P(dp+) transgene is clearly less restrictive than ref(2)P(dpp′) (P = 5.10−4), but not clearly different from an unfunctional allele (value 0). Moreover, the size of inoculum necessary to infect flies increases with mRNA levels for the ref(2)P(dpp′) transgene. On the other hand, increasing the quantity of mRNA levels for ref(2)P(dp+) has no effect, revealing that this allele cannot inhibit the strain 23 viral cycle. Therefore, in the case of viral strain 23, a ref(2)P allele carrying the d and p mutations behaves like a deficiency, while an additionnal p′ mutation generates a restrictive allele. The Q43L mutation p′ is essential to the restrictive character with virus strain 23 but not with A3, thus highlighting the difference in sensitivity of each viral strain to variants of the PB1 domain.
A mutated PB1 domain is sufficient to define a restrictive allele:
As shown above, dpp′ mutations are sufficient to characterize ref(2)P alleles in viral functions. As these mutations are comprised within the conserved PB1 domain, we then tested whether the PB1 domain is sufficient in itself to recapitulate the effects observed with the full-length Ref(2)P protein. Two new ref(2)P transgenes were constructed. They encode C-terminal truncated Ref(2)P proteins retaining only the first 91 amino acids. The ref(2)P(+++)Δ92-599 transgene contains the PB1 domain from ref(2)P(+++)O1, while the recombinant ref(2)P(dpp′)Δ92-599 includes the dpp′ mutations (Figure 1B).
Neither of these two trangenes has the capacity to restore the fertility of Su(P)ste ref(2)P-deficient male flies (data not shown). The host function is therefore lost due to C-terminal truncation. Therefore, the fly function of ref(2)P cannot be recapitulated by the PB1 domain.
As shown in Figure 4A, ref(2)P(dpp′)Δ92-599 inhibits viral multiplication as efficiently as the full-length restrictive allele [P = 0.83 when compared to ref(2)P(dpp′)O1]. On the contrary, ref(2)P(+++)Δ92-599 is permissive and allows improvement of viral cycling, although it is not as efficient as a full-length permissive allele [ref(2)P(+++)O1 (P = 0.043)]. From these results, we conclude that a PB1 domain of Ref(2)P containing the dpp′ mutations is sufficient to direct the restrictive effect. Nevertheless, the fact that ref(2)P(+++)Δ92-599 is less permissive than the ref(2)P(+++) allele suggests that the PB1 domain alone does not permit expression of the full permissive character.
Evolution of the PB1 domain:
Since the PB1 domain is sufficient to completely define the restrictive character, it was considered to describe the restrictivity site evolution. Using a D. simulans haplotype as an external reference and 14 natural sequenced alleles (Wayne et al. 1996), a tree was built (Figure 5). Except for the typical alleles ref(2)P(+++)O1 and ref(2)P(dpp′)Pa, these alleles constitute a random sample for ref(2)P at the scale of the D. melanogaster species (Kreitman 1983; Kreitman and Aguade 1986). Figure 5 shows that all the alleles containing mutations within the PB1 domain, including the restrictive alleles, define a monophyletic tree.
Figure 5.—
Evolution of the Ref(2)P PB1 domain. This tree was built according to the principle of parsimony. The external reference is D. simulans Ref(2)P (Wayne et al. 1996). No synonymous mutation is observed in the PB1 coding sequence among the 14 fully sequenced haplotypes. The number of sequenced alleles corresponding to each category is in parentheses.
Among the 14 sequenced haplotypes of D. melanogaster, 7 are identical to the ancestral allele in the PB1 domain. These 7 ref(2)P(+++) alleles are permissive, suggesting that the ancestral allele was a ref(2)P(+++) permissive. In addition, our study suggests that the mutations necessary to obtain the currently encountered restrictive alleles would have appeared in the following order: p′, p, and, finally, d.
DISCUSSION
Infection of Drosophila populations by the sigma rhabdovirus is controled by different fly genes, including ref(2)P. We aimed to study how ref(2)P polymorphic sites found in natural populations affect viral infection. By determining the effect of recombinant alleles carrying various combinations of these polymorphic sites, we demonstrate that it is possible to isolate the fly- and virus-related functions of Ref(2)P. Our genetic assay offers a rare opportunity to study independently two unknown functions of the same protein.
It had previously been shown that the PB1 domain is essential to Ref(2)P activities. Indeed, the ref(2)Pod2 allele that leads to synthesis of a wild-type quantity of protein without the PB1 domain mimics a ref(2)P deficiency (Gay and Contamine 1993; Wyers et al. 1995). Here we demonstrate that the PB1 domain bears the viral function of Ref(2)P (Figure 4A). It recapitulates the full ref(2)P restrictive effect when carrying the d, p, and p′ mutations. Similarly, the permissive ref(2)P(+++)Δ92-599 allele mimics a permissive allele even if it is not as efficient in improving viral multiplication.
Nevertheless, fly functionality is affected by C-terminal truncation of the Ref(2)P protein and hence is not carried by an isolated PB1 domain. But functionality for Drosophila in our study was tested only through male fertility. The molecular mechanisms involved in this sterility are unknown. Recent studies have shown a conserved interaction between Ref(2)P and atypical protein kinase C (DaPKC) (Avila et al. 2002). Moreover, simultaneous gene silencing of ref(2)P and DaPKC results in a reduction of the expression of drosomycin, a compound implicated in innate immunity and activated by the Toll pathway (Goto et al. 2003). Thus, additional functions of Ref(2)P, other than those involved in male fertility, seem possible and it would be of interest to test if mutations affecting ref(2)P modulate the Toll pathway in a way similar to what was observed when focusing on sigma infection.
One could hypothesize that Ref(2)P modulates sensitivity to sigma through the Toll pathway. In that case, the Toll pathway could be rendered more efficient by restrictive mutations. However, since we have shown here that the strength and the type of effect of ref(2)P alleles depends on the sigma strain (Figure 4), we do not favor this hypothesis. If d, p, and p′ mutations were to decrease the Toll-dependent immunity, these mutations should be counterselected in uninfected flies, which was not observed in Languedoc populations (Fleuriet and Periquet 1993). It is still possible that PB1 serves as a sensor to sigma virus infection that would regulate the Toll pathway and this could be tested.
Our work shows that the PB1 domain of Ref(2)P is necessary for both viral and host functions but is sufficient only for viral functions. PB1 domains are usually involved in protein–protein interactions. Indeed, two types of PB1 domains can be distinguished, type I and type II. For the atypical protein kinase C, it has been reported that its heterodimerization with the mammalian orthologs of Ref(2)P, ZIP/p62 occurs through a PB1 type I–PB1 type II interaction (Hirano et al. 2004). Ref(2)P also contains both type I and type II subdomains in its PB1 domain (data not shown), suggesting that, in our system, it may form heterodimers with another protein.
Which type of protein might this be? Since the PB1 domain carries the virus-related function of Ref(2)P, any sigma viral protein may be an interesting candidate. A physical interaction has previously been shown to occur between the N or P viral proteins and Ref(2)P (Wyers et al. 1993), but no PB1 domain is detectable in N or P (data not shown). Ref(2)P may form oligomers directly with viral proteins other than N or P, or with N or P through an intermediate protein.
One may speculate in any case that such an interaction could play a role in the control of viral cycling. Multiplication of the sigma virus increases with the quantity of permissive ref(2)P products (Figure 2). Moreover, while the sigma virus is capable of replication in ref(2)P-deficient flies, only 7% (Figure 2, 1/15.1) of the number of virus cycles normally occurring in permissive homozygous flies take place. Therefore, we conclude that the sigma virus uses the Ref(2)P permissive protein to improve the efficiency of a viral function.
Our data suggest that the dominant relationship between restrictive and permissive ref(2)P alleles differs, depending on the viral strain used to infect flies (Figure 2). If, as discussed above, an interaction between Ref(2)P and a viral product mediates the aforementioned viral function, it is then possible to interpret this observation as a variation of affinity of the viral product for the permissive vs. restrictive protein. In the case of viral strain 23, the viral product would preferentially interact with permissive Ref(2)P when compared to restrictive Ref(2)P. On the other hand, the A3 protein would display better affinity for the restrictive than for the permissive protein. In addition, the advantage due to permissive Ref(2)P is lower for the virus 23 than for the A3 virus. It could be the result of general lower affinity of viral products to Ref(2)P. However, it remains possible that another Drosophila gene, which would inhibit the virus 23, could be present on our reference permissive chromosomes.
The ref(2)P gene is not an essential gene. Indeed, apart from male sterility in Su(P)ste flies (Bichon et al. 2001), no obvious phenotype is observed in the case of ref(2)P homozygous loss of function. Such a gene can therefore accumulate mutations. Many mutations, especially insertion or deletion ones in the coding sequence, can inactivate the gene, while only specific mutations can create a restrictive allele from an ancestral permissive allele. However, among the 31 natural haplotypes currently known (Dru et al. 1993; Wayne et al. 1996), no unfunctional allele has been observed while eight restrictive alleles were found. This discrepancy evidences the advantage of restrictive over loss-of-function alleles for Drosophila. Indeed, as shown by this study, restrictive alleles are fly functional and much more efficient in inhibiting viral cycles than fly-unfunctional alleles.
To explain the strong polymorphism at the ref(2)P locus, the transient selection hypothesis was proposed (Wayne et al. 1996), which implies that the virus and the ref(2)P allele undergo co-evolution. According to this hypothesis, in a fly population infected by a virus sensitive to a restrictive allele, the frequency of restrictive alleles would increase and the frequency of the sensitive virus decrease until the fly population is cured. It is worth noting that even if a viral clone multiplies in homozygous permissive flies, the resulting viral population contains spontaneous viral mutants able to multiply efficiently in restrictive flies (Contamine 1981). In this case, it should be possible to isolate viral strains displaying a different sensitivity to a particular restrictive allele. Indeed, we have shown that viral strain 23 is less sensitive to the typical restrictive ref(2)P(dpp′) allele than strain A3.
During the transient selection wave, the adapted viruses invaded the fly population. Then, a new mutation at the ref(2)P locus would be necessary to generate a new restrictive allele able to inhibit the new virus. The transient selection wave is based on frequency-dependent selection; i.e., pressure of selection decreases as frequency of the selected allele increases (Ayala and Campbell 1974). Pressure of selection being the presence of viruses that affects viability of eggs, the pressure decreases as viruses are counterselected through an increase in restrictive allele frequency. In other words, the fly population would be cured of infection by the sensitive virus before fixation of restrictive alleles could occur, as observed in Languedoc populations (Fleuriet and Periquet 1993).
Evolution of Ref(2)P viral functions can be studied according to the PB1 domain polymorphism (Figure 5), since this domain is essential and sufficient for viral functions. The tree in Figure 5 built according to the parsimony principle suggests that none of the current variants in the PB1 domain carries a single mutation when compared to the ancestral permissive allele. On the contrary, evolutionarily selected mutations seem to be added on the alleles that already carry mutations in the PB1 domain (i.e., not on the ancestral allele). Most likely, all the ref(2)P variants at the PB1 domain have been restrictive alleles at some time during co-evolution between ref(2)P and the sigma virus. In addition, if among the 14 complete and available ref(2)P sequences used to construct the tree, we examine only those based on a genomic sequence [i.e., we exclude the permissive ref(2)PO2 and the restrictive ref(2)Pn alleles, which correspond to cDNA sequencing (Wayne et al. 1996)], it is possible to take into consideration the different types of mutations outside the PB1 domain: N for amino acid change (nonsynonymous), S for synonymous mutation, and I for mutation located in an intron. When comparing these alleles to the root of the tree, the six permissive alleles that do not carry PB1-located mutations display 7N, 4S, and 23I. On the other hand, the six alleles harboring PB1-located mutations carry 2N, 0S, and 5I. The lower polymorphism observed among PB1-mutated alleles is indeed in agreement with strong evolutionary selection of mutations occurring in this domain and with a recent origin of current restrictive alleles. Our tree suggests that, first, the p′ mutation would have generated a restrictive allele from the ancient permissive allele. After viral adaptation, pressure of selection would have favored a new restrictive allele carrying a p mutation, and, eventually, a d mutation. Our data (Figure 5) suggest that co-evolution between the sigma virus and its host would be the result of six independent selection waves and would have led to the current alleles.
Nowadays, the A3 virus has the capacity to use the Ref(2)P(+pp′) variant as efficiently as the Ref(2)P(+++) protein, which is similar to the ancestral permissive protein (data not shown). Therefore, viral adaptation might enhance viral cycle efficiency with restrictive Ref(2)P without losing efficiency in the presence of permissive alleles. To maintain a highly beneficial effect of the ancestral allele would be important for the virus because this allele remains the most frequent in fly populations. As observed (Figure 5), the result of successive transient selection waves would be an increase in divergence between the ancestral permissive allele and the current restrictive allele. A high divergence would lead to a more difficult viral adaptation. A consequence would be a longer half-life of restrictive alleles and a higher frequency of the most divergent restrictive alleles in the species. Eventually, it may become impossible for the virus to adapt to a strongly divergent restrictive allele without modifying its relationship with the ancestral allele.
Adaptation of Drosophila to the sigma virus through evolution of Ref(2)P reveals an example of a constitutive immunity. The immunity driven by Ref(2)P is much more specific than inducible or constitutive innate immunity. Another example of this constitutive immunity includes CCR5 involvement in resistance to the HIV (Samson et al. 1996). Models for studying this type of immunity are rare but could be of importance in virology. Because insects have no adaptative immunity they provide good models for constitutive immunity. A better understanding of Drosophila and sigma virus interaction including genes other than ref(2)P could shed light onto the general mechanisms of co-evolution.
Acknowledgments
We thank Jean-Michel Rossignol for his useful comments on the manuscript. P.D. was the recipient of a fellowship from the Ministère de l'Education Nationale, de l'Enseignement supérieur et de la Recherche.
References
- Avila, A., N. Silverman, M. T. Diaz-Meco and J. Moscat, 2002. The Drosophila atypical protein kinase C-Ref(2)P complex constitutes a conserved module for signaling in the toll pathway. Mol. Cell. Biol. 22: 8787–8795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayala, F. J., and C. A. Campbell, 1974. Frequency-dependent selection. Annu. Rev. Ecol. Syst. 5: 115–138. [Google Scholar]
- Bichon, A., N. Boukhatem, P. Gay, P. Dru, H. Terzian et al., 2001. Genetic and molecular features of Su(P), a gene that interacts with ref(2)P in male fertility of Drosophila melanogaster. Mol. Genet. Genomics 265: 354–361. [DOI] [PubMed] [Google Scholar]
- Brun, G., and N. Plus, 1980. The viruses of Drosophila, pp. 625–702 in The Genetics and Biology of Drosophila, edited by M. Ashburner and T. R. F. Wright. Academic Press, London.
- Bussereau, F., 1970. Study of CO2 sensitivity symptom induced by sigma virus in Drosophila. I. Influence of inoculation place on delay of symptom appearance. Ann. Inst. Pasteur (Paris) 118: 367–385. [PubMed] [Google Scholar]
- Bussereau, F., 1971. A study of CO2 sensitivity produced by vesicular stomatitis virus in Drosophila melanogaster. Ann. Inst. Pasteur (Paris) 121: 223–239. [PubMed] [Google Scholar]
- Contamine, D., 1981. Role of the Drosophila genome in sigma virus multiplication. I. Role of the ref(2)P: selection of host-adapted mutants at the non-permissive allele ref(2)Pp. Virology 114: 474–488. [DOI] [PubMed] [Google Scholar]
- Contamine, D., 1984. The late functions of Drosophila sigma virus. Arch. Virol. 82: 31–47. [DOI] [PubMed] [Google Scholar]
- Contamine, D., A. M. Petitjean and M. Ashburner, 1989. Genetic resistance to viral infection: the molecular cloning of a Drosophila gene that restricts infection by the rhabdovirus sigma. Genetics 123: 525–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dezelee, S., F. Bras, D. Contamine, M. Lopez-Ferber, D. Segretain et al., 1989. Molecular analysis of ref(2)P, a Drosophila gene implicated in sigma rhabdovirus multiplication and necessary for male fertility. EMBO J. 8: 3437–3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dru, P., F. Bras, S. Dezelee, P. Gay, A. M. Petitjean et al., 1993. Unusual variability of the Drosophila melanogaster ref(2)P protein which controls the multiplication of sigma rhabdovirus. Genetics 133: 943–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleuriet, A., 1988. Maintenance of a hereditary virus, the sigma virus, in populations of its host, D. melanogaster, pp. 1–30 in Evolutionnary Biology, edited by M. K. Hecht and B. Wallace. Plenum Press, New York.
- Fleuriet, A., 1994. Female characteristics in the Drosophila melanogaster-sigma virus system in natural populations from Languedoc (southern France). Arch. Virol. 135: 29–42. [DOI] [PubMed] [Google Scholar]
- Fleuriet, A., and G. Periquet, 1993. Evolution of the Drosophila melanogaster-sigma virus system in natural populations from Languedoc (southern France). Arch. Virol. 129: 131–143. [DOI] [PubMed] [Google Scholar]
- Gay, P., 1978. Drosophila genes which intervene in multiplication of sigma virus. Mol. Gen. Genet. 159: 269–283. [DOI] [PubMed] [Google Scholar]
- Gay, P., and D. Contamine, 1993. Study of the ref(2)P locus of Drosophila melanogaster. II. Genetic studies of the 37DF region. Mol. Gen. Genet. 239: 361–370. [DOI] [PubMed] [Google Scholar]
- Gay, P., and C. Ozolins, 1968. Hereditary transmission of sigma virus by Drosophila bearing the “refractory” gene. I. Study of a P- g- virus strain. Ann. Inst. Pasteur (Paris) 114: 29–48. [PubMed] [Google Scholar]
- Goto, A., S. Blandin, J. Royet, J. M. Reichhart and E. A. Levashina, 2003. Silencing of Toll pathway components by direct injection of double-stranded RNA into Drosophila adult flies. Nucleic Acids Res. 31: 6619–6623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillemain, A., and N. Plus, 1954. Contrôle génique de la multiplication du virus de la sensibilité héréditaire au CO2 chez Drosophila melanogaster. Caryologia (Suppl.): 1211–1213.
- Hirano, Y., S. Yoshinaga, K. Ogura, M. Yokochi, Y. Noda et al., 2004. Solution structure of atypical protein kinase C PB1 domain and its mode of interaction with ZIP/p62 and MEK5. J. Biol. Chem. 279: 31883–31890. [DOI] [PubMed] [Google Scholar]
- Klemenz, R., U. Weber and W. J. Gehring, 1987. The white gene as a marker in a new P-element vector for gene transfer in Drosophila. Nucleic Acids Res. 15: 3947–3959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitman, M., 1983. Nucleotide polymorphism at the alcohol dehydrogenase locus of Drosophila melanogaster. Nature 304: 412–417. [DOI] [PubMed] [Google Scholar]
- Kreitman, M. E., and M. Aguade, 1986. Excess polymorphism at the Adh locus in Drosophila melanogaster. Genetics 114: 93–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli, U. K., 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685. [DOI] [PubMed] [Google Scholar]
- Marchler-Bauer, A., J. B. Anderson, C. DeWeese-Scott, N. D. Fedorova, L. Y. Geer et al., 2003. CDD: a curated Entrez database of conserved domain alignments. Nucleic Acids Res. 31: 383–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson, H. M., C. R. Preston, R. W. Phillis, D. M. Johnson-Schlitz, W. K. Benz et al., 1988. A stable genomic source of P element transposase in Drosophila. Genetics 118: 461–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin, G. M., and A. C. Spradling, 1982. Genetic transformation of Drosophila with transposable element vectors. Science 218: 348–353. [DOI] [PubMed] [Google Scholar]
- Samson, M., F. Libert, B. J. Doranz, J. Rucker, C. Liesnard et al., 1996. Resistance to HIV-1 infection in Caucasian individuals bearing mutant alleles of the CCR-5 chemokine receptor gene. Nature 382: 722–725. [DOI] [PubMed] [Google Scholar]
- Wayne, M. L., D. Contamine and M. Kreitman, 1996. Molecular population genetics of ref(2)P, a locus which confers viral resistance in Drosophila. Mol. Biol. Evol. 13: 191–199. [DOI] [PubMed] [Google Scholar]
- Wyers, F., P. Dru, B. Simonet and D. Contamine, 1993. Immunological cross-reactions and interactions between the Drosophila melanogaster ref(2)P protein and sigma rhabdovirus proteins. J. Virol. 67: 3208–3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyers, F., A. M. Petitjean, P. Dru, P. Gay and D. Contamine, 1995. Localization of domains within the Drosophila Ref(2)P protein involved in the intracellular control of sigma rhabdovirus multiplication. J. Virol. 69: 4463–4470. [DOI] [PMC free article] [PubMed] [Google Scholar]