Abstract
Genetic analyses of nine traits associated with stem water-soluble carbohydrate (SWSC) accumulation and remobilization at grain-filling period under drought stress (DS) and well-watered (WW) conditions were undertaken using doubled haploid lines (DHLs) derived from two Chinese common wheat cultivars. Some significantly and very significantly positive correlation was observed among nine traits associated with SWSC. Higher phenotypic values for most traits were detected under DS. Broad sense heritabilities (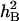 ) of the traits showed wide fluctuations between two water treatments. A total of 48 additive and 62 pairs of epistatic QTL for nine traits were identified as distributing on all 21 chromosomes. A majority of QTL involved significant additive and epistatic effects with interactions of QTL and environments (QEIs). Two additive and two pairs of epistatic loci involved only QEIs without corresponding significant additive or epistatic effects. The contributions of the additive QEIs were two- to fourfolds higher than those of their corresponding additive QTL. Most of the additive QEIs for traits associated with SWSC interacted with DS. In addition, some QTL for the grain-filling efficiencies and thousand-grain weight were colocated in the same or adjacent chromosome intervals with QTL for accumulation and remobilization efficiency of SWSC before 14 days after flowering.
) of the traits showed wide fluctuations between two water treatments. A total of 48 additive and 62 pairs of epistatic QTL for nine traits were identified as distributing on all 21 chromosomes. A majority of QTL involved significant additive and epistatic effects with interactions of QTL and environments (QEIs). Two additive and two pairs of epistatic loci involved only QEIs without corresponding significant additive or epistatic effects. The contributions of the additive QEIs were two- to fourfolds higher than those of their corresponding additive QTL. Most of the additive QEIs for traits associated with SWSC interacted with DS. In addition, some QTL for the grain-filling efficiencies and thousand-grain weight were colocated in the same or adjacent chromosome intervals with QTL for accumulation and remobilization efficiency of SWSC before 14 days after flowering.
WHEAT (Triticum aestivum L.), one of the important staple food crops, is grown under a broad range of environmental conditions in terms of water regimes, climatic factors, and soil types. As water resources for agronomic use become more limiting, drought will increasingly affect yield and yield stability of dryland wheat in arid and semiarid areas. Currently, in these regions wheat crops often suffer from water deficit during the growing season, leading to substantial yield reductions (Ehdaie et al. 1988; Ehdaie and Waines 1989). Therefore, drought tolerance, as well as yield, is a major objective for breeders.
Progress in increasing grain yield and its stability under different water-stressed conditions by direct selection is difficult due to low heritability and significant genotype × environment (G × E) interactions (Ceccarelli et al. 1991; Yin et al. 1999; Teulat et al. 2002). As an alternative, a multitude of morpho-physiological characters have been suggested as associated traits for increasing grain yield under drought conditions (Teulat et al. 2002; Slafer et al. 2005). Among those traits, water-soluble carbohydrates (WSC) of leaves or stems (culm and leaf sheath) have been considered an important physiological trait indicative of drought tolerance because of dual functions, i.e., not only acting in osmotic regulation as the osmolyte under adverse environmental conditions, but also contributing to grain growth and development as the dominant carbon source for grain yield when active photosynthesis is inhibited by terminal drought [drought stress (DS) during grain filling] (Blum 1996; Setter et al. 1998; Diab et al. 2004; Ehdaie et al. 2006; van Herwaarden et al. 2006). There have been many detailed illustrations of the physiological effects of different environmental conditions on WSC accumulation in wheat (Ehdaie and Waines 1989; Virgona and Barlow 1991; Bancal and Triboi 1993; Galiba et al. 1997; Kerepesi and Galiba 2000). Wheat grown under DS may depend more on stem reserves of WSC for grain filling, because current assimilates of flag leaves alone cannot support canopy respiration and grain growth due to water deficit during grain filling (Rawson et al. 1983). Recent studies also demonstrate that the remobilization of pre-anthesis-stored carbohydrate reserves in wheat stem before flowering is promoted by water deficit, which, during grain filling, can enhance plant senescence, accelerate grain filling, and improve yield in cases where senescence is unfavorably delayed by heavy use of nitrogen (Yang et al. 2000, 2001). More detailed studies found that stem water-soluble carbohydrates (SWSCs) accumulated before flowering, and during the early periods after flowering usually accounted for 10–30% of the stem dry weight, depending on cultivars and environments, and contributed up to 70% or more of the grain weight under terminal drought conditions (Bidinger et al. 1977; Setter et al. 1998; Yang et al. 2001).
To develop SWSC as an effective selection criterion, and to examine the feasibility of developing molecular markers for use in marker-assisted selection in breeding programs, it is necessary to understand the molecular genetic basis of SWSC in wheat. Additionally, this understanding would provide researchers with knowledge of how genes and biochemical pathways controlling SWSC are regulated. However, current knowledge of wheat is limited. In other crop species, WSC is a quantitative trait (Galiba et al. 1997; Teulat et al. 2001; Nagata et al. 2002; Takai et al. 2005; Thévenot et al. 2005). Molecular quantitative genetics is a useful approach for studying complex quantitative traits by describing the characteristics of continuous phenotypic distributions and for estimating the number of loci involved, the average gene action, and the degree of interaction between quantitative trait loci (QTL) and environment (Tanksley 1993). The application of this technique may contribute to the identification of loci affecting SWSC accumulation and remobilization. Thévenot et al. (2005) reported that carbohydrate composition and related metabolizable enzymes in mature grains of maize seemed to be controlled predominantly by additive QTL, as well as by QTL interactions. Moreover, numerous candidate genes of the carbohydrate synthetic pathway colocated with QTL. In barley grown in a drought environment, additive QTL for leaf WSC shared some chromosome zones with QTL for plant water status and/or osmotic adjustment by forming clusters of QTL (Teulat et al. 2001). Under normal conditions, additive QTL for SWSC in rice showed a strong association with number of days to heading (Nagata et al. 2002) and were tightly linked to QTL for spanicle fertility (Takai et al. 2005). Galiba et al. (1997) located a gene responsible for SWSC during cold acclimation on chromosome 5A of wheat. Although SWSC proved to be quantitative, no study has been undertaken to investigate QTL × environment interaction (QEI) variability and effects on agronomic traits of crops (Crossa et al. 1999; Hemamalini et al. 2000; Asíns 2002; Campbell et al. 2003).
Our research used a doubled haploid line (DHL) population grown under two water regimes, DS and well watered (WW), to map QTL for accumulation and remobilization of SWSC, as well as grain filling at different developmental stages. The objectives were (1) to detect additive and epistatic QTL controlling accumulation and remobilization of SWSC under two water regimes and (2) to analyze additive QEIs (A-QEIs) and epistatic QEIs (E-QEIs) of these traits. The purpose was to gain further insights into the molecular basis of response to water deficit and to develop practical markers for use in marker-assisted selection in breeding drought-tolerant wheat.
MATERIALS AND METHODS
Plant materials and environmental designs:
The wheat DHL population was the same as used in previous studies (Jing et al. 1999; Hao et al. 2003; Zhou et al. 2005). It was derived from a Hanxuan 10 × Lumai 14 cross. Hanxuan 10 is a drought-tolerant cultivar from Shanxi Academy of Agricultural Sciences, released in 1966 and still grown in arid and barren areas. Lumai 14 is a high-yielding cultivar adapted to abundant water and fertile conditions from Yantai Institute of Agricultural Sciences, Shandong Province, and was widely grown during the 1990s in northern China. A total of 150 lines were grown on the experimental farm (39°48′ N, 116°28′ E, 46 m altitude) of the Institute of Crop Sciences, Chinese Academy of Agricultural Sciences, Beijing. The population was sown in October 2004 and harvested in June 2005. The experimental field was divided into two parts for different water treatments. Field design of each part consisted of randomized complete blocks with three replications. Each plot was two rows of 2 m with 30 cm between rows. One water regime was rain fed and was treated as the DS environment with a total of 133.4 mm rainfall during the growing season; the other regime was WW with 90 mm applied at the pre-overwintering, jointing, flowering, and grain-filling stages, respectively.
Assays of stem-water-soluble carbohydrates and thousand-grain weight:
In each genotype/line, main stems with the same heading date were tagged as selected samples. Five main shoots were cut at the soil surface at three phenological stages, viz., flowering, grain filling [∼14 days after flowering (DAF)], and maturity. Leaf blades and spikes were removed from samples and main stems retaining only the culm and leaf sheath were immediately put into liquid nitrogen and dehydrated in a refrigerated-vacuum evaporator at 8.106 kPa air pressure and −60° for 24 hr. After dehydration, samples were treated at 105° for 20 min and further dried at 80° until a constant dry weight. Samples were cut into pieces 1∼2 mm in length. SWSC were extracted according to a modified procedure described by Wardlaw and Willenbrink (1994). Extractions were performed with 0.1–0.2 g dry material for each sample with three replications. Samples were boiled in 40 ml ddH2O for 40 min. Stem fractions were filtered and filtrates were transferred to volumetric flasks (50 ml) and brought to 50 ml by addition of ddH2O. Total amounts of SWSC (mg WSC/100 mg dry weight) were determined as fructose equivalents using the anthrone colorimetric assay (Yemm and Willis 1954) at 620 nm on a LG-721 spectrophotometer. After obtaining assay data, including SWSC at the earlier flowering stage (SWSCF), SWSC at the grain-filling stage (SWSCG), and SWSC at the maturity stage (SWSCM), accumulating efficiency of SWSC (AESWC) and remobilization efficiency of SWSC (RESWC) were estimated as percentages by [(SWSCG − SWSCF)/SWSCG] × 100%, and [(SWSCG − SWSCM)/SWSCG] × 100%, respectively.
Five spikes corresponding to main stem samples were collected at the grain filling and maturity stages for each genotype. After all spikes were dried naturally, grains of each genotype were threshed, weighed, and numbered to obtain thousand-grain weight at the grain-filling stage (TGWG) and thousand-grain weight at the maturity stage (TGWM) (g/1000 grains). In addition, the grain-filling efficiencies at the early period (before 14 DAF) (GFEE) and the grain-filling efficiencies at the late period (from 14 DAF to grain maturity) (GFEL) were assessed as percentages by [TGWG/TGWM] × 100% and [(TGWM − TGWG)/TGWM] × 100%, respectively.
Statistical analysis:
Analyses of variance (ANOVA) of the data were conducted by the SPSS version 11.0 statistical package to assess the total and residual variances among DHLs for all traits. Significant differences (P ≤ 0.05) between traits under the two water regimes were determined by two-tailed F-test. Trait data were subjected to one-way ANOVA, treating water environments as random effects and genotypes as fixed effects. Broad sense heritabilities (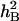 ) were computed from the estimates of genetic (
) were computed from the estimates of genetic (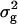 ) and residual (
) and residual (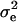 ) variances derived from the expected mean squares as
) variances derived from the expected mean squares as 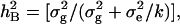 where k is the number of replications. Phenotypic correlations among the traits under the two water regimes were performed using genotypic means.
where k is the number of replications. Phenotypic correlations among the traits under the two water regimes were performed using genotypic means.
QTL identification:
A genetic linkage map, consisting of 395 marker loci, including 132 amplified fragment length polymorphisms and 263 simple sequence repeats, was established from data on 150 DHLs using MAPMAKER/Exp version 3.0 software (Hao et al. 2003; Zhou et al. 2005). The map covered 3904 cM with an average distance of 9.9 cM between adjacent markers. On the basis of a genetic linkage map, QTL for nine target traits, including SWSCF, SWSCG, SWSCM, AESWC, RESWC, TGWG, TGWM, GFEE, and GFEL, were detected using the QTLMapper version 1.0 (Wang et al. 1999) set for composite interval mapping of a mixed linear model. The closest marker to each local LOD peak (putative QTL) was used as a cofactor to control the genetic background while testing at a position of the genome. The threshold LOD score to declare the presence of a QTL was 2.50, and the significance level was P ≤ 0.005 for identifying additive and epistatic effects of QTL and QEIs effects. QTL were named according to the rule of “QTL + trait + research department + chromosome” (McIntosh et al. 1999).
RESULTS
Phenotypic variation among DHLs:
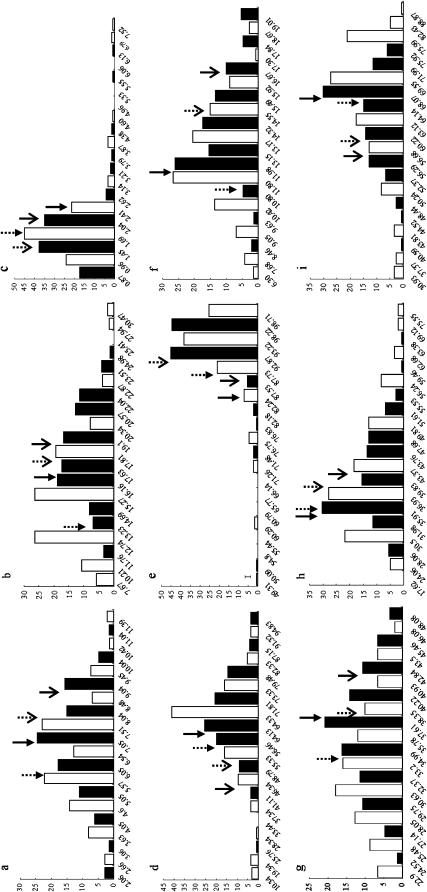
The genotypic means of DHLs and the parents for nine target traits, along with broad sense heritabilities under the DS and WW conditions, are summarized in Table 1. The parents, Hanxuan 10 and Lumai 14, differed significantly in the identified traits. Phenotypic values of Lumai 14 for most traits other than SWSCM, AESWC, and GFEL were much higher than those of Hanxuan 10 under both water regimes. The mean values of DHLs were intermediate between the parents for most traits. Some lines had more extreme values than the parents, showing substantial transgressive segregation. In addition, all target traits showed considerable phenotypic variations and continuous distributions under both water regimes (Figure 1), indicating their quantitative bases. These results suggested that favorable alleles governing the target traits were divided between the parents.
TABLE 1.
Phenotypic data for the parents and DHLs under drought-stressed and well-watered conditions
| Parents
|
DH population lines
|
|||||
|---|---|---|---|---|---|---|
| Traita | Hanxuan 10 | Lumai 14 | Mean ± SD | Minimum | Maximum |
 b b
|
| SWSCF | 6.84/5.59 | 8.95/7.80 | 6.43* ± 1.85/5.96 ± 1.88 | 1.06/1.70 | 10.99/11.34 | 0.49/0.51 |
| SWSCG | 17.22/13.18 | 18.61/17.64 | 17.38** ± 3.08/14.23 ± 4.33 | 10.29/5.14 | 24.93/30.42 | 0.27/0.63 |
| SWSCM | 2.39/1.68 | 2.28/1.60 | 1.46 ± 0.82/1.55 ± 1.02 | 0.28/0.23 | 6.08/7.47 | 0.36/0.59 |
| AESWC | 59.59/57.84 | 44.29/51.32 | 62.09** ± 12.40/56.36 ± 15.34 | 18.09/10.27 | 94.78/91.27 | 0.40/0.57 |
| RESWC | 85.99/88.10 | 87.78/91.21 | 91.17** ± 6.34/88.12 ± 8.12 | 44.74/43.82 | 98.17/98.66 | 0.35/0.60 |
| TGWG | 12.87/11.94 | 17.71/14.53 | 13.39** ± 2.36/11.73 ± 2.46 | 7.29/4.93 | 18.96/18.62 | 0.48/0.52 |
| TGWM | 37.74/33.83 | 41.48/37.93 | 35.59** ± 5.61/31.67 ± 5.97 | 21.90/20.33 | 48.03/46.03 | 0.47/0.53 |
| GFEE | 34.10/35.29 | 42.69/38.30 | 38.09 ± 7.08/38.45 ± 11.30 | 24.13/11.18 | 63.33/75.50 | 0.11/0.65 |
| GFEL | 65.90/64.71 | 57.31/61.70 | 61.91 ± 7.08/61.55 ± 11.30 | 36.67/24.50 | 75.87/88.82 | 0.11/0.65 |
The numbers at the left of the slash (“/”) are the phenotypic values of traits identified under DS, and the numbers at the right indicate WW conditions. *P = 0.05 and **P = 0.01.
SWSCF, stem water-soluble carbohydrates at the flowering stage; SWSCG, stem water-soluble carbohydrates at the grain-filling stage; SWSCM, stem water-soluble carbohydrates at the maturity stage; AESWC, accumulation efficiency of stem water-soluble carbohydrates; RESWC, remobilization efficiency of stem water-soluble carbohydrates; TGWG, thousand-grain weight at the grain-filling stage (∼14 DAF), TGWM, thousand-grain weight at the maturity stage; GFEE, grain-filling efficiency at the early stage (before 14 DAF); and GFEL, grain-filling efficiency at the late stage (from 14 DAF to grain maturity).
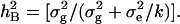
Figure 1.—
Frequency distributions of the DHLs for different traits under two water regimes. a–i represent traits SWSCF, SWSCG, SWSCM, AESWC, RESWC, TGWG, TGWM, GFEE, and GFEL, respectively. The histograms with solid and the open bars represent the frequencies of trait values of DHLs under DS and WW conditions. Solid and open arrowheads represent Hanxuan 10 and Lumai 14, respectively, whereas the continuous and broken arrow shafts represent DS and WW, respectively.
On the basis of the ANOVA, it was apparent that phenotypic means of the DHLs and parents for most traits were highly affected by the DS. The mean values of most of the traits under DS conditions were significantly higher than those under WW conditions. Some traits, such as SWSCF, SWSCG, AESWC, RESWC, TGWG, and TGWM, showed highly significant differences (P < 0.01) (Table 1). The 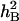 for all traits under DS were generally lower than those under the WW regime. In the WW environment,
for all traits under DS were generally lower than those under the WW regime. In the WW environment, 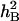 of the target traits varied from 0.51 to 0.65, whereas under DS,
of the target traits varied from 0.51 to 0.65, whereas under DS, 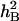 varied from 0.11 to 0.49. The greatest differences in
varied from 0.11 to 0.49. The greatest differences in 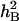 between the two water environments involved SWSCG, SWSCM, RESWC, GFEE, and GFEL (Table 1). This indicated that environmental factors had a large influence on the inheritances of these complex traits.
between the two water environments involved SWSCG, SWSCM, RESWC, GFEE, and GFEL (Table 1). This indicated that environmental factors had a large influence on the inheritances of these complex traits.
Correlation analyses for identified traits:
Correlations among all traits under the two water regimes are given in Table 2. The traits associated with SWSC at different growth stages, e.g., SWSCF, SWSCG, and SWSCM, were poorly correlated with each other, with the exception of the highly significant correlation (r2 = 0.297**; * and ** represent the significant difference of 0.05 and 0.01 level of probability, respectively) between SWSCF and SWSCG under WW conditions. SWSCF showed a highly significant negative correlation with AESWC under both DS (r2 = −0.823**) and WW (r2 = −0.548**) conditions, but had lower correlations with the other traits. However, SWSCG showed a high and positive correlation with AESWC (r2 = 0.444** and 0.479**) and RESWC (r2 = 0.398** and 0.357**) in both environments, TGWM (r2 = 0.239** under DS), TGWG (r2 = 0.198* under WW), and GFEL (r2 = 0.195* under DS). This suggested that SWSCG could play an important role in grain filling of wheat, especially under DS conditions. SWSCM had a negative correlation with most of the other traits, except TGWM and GFEL under both water regimes, and even showed a highly significant negative correlation with GFEE (r2 = −0.223** under WW) and RESWC (r2 = −0.937** and −0.863** under DS and WW). AESWC was significantly correlated with GFEE (r2 = −0.196*) and GFEL (r2 = 0.196*) under DS, whereas the effects were the opposite for RESWC with GFEE (r2 = 0.182*) and GFEL (r2 = −0.182*) under WW conditions. This indicated that SWSC accumulation before flowering could be of more importance to grain filling under DS than under WW conditions.
TABLE 2.
Correlation coefficients for traits under two water regimes
| SWSCF | SWSCG | SWSCM | AESWC | RESWC | TGWG | TGWM | GFEE | GFEL | |
|---|---|---|---|---|---|---|---|---|---|
| SWSCF | 1 | 0.095 | −0.068 | −0.823** | 0.070 | 0.160 | 0.082 | 0.104 | −0.104 |
| SWSCG | 0.297** | 1 | −0.128 | 0.444** | 0.398** | 0.010 | 0.239** | −0.195* | 0.195* |
| SWSCM | −0.052 | 0.030 | 1 | 0.088 | −0.937** | −0.048 | 0.085 | −0.129 | 0.129 |
| AESWC | −0.548** | 0.479** | −0.045 | 1 | 0.057 | −0.097 | 0.099 | −0.196* | 0.196* |
| RESWC | 0.196* | 0.357** | −0.863** | 0.195* | 1 | 0.044 | −0.010 | 0.060 | −0.060 |
| TGWG | 0.040 | 0.198* | −0.152 | 0.048 | 0.167 | 1 | 0.428** | 0.593** | −0.593** |
| TGWM | −0.008 | 0.142 | 0.205* | 0.034 | −0.110 | −0.004 | 1 | −0.461 | 0.461 |
| GFEE | 0.075 | 0.074 | −0.223** | −0.006 | 0.182* | 0.733** | −0.653** | 1 | −1.000* |
| GFEL | −0.075 | −0.074 | 0.223* | 0.006 | −0.182* | −0.773** | 0.653** | −1.000* | 1 |
Numbers in the upper right segment apply to DS; those at the lower left are for WW. *P = 0.05 and **P = 0.01.
Additive QTL and additive QTL × environment interactions:
A total of 48 additive QTL were detected for the nine traits. Map locations and additive effects of the QTL and interaction effects between additive QTL and environments are summarized in Table 3. Except chromosomes 2B, 3D, 4D, 5D, and 6D, the other 16 of 21 chromosomes were mapped QTL for 9 traits (Figure 2). Among them, 23 additive QTL showed interacting effects with environment, whereas 25 showed no interactions. In addition, two QTL were detected on the basis of interaction effects with environments. The phenotypic variance explained by the additive QTL and by the QEIs varied from 1.06 to 7.53% and from 3.88 to 19.43%, respectively, depending on the trait.
TABLE 3.
Additive and interacting effects of QTL × environment of identified QTL for the traits associated with SWSC and TWG
| Trait | QTL | Flanking marker | Sitea (cM) | LOD | Ab | H2(A) (%)b | AE1c | H2(AE1) (%)c |
|---|---|---|---|---|---|---|---|---|
| SWSCF | QSwscf.cgb-1A.1 | WMC59–WMC254 | 10 | 10.26 | −0.456** | 4.34 | 0.526** | 11.55 |
| QSwscf.cgb-1D.1 | WMC432–WMC222 | 0 | 3.72 | 0.299** | 1.87 | 0.336* | 4.71 | |
| QSwscf.cgb-2D.1 | WMC453.1–WMC18 | 0 | 7.77 | −0.404** | 3.41 | −0.421** | 7.40 | |
| QSwscf.cgb-2D.2 | WMC41–WMC170 | 0 | 3.11 | 0.225* | 1.06 | |||
| QSwscf.cgb-4A | WMC420–Xgwm601 | 3 | 5.44 | −0.338** | 2.39 | 0.305* | 3.88 | |
| QSwscf.cgb-4B.1 | Xgwm368–Xgwm107 | 15 | 4.93 | −0.459** | 8.80 | |||
| QSwscf.cgb-7B.1 | CWM467–CWM466 | 0 | 7.52 | −0.436** | 3.97 | −0.387** | 6.25 | |
| QSwscf.cgb-7D | Xgdm88–WMC463 | 0 | 3.41 | 0.354** | 2.62 | |||
| SWSCG | QSwscg.cgb-4A | Xgwm610–Xgwm397 | 4 | 5.29 | −0.703** | 5.60 | 0.739* | 10.50 |
| SWSCM | QSwscm.cgb-1A.1 | CWM516–CWM517 | 0 | 4.09 | −0.240** | 7.53 | ||
| QSwscm.cgb-6B.1 | Xgwm219–WMC341 | 0 | 3.18 | −0.138* | 2.49 | 0.218* | 12.43 | |
| AESWC | QAeswc.cgb-1A.1 | P3156-250–WMC59 | 0 | 4.01 | 2.965** | 7.73 | ||
| QAeswc.cgb-2A.1 | Xgwm372–Xgwm448 | 0 | 4.76 | −4.080** | 7.32 | 3.306** | 9.61 | |
| QAeswc.cgb-3B.1 | P3622-400–P2076-147 | 4 | 4.41 | −3.251** | 4.65 | |||
| QAeswc.cgb-5A | P2470-280–Xgwm154 | 0 | 5.89 | −2.509** | 2.77 | 3.770** | 12.50 | |
| QAeswc.cgb-7B.1 | WMC311–CWM467 | 2 | 5.13 | 2.059** | 1.86 | 2.416* | 5.13 | |
| QAeswc.cgb-7D.1 | WMC436–Xgwm44 | 9 | 5.16 | 3.405** | 5.10 | |||
| RESWC | QReswc.cgb-1A | Xgwm135–CWM516 | 4 | 4.18 | 1.758** | 6.24 | ||
| QReswc.cgb-3B | Xgwm547–Xgwm181 | 1 | 3.14 | −1.357** | 3.72 | |||
| QReswc.cgb-7A.1 | P2071-180–Xgwm260 | 3 | 4.45 | 1.858** | 6.97 | −1.798** | 13.05 | |
| TGWG | QTgwg.cgb-2A.1 | P2478-160–WMC27.2 | 16 | 4.48 | 0.602** | 5.19 | ||
| QTgwg.cgb-4A.1 | CWM145–P3613-190 | 18 | 3.21 | −0.414** | 2.45 | 0.429* | 5.27 | |
| QTgwg.cgb-5B | P4138-238–P5166-275 | 20 | 3.55 | −0.551** | 4.35 | |||
| QTgwg.cgb-6A.1 | Xgwm334–WMC297 | 1 | 3.01 | 0.508** | 3.69 | |||
| QTgwg.cgb-7A | WMC488–P2071-180 | 0 | 4.89 | 0.345** | 1.70 | −0.461** | 6.08 | |
| TGWM | QTgwm.cgb-2D.1 | P5874-138–WMC41 | 20 | 6.98 | 1.505** | 5.48 | ||
| QTgwm.cgb-3A.1 | P3614-280–EST47 | 1 | 7.43 | 1.138** | 4.20 | 1.010** | 4.94 | |
| QTgwm.cgb-3A.2 | CWM48.1–WMC532 | 10 | 6.72 | 1.630** | 6.43 | |||
| QTgwm.cgb-3A.3 | Xgwm391–P8422-170 | 1 | 3.08 | −0.973** | 2.29 | |||
| QTgwm.cgb-3B.1 | P3622-400–P2076-147 | 6 | 2.72 | −1.107** | 2.97 | |||
| QTgwm.cgb-6A.1 | CWM306–Xpsp3071 | 8 | 3.28 | 0.856** | 1.77 | 0.965** | 4.51 | |
| QTgwm.cgb-6A.2 | P3465-460–P3526-130 | 14 | 8.32 | −1.567** | 5.94 | |||
| GFEE | QGfee.cgb-1B.1 | WMC44–Xgwm259 | 10 | 2.96 | 1.628** | 2.52 | ||
| QGfee.cgb-2A1 | WMC522–WMC453.2 | 2 | 9.62 | 2.682** | 6.83 | −2.071** | 8.14 | |
| QGfee.cgb-2A-.2 | WMC27.2–P5166-248 | 0 | 2.98 | 1.787** | 3.03 | |||
| QGfee.cgb-4B | Xgwm107–Xgwm513 | 0 | 10.63 | 2.358** | 5.28 | −2.249** | 9.60 | |
| QGfee.cgb-5A.1 | Xgwm156–Xgwm415 | 4 | 5.77 | 1.763** | 2.95 | −3.199** | 19.43 | |
| QGfee.cgb-5A.2 | Xgwm205.1–Xgwm443 | 8 | 4.35 | −2.456** | 5.73 | 2.047** | 7.96 | |
| QGfee.cgb-6A | Xgwm334–WMC297 | 1 | 2.92 | 1.452** | 2.00 | |||
| QGfee.cgb-6B | P3470-210–WMC269.3 | 21 | 3.27 | 1.373** | 1.79 | |||
| QGfee.cgb-7B.1 | WMC269.4–P3446-380 | 5 | 3.67 | −1.676** | 2.67 | |||
| GFEL | QGfel.cgb-1B.1 | WMC44–Xgwm259 | 10 | 2.96 | −1.628** | 2.52 | ||
| QGfel.cgb-2A.1 | WMC522–WMC453.2 | 2 | 9.62 | −2.682** | 6.83 | 2.071** | 8.14 | |
| QGfel.cgb-2A.2 | WMC27.2–P5166-248 | 0 | 2.98 | −1.787** | 3.03 | |||
| QGfel.cgb-4B | Xgwm107–Xgwm513 | 0 | 10.63 | −2.358** | 5.28 | 2.249** | 9.60 | |
| QGfel.cgb-5A.1 | Xgwm156–Xgwm415 | 4 | 5.77 | −1.763** | 2.95 | 3.199** | 19.43 | |
| QGfel.cgb-5A.2 | Xgwm205.1–Xgwm443 | 8 | 4.35 | 2.456** | 5.73 | −2.047** | 7.96 | |
| QGfel.cgb-6A | Xgwm334–WMC297 | 1 | 2.92 | −1.452** | 2.00 | |||
| QGfel.cgb-6B | P3470-210–WMC269.3 | 21 | 3.27 | −1.373** | 1.79 | |||
| QGfel.cgb-7B.1 | WMC269.4–P3446-380 | 5 | 3.67 | 1.676** | 2.67 |
Genetic distance between the most likely position of the putative QTL and the left flanking marker in the marker interval.
A, the additive effect. A positive value indicates the Hanxuan 10 allele having a positive effect on the trait, and a negative value represents the Lumai 14 allele having positive effect; *P = 0.005 and **P = 0.001; H2(A) (%) indicates the proportion of phenotypic variance explained by additive QTL.
E1, the DS environment; AE1, the additive QTL × environment interaction in DS; the absolute interaction effect value of additive QTL × environment in the WW environment is the same as in the DS environment, but the effect origin is reversed; H2(AE1) (%) indicates the phenotypic variance explained by additive QTL × environment interaction.
Figure 2.—
Chromosome locations of additive QTL for the nine traits associated with SWSC in DHLs. (▪) SWSCF, (□) SWSCG, ( ) SWSCM, (♦) AESWC, (⋄) RESWC, (•) TGWG, (○) TGWM, (▴) GFEE, and (▵) GFEL, respectively.
) SWSCM, (♦) AESWC, (⋄) RESWC, (•) TGWG, (○) TGWM, (▴) GFEE, and (▵) GFEL, respectively.
For SWSC content at three different growth stages, 10 additive QTL included 7 for SWSCF, 1 for SWSCG, and 2 for SWSCM located on different regions of chromosomes 1A, 1D, 2D, 4A, 4B, 6B, 7B, and 7D, with phenotypic variation ranging from 1.06 to 7.53%. No chromosome region had QTL controlling SWSC contents at all three growth stages. Among these QTL, 7 were conferred by favorable alleles from Lumai 14, whereas the other 3 were from Hanxuan 10. In addition, 7 of the 10 additive QTL were identified with A-QEIs. Five A-QEIs were associated with DS, explaining from 3.88 to 12.43% of the phenotypic variation, whereas the other two were associated with WW, explaining 7.40 and 6.25% of the variation. One QTL, QSwscf.cgb-4B.1, was detected with only an A-QEI effect, enhanced by WW conditions with an 8.80% contribution to the phenotypic variation.
The five QTL showing significant associations with AESWC explained from 1.86 to 7.32% of the phenotypic variation. Three loci, mapping on chromosomes 2A, 3B, and 5A, derived their additive effects from favorable alleles of Lumai 14, whereas the additive effects of the other two, located on 7B and 7D, came from favorable alleles in Hanxuan 10. Three QTL, QAeswc.cgb-2A.1, QAeswc.cgb-5A, and QAeswc.cgb-7B.1, among the five loci were involved in significant or very significant interactions with DS, accounting for 9.61, 12.50, and 5.13% of the phenotypic variation, respectively. One QTL, QAeswc.cgb-1A.1, had only an A-QEI effect, explaining 7.73% of the phenotypic variation in the DS environment.
Three QTL controlling RESWC were located on chromosomes 1A, 3B, and 7A, accounting for from 3.72 to 6.97% of the phenotypic variation. The favorable allele of QReswc.cgb-3B came from Lumai 14, and the other two favorable alleles were from Hanxuan 10. Only one of the QTL significantly interacted with WW, explaining 13.05% the phenotypic variation.
Twelve chromosomal regions on linkage groups 2A, 2D, 3A, 3B, 4A, 5B, 6A, and 7A were associated with TGW at the grain filling and maturity stages; these included five QTL for TGWG and seven for TGWM. No consistent QTL was detected for TGW. These loci accounted for 1.70–6.43% of the phenotypic variation. Five of them were favorable alleles from Lumai 14, and the other seven were from Hanxuan 10. Moreover, three QTL were involved in A-QEIs with DS, explaining a phenotypic variation from 4.51 to 5.27%, whereas another locus was associated with the A-QEI with WW, explaining 6.08% of the phenotypic variation.
Nine apparently common QTL associated, respectively, with GFEE and GFEL were located on chromosomes 1B, 2A, 4B, 5A, 6A, 6B, and 7B. Between the corresponding QTL for GFEE and GFEL, the effect values of the QTL, including the additive and A-QEI effects, and their contributory percentages were equivalent, but the effect directions between the two traits were opposite. These QTL for any one of the two traits explained phenotypic variation ranging from 1.79 to 6.83%. The favorable alleles of the two QTL controlling GFEE, QGfee.cgb-5A.2 and QGfee.cgb-7B.1, came from Lumai 14, and the other seven were from Hanxuan 10. Furthermore, four QTL for both GFEE and GFEL involved A-QEIs, accounting for phenotypic variation ranging from 7.96 to 19.43%. The A-QEI effects of three QTL for GFEE were identified under WW and one QTL under DS; on the contrary, the A-QEI effects of three QTL for GFEL were identified under DS and one QTL under WW.
Epistatic QTL and epistatic QTL × environment interactions:
All nine traits were influenced by epistatic effects of the additive × additive type and the interacting effects of the E-QEIs. A total of 64 pairs of epistatic QTL were identified (Table 4). These epistatic QTL showed more interaction trends of nonallelic genes between genomes A and B, explaining phenotypic variations ranging from 0.84 to 8.26%. Among these QTL, 48 pairs involved only epistatic effects, 14 pairs were concerned not only with epistatic effects but also with E-QEI effects, and two pairs involved only E-QEI effects.
TABLE 4.
Epistatic effects and interacting effects of epistatic QTL × environment of identified QTL for the traits associated with SWSC and TWG
| Trait | QTLia | Flanking marker | Site (cM)b | QTLja | Flanking marker | Site(cM)b | LOD | AAc | H2(AA) (%)c | AAE1d | H2(AAE1) (%)d |
|---|---|---|---|---|---|---|---|---|---|---|---|
| SWSCF | QSwscf.cgb-1A.2 | P5522-280–P6934-90 | 0 | QSwscf.cgb-6B | WMC417.2–P4232-550 | 14 | 6.23 | −0.501** | 2.97 | ||
| QSwscf.cgb-1B.1 | Xgwm131–WMC156 | 0 | QSwscf.cgb-1B.2 | P1142-430–P3470-180 | 2 | 4.85 | −0.387** | 1.77 | 0.368* | 3.21 | |
| QSwscf.cgb-1B.3 | CWM90–EST49 | 0 | QSwscf.cgb-3A.1 | WMC388–WMC21 | 0 | 4.55 | −0.281** | 0.94 | |||
| QSwscf.cgb-2B | P4233-160–CWM529 | 2 | QSwscf.cgb-5A.1 | Xgwm410–WMC340 | 7 | 2.97 | −0.383** | 1.73 | |||
| QSwscf.cgb-2D.2 | WMC41–WMC170 | 0 | QSwscf.cgb-2D.3 | WMC181–P3470-220 | 8 | 3.73 | −0.266** | 0.84 | |||
| QSwscf.cgb-3A.2 | WMC21–WMC505.2 | 0 | QSwscf.cgb-5A.2 | P2470-280–Xgwm154 | 0 | 2.91 | −0.303** | 1.09 | |||
| QSwscf.cgb-3B | CWM539.1–WMC236 | 0 | QSwscf.cgb-7B.2 | P1123-235–Xgwm611 | 5 | 2.74 | −0.273** | 0.88 | |||
| QSwscf.cgb-3D | WMC437–WMC529.1 | 15 | QSwscf.cgb-5B | Xgwm371–Xgwm335 | 3 | 3.85 | 0.471** | 2.63 | |||
| QSwscf.cgb-7B.1 | CWM467–CWM466 | 0 | QSwscf.cgb-7B.3 | Xgwm611–Xgwm302 | 6 | 4.86 | −0.452** | 2.42 | |||
| SWSCG | QSwscg.cgb-1B | Xgwm268–WMC44 | 22 | QSwscg.cgb-7A.1 | Xgwm635.2–Xgwm635.1 | 0 | 4.21 | 0.667** | 1.78 | ||
| QSwscg.cgb-1D | CWM1–WMC432 | 0 | QSwscg.cgb-3B | P3716-150–Xgwm644.2 | 0 | 4.58 | 0.707** | 2.00 | −0.703* | 3.95 | |
| QSwscg.cgb-2A.1 | P1138-490–WMC264.2 | 1 | QSwscg.cgb-6A.1 | P3465-460–P3526-130 | 18 | 2.78 | −0.749** | 2.24 | |||
| QSwscg.cgb-2A.2 | P3613-170–CWM276 | 0 | QSwscg.cgb-7A.2 | WMC301–WMC9 | 15 | 3.86 | −0.708* | 4.01 | |||
| QSwscg.cgb-2B | WMC441–WMC344 | 5 | QSwscg.cgb-6B | WMC182–Xgwm644.1 | 0 | 7.07 | 1.184** | 5.61 | −1.060** | 8.99 | |
| QSwscg.cgb-3B | Xgwm547–Xgwm181 | 0 | QSwscg.cgb-7A.3 | P3156-360–WMC83 | 0 | 3.78 | 0.785** | 2.46 | |||
| QSwscg.cgb-6A.2 | EST25–CWM88 | 0 | QSwscg.cgb-7A.4 | CWM48.2–CWM462.1 | 0 | 9.13 | −0.901** | 3.25 | 1.022** | 8.36 | |
| SWSCM | QSwscm.cgb-1A.2 | WMC20–WMC93 | 3 | QSwscm.cgb-4A | CWM145–P3613-190 | 18 | 4.95 | 0.243** | 4.71 | ||
| QSwscm.cgb-1B.1 | P4133-170–Xgwm582 | 4 | QSwscm.cgb-1B.2 | WMC156–P3446-183 | 0 | 4.88 | 0.144* | 1.65 | |||
| QSwscm.cgb-2A.1 | Xgwm68.2–Xgwm71.1 | 0 | QSwscm.cgb-6D | Xgwm325–WMC113 | 0 | 3.60 | −0.197** | 3.09 | |||
| QSwscm.cgb-2A.2 | Xpsp3088–WMC296 | 5 | QSwscm.cgb-5A.2 | Xgwm595–WMC410 | 0 | 4.08 | −0.207** | 3.42 | |||
| QSwscm.cgb-2B.1 | P4233-160–CWM529 | 0 | QSwscm.cgb-4B | WMC47–P3459-220 | 42 | 3.88 | −0.199** | 3.16 | |||
| QSwscm.cgb-2B.2 | P3470-245–Xgwm429 | 5 | QSwscm.cgb-3B.2 | P2449-185–WMC231 | 19 | 3.86 | −0.138* | 1.52 | 0.214* | 7.30 | |
| QSwscm.cgb-3B.1 | P3156-185–P5138-100 | 6 | QSwscm.cgb-5B | Xgwm67–Xgwm213 | 0 | 6.58 | −0.219** | 3.82 | |||
| QSwscm.cgb-5A.1 | P3616-185–P3616-195 | 10 | QSwscm.cgb-7B | Xpsp3033–Xgwm297 | 8 | 3.43 | 0.245** | 4.79 | |||
| QSwscm.cgb-6B.1 | Xgwm219–WMC341 | 0 | QSwscm.cgb-6B.2 | P3470-210–WMC269.3 | 9 | 5.39 | −0.187** | 2.79 | |||
| AESWC | QAeswc.cgb-1A.1 | P3156-250–WMC59 | 0 | QAeswc.cgb-1A.2 | WMC385–P8222-250 | 0 | 7.74 | 2.016* | 1.16 | ||
| QAeswc.cgb-1A.2 | P3156-250–WMC59 | 2 | QAeswc.cgb-5D | Xgdm43–Xgwm174 | 0 | 5.65 | 2.285** | 1.49 | |||
| QAeswc.cgb-2A.2 | P3715-309–P1138-490 | 0 | QAeswc.cgb-3B.2 | P3716-150–Xgwm644.2 | 1 | 4.87 | −2.789** | 2.21 | 2.965* | 5.00 | |
| QAeswc.cgb-3B.1 | P3622-400–P2076-147 | 4 | QAeswc.cgb-3B.3 | WMC291–P3156-185 | 0 | 4.02 | −2.128* | 1.29 | |||
| QAeswc.cgb-4A | Xgwm265–WMC161 | 2 | QAeswc.cgb-4B | Xgwm107–Xgwm513 | 9 | 6.55 | −2.468** | 1.73 | 3.999** | 9.10 | |
| QAeswc.cgb-5B.1 | Xgwm371–Xgwm335 | 5 | QAeswc.cgb-5B.2 | P8143-349–P2454-195 | 1 | 4.08 | −3.173** | 2.86 | |||
| QAeswc.cgb-5B.3 | P5166-275–P8143-349 | 0 | QAeswc.cgb-7A | Xgwm635.2–Xgwm635.1 | 1 | 4.83 | 3.564** | 7.23 | |||
| QAeswc.cgb-6B | P3516-205–P8966-195 | 3 | QAeswc.cgb-7D.2 | Xgwm44–Xgwm121 | 27 | 6.78 | −3.367** | 3.22 | 3.372** | 6.47 | |
| QAeswc.cgb-7B.2 | CWM467–CWM466 | 0 | QAeswc.cgb-7B.3 | Xgwm611–Xgwm302 | 26 | 3.47 | 2.510* | 1.79 | |||
| RESWC | QReswc.cgb-2A.1 | P5858-610–Xgwm328 | 0 | QReswc.cgb-4A | CWM145–P3613-190 | 0 | 3.56 | −1.269** | 3.95 | ||
| QReswc.cgb-2A.2 | WMC27.2–P5166-248 | 17 | QReswc.cgb-2D | P4233-175–P6411-410 | 25 | 3.47 | −1.582** | 6.13 | |||
| QReswc.cgb-7A.1 | P2071-180–Xgwm260 | 3 | QReswc.cgb-7A.2 | WMC301–WMC9 | 15 | 3.91 | −1.157* | 3.28 | |||
| TGWG | QTgwg.cgb-1B | P3622-280–P3442-309 | 0 | QTgwg.cgb-5A | WMC524–Xgwm595 | 0 | 4.28 | 0.592** | 5.16 | ||
| QTgwg.cgb-2A.2 | Xgwm122–CWM138.2 | 4 | QTgwg.cgb-2B | WMC474–Xgwm374 | 0 | 8.12 | 0.552** | 4.49 | −0.434* | 5.55 | |
| QTgwg.cgb-3A.1 | EST47–P3716-170 | 0 | QTgwg.cgb-3A.2 | WMC532–Xgwm369 | 1 | 3.13 | −0.449** | 2.97 | |||
| QTgwg.cgb-4A.2 | CWM145–P3613-190 | 9 | QTgwg.cgb-4A.3 | P4232-260–P2454-270 | 3 | 3.84 | −0.749** | 8.26 | |||
| QTgwg.cgb-6A.2 | Xgwm334–WMC297 | 0 | QTgwg.cgb-6A.3 | P3474-260–CWM162 | 2 | 5.81 | −0.627** | 5.79 | |||
| QTgwg.cgb-6A.4 | WMC179.1–WMC256 | 21 | QTgwg.cgb-6A.4 | P3465-460–P3526-130 | 4 | 3.82 | 0.588** | 5.09 | |||
| TGWM | QTgwm.cgb-2A | P3613-170–CWM276 | 5 | QTgwm.cgb-3B.3 | Xgwm299–CWM539.1 | 1 | 4.66 | −1.488** | 4.10 | ||
| QTgwm.cgb-2B.1 | P6411-216–P3470-245 | 0 | QTgwm.cgb-7B.4 | WMC276–Xgwm577 | 1 | 11.11 | 1.890** | 6.61 | |||
| QTgwm.cgb-2B.2 | WMC344–WMC474 | 2 | QTgwm.cgb-3B.4 | Xgwm340–Xgwm247 | 2 | 8.37 | −1.487** | 4.09 | |||
| QTgwm.cgb-2D.2 | WMC181–P3470-220 | 0 | QTgwm.cgb-7D | Xgwm44–Xgwm121 | 7 | 7.44 | 1.457** | 3.93 | |||
| QTgwm.cgb-3A.1 | P3614-280–EST47 | 0 | QTgwm.cgb-7A.2 | CWM462.2–Xgwm635.2 | 0 | 10.87 | 1.220** | 2.75 | |||
| QTgwm.cgb-3B.2 | P2478-155–WMC505.1 | 0 | QTgwm.cgb-7B.3 | Xgwm297–Xgwm68.1 | 16 | 6.40 | −1.319** | 3.22 | |||
| QTgwm.cgb-3B.1 | P3622-400–P2076-147 | 0 | QTgwm.cgb-4A.1 | P3446-205–CWM145 | 1 | 6.45 | 1.071** | 2.12 | |||
| QTgwm.cgb-4A.2 | P5611-136–P4232-260 | 4 | QTgwm.cgb-7B.2 | WMC526–WMC273 | 2 | 4.25 | −1.117** | 2.31 | |||
| QTgwm.cgb-4D | Xgwm192–WMC331 | 24 | QTgwm.cgb-7B.1 | P6401-238–WMC269.4 | 25 | 4.07 | −0.823** | 1.25 | |||
| QTgwm.cgb-6A.3 | P4232-400–CWM306 | 33 | QTgwm.cgb-7A.1 | P3465-175–CWM48.2 | 7 | 6.08 | −1.004** | 1.86 | −1.132** | 4.74 | |
| QTgwm.cgb-6B.1 | Xgwm219–WMC341 | 0 | QTgwm.cgb-6B.2 | P3470-210–WMC269.3 | 21 | 4.09 | −0.805** | 1.20 | |||
| GFEE | QGfee.cgb-1A | Xpsp3027–Xgwm164 | 0 | QGfef.cgb-2D.2 | P5874-138–WMC41 | 6 | 3.00 | 1.720** | 1.96 | ||
| QGfee.cgb-1B.3 | WMC269.2–CWM90 | 15 | QGfef.cgb-1B.2 | WMC44–Xgwm259 | 6 | 5.92 | 1.905** | 2.41 | |||
| QGfee.cgb-1D | Xgdm33–P3470-527 | 4 | QGfef.cgb-2D.1 | Xgwm261–WMC112 | 0 | 7.93 | −2.459** | 4.01 | 1.924** | 4.91 | |
| Qgfee.cgb-2A.3 | P3613-170–CWM276 | 8 | QGfef.cgb-7B.2 | WMC311–CWM467 | 2 | 5.97 | −1.978** | 2.60 | 1.709* | 3.88 | |
| QGfee.cgb-3B | Xgwm72–Xgwm274 | 0 | QGfef.cgb-7B.3 | Xgwm297–Xgwm68.1 | 16 | 6.96 | 2.502** | 4.15 | |||
| GFEL | QGfel.cgb-1A | Xpsp3027–Xgwm164 | 0 | QGfel.cgb-2D.2 | P5874-138–WMC41 | 6 | 3.00 | −1.720** | 1.96 | ||
| QGfel.cgb-1B.3 | WMC269.2–CWM90 | 15 | QGfel.cgb-1B.2 | WMC44–Xgwm259 | 6 | 5.92 | −1.905** | 2.41 | |||
| QGfel.cgb-1D | Xgdm33–P3470-527 | 4 | QGfel.cgb-2D.1 | Xgwm261–WMC112 | 0 | 7.93 | 2.459** | 4.01 | −1.924** | 4.91 | |
| QGfel.cgb-2A.3 | P3613-170–CWM276 | 8 | QGfel.cgb-7B.2 | WMC311–CWM467 | 2 | 5.97 | 1.978** | 2.60 | −1.709* | 3.88 | |
| QGfel.cgb-3B | Xgwm72–Xgwm274 | 0 | QGfel.cgb-7B.3 | Xgwm297–Xgwm68.1 | 16 | 6.96 | −2.502** | 4.15 |
QTLi and QTLj are a pair of QTL detected by two-dimensional searching.
Genetic distance between the most likely position of the putative QTL and the left flanking marker in the marker interval.
AA, the direction of the epistatic effect: a positive value means that the parent-type effect is greater than the recombinant-type effect, and the negative value means that the parent-type effect is less than the recombinant-type effect; *P = 0.005 and **P = 0.001; H2(AA) (%) indicates the phenotypic variance explained by epistatic QTL.
E1, the DS environment; AAE1, the interaction effect of epistatic QTL × environment in the DS environment; the absolute effect value of QTL × environment interaction in the WW environment is the same as in the DS environment, but the effect origin is reversed; H2(AAE1) (%) indicates the phenotypic variance explained by an epistatic QTL × environment interaction.
A total of 24 pairs of significantly epistatic QTL were detected for SWSC at three different development stages, including 9 pairs each for SWSCF and SWSCM and 6 pairs for SWSCG. Among the 24 pairs of significantly epistatic QTL, 8 pairs had positive effects—i.e., the parent-type effect was higher than the recombinant-type effect—whereas the other 16 pairs showed negative effects where recombinant-type effects were higher than parent-type effects. These epistatic QTL accounted for phenotypic variations ranging from 0.84 to 5.61%. Three pairs involved E-QEIs under DS, explaining phenotypic variations varying from 3.21 to 8.36%, whereas two pairs involved E-QEIs under WW, explaining 3.95 to 8.99% of the phenotypic variations. Furthermore, one pair of QTL, QSwscg.cgb-2A.2 and QSwscg.cgb-7A.2, detected only E-QEI effects with WW, explaining 4.01% of the phenotypic variation.
Eight pairs of epistatic QTL involved AESWC. As for SWSCG, AESWC was involved in three types of interactions. Five pairs showed only epistatic effects, including three pairs with positive effects and two pairs with negative effects, explaining phenotypic variations of 1.16–2.86%. The other three pairs involved both epistatic and E-QEI effects. These loci explained phenotypic variations of 1.73–3.22% with negative effects and also explained phenotypic variations ranging from 5.00 to 9.10% by E-QEIs with DS. Furthermore, one pair detected only an E-QEI effect under DS, explaining 7.23% of the phenotypic variation.
Three pairs of epistatic QTL were detected for RESWC, and these did not interact with environment. The effects of all epistatic loci were negative, explaining phenotypic variations of 3.28–6.13%.
A total of 17 pairs of epistatic QTL involved TGW at both grain filling and maturity; these included 6 pairs for TGWG and 11 pairs for TGWM. These epistatic QTL accounted for phenotypic variations ranging from 1.20 to 8.26%. Among them, 7 pairs showed positive effects and 10 pairs were negative. Only one pair each for TGWG and TGWM involved E-QEIs with WW, accounting for 5.55 and 4.74% of the phenotypic variation, respectively.
Five pairs of epistatic QTL were identified, respectively, for both GFEE and GFEL. As for additive effects for GFEE and GFEL, the effect values, including the epistatic and the E-QEIs or the phenotypic variations, were approximately equivalent between corresponding epistatic QTL for the two traits. However, the effect directions of the specific epistatic loci between the two traits were appropriately opposite. Among the five pairs of epistatic QTL for each trait, three pairs showed only epistatic effects with the same direction, explaining phenotypic variations ranging from 1.96 to 4.15%. Positive effects were shown for GFEE, and negative effects for GFEL. The other two pairs had both epistatic and E-QEI effects at the same time. The epistatic effects were negative for GFEE and positive for GFEL, accounting for 4.01 and 2.60% of the phenotypic variations, respectively. Moreover, the E-QEI effects with DS for GFEE and with WW for GFEL explained 4.91 and 3.88% of the phenotypic variations, respectively.
Distribution of the additive and the epistatic QTL:
In this study, 48 significantly additive QTL for nine traits were distributed on 16 chromosomes in the DHL population. An interesting feature was the highly concentrated distribution of the additive QTL in a few chromosomal regions and the existence of QTL hot spots, namely, the chromosomal regions shared by multiple QTL (Table 3, Figure 2). For example, the additive QTL involved in AESWC and TGWM, QAeswc.cgb-3B.1 and QTgwm.cgb-3B.1, were identified within the same chromosome 3B interval, P3622-400–P2076-147. Similarly, the additive QTL for TGWG, GFEE, and GFEL were colocated in the same marker interval, Xgwm334–WMC297, on chromosome 6A. The same locations always occurred for the traits GFEE and GFEL. However, QTL clustering occurred in several neighboring marker intervals. For example, on chromosome 1A, the chromosomal region flanking markers from Xgwm135 to CWM517 were shared by QTL for RESWC and SWSCM; QTL for TGWG, GFEE, and GFEL, for TGWM and SWSCF, for TGWG and RESWC, and for AESWC and SWSCF shared neighboring intervals with flanking markers from P2478-160 to P5166-248 on chromosome 2A, from P5874-138 to WMC170 on chromosome 2D, from WMC488 to Xgwm260 on chromosome 7A, and from WMC311 to CWM466 on chromosome 7B, respectively. Two important chromosomal regions on the long arm of chromosome 2A and the short arm of chromosome 5A, i.e., WMC522–P5166-248 and Xgwm156–Xgwm443, carried QTL for GFEE and GFEL, where the QTL for TGWG and AESWC were also colocated. Similarly, clustered distributions on some chromosomal regions were also found for loci associated with 64 pairs of epistatic QTL. Most of them were distributed in the same, or adjacent, regions to the significantly additive QTL (Table 4), further increasing the QTL density in the clustered regions. This indicated that specific hot-spot regions might carry genes controlling traits associated with SWSC.
DISCUSSION
Phenotypic variation under two water management conditions:
In this study, variation in SWSC among DHLs and their parents at three developmental stages were approximately coincident under two water management conditions, whereas by ANOVA, it was clear that phenotypic means for most traits were more affected by DS. The means under DS were significantly higher than those under WW (Table 1). It seemed that DS not only increased SWSC content to a certain degree, but also improved TGW of the main stem spike. A suggestion has been made that the proportion of retranslocated pregrain filling biomass increases with water deficit, so that yield reduction associated with reduced post-anthesis assimilation is partly offset by increasing retranslocation (Bidinger et al. 1977). On the other hand, if mild soil drying during the grain-filling period of rice and wheat is controlled properly, it can enhance whole-plant senescence and lead to faster and better remobilization of pre-anthesis-stored carbon from vegetative tissues to the grains. Such gains may outweigh the loss of photosynthesis and the shortened grain-filling period and increase grain yield and harvest index in cases where plant senescence is unfavorably delayed (Yang et al. 2000, 2001, 2002; Yang and Zhang 2006).
In contrast, SWSCG showed significantly positive correlations with AESWC, RESWC, TGWG, TGWM, and GFEL (Table 2), suggesting that SWSCG could play a key function in the subsequent release of carbohydrates from stem to grain. However, a majority of the corresponding traits had lower correlation coefficients between the traits associated with the SWSC and TGW. It seemed that the remobilization mechanism of WSC is complex and not all of the SWSC completely contributes to the improvement of grain yield. The possible fate of those reserves could be consumed in respiration and translocation to the grains or other parts of the plant (McCullough and Hunt 1989; Cruz-Aguado et al. 2000). Likewise, Evans and Wardlaw (1996) reported that there was no simple relationship between grain yield and the amount of reserves mobilized during grain filling in wheat, because the accumulation of stem reserves seems to be sensitive to environmental conditions and source-sink status.
Genetic dissections and pleiotropy of QTL:
Although a wealth of information produced by a multitude of previous studies has considerably improved our understanding of the physiological function of SWSC, as well as its application in wheat-breeding programs, the genetic basis of SWSC and its associated traits at the molecular level seems to be rather obscure because of the complexity. Fortunately, in other cereals such as barley (Teulat et al. 2001), rice (Nagata et al. 2002; Takai et al. 2005), and maize (Thévenot et al. 2005), some additive QTL controlling carbohydrates stored in leaves, culms with leaf sheaths, or seeds have been addressed. These loci are located on different chromosome regions with different genetic effects. In addition, QTL mapping for TGW and associated traits for grain filling have been studied in detail (Börner et al. 2002; Campbell et al. 2003; Takai et al. 2005; Dilbirligi et al. 2006). We identified a total of 48 additive QTL and 62 pairs of epistatic QTL with significant contributory percentages covering nine traits. Among these loci, 10 QTL for SWSC content at three development stages mapped on different regions of seven chromosomes—1A, 1D, 2D, 4A, 6B, 7B, and 7D—with different additive effects and contribution percentages (Table 3). The lack of overlapping intervals for these loci suggested that SWSC could be controlled by different genes at different growth stages. In a previous study, despite a gene responsible for SWSC during cold acclimation located on the long arm of chromosome 5A of wheat (Galiba et al. 1997), we did not identify any significantly additive loci for SWSC on the long arm of 5A except three loci concerned with epistatic effects on SWSC (Table 4). It is likely that different conditions induce gene expression at different loci. Interestingly, Verma et al. (2004) mapped several QTL for grain yield and flag leaf senescence near the markers Xgwm30 and Xgwm539 on chromosome 2D in winter wheat, whereas in this study, three additive QTL for SWSCF and TGWM and eight loci associated with epistasis for SWSCF, TGWM, RESWC, GFEE, and GFEL were adjacent to markers Xgwm30 and Xgwm539 on chromosome 2D. In addition, phenotypic correlation analyses among these traits were found to be similar in our work and previous studies (Yang et al. 2000; 2001; 2002; Yang and Zhang 2006). It was hypothetical that the genetics of SWSC content, SWSC remobilization, grain filling, grain yield, and leaf senescence could be related. Many chromosome linkage groups mapped for traits associated with SWSC have been also reported to share QTL for yield in wheat (Kato et al. 2000; Börner et al. 2002; Quarrie et al. 2005; Dilbirligi et al. 2006; Marza et al. 2006), and some loci for TGW were relatively consistent with previous studies, especially those on chromosome 3A (Campbell et al. 2003; Dilbirligi et al. 2006).
Some QTL for TGW, GFEE, and GFEL were colocated to the same or adjacent intervals with QTL for SWSCF, SWSCG, SWSCM, AESWC, and RESWC (Table 3, Figure 2). For example, QTL for TGWM and SWSCF shared the 0.8-cM P5874-138–WMC41 interval on chromosome 2D. Another interval on chromosome 3B, P3622-400–P2076-147, was shared by QTL for AESWC and TGWM. Interestingly, these additive QTL were not involved in QEIs, indicating they could act independently of the environment. Takai et al. (2005) reported a QTL for percentage grain filling per panicle tightly linked to a QTL controlling WSC content in the culm and leaf sheaths in rice. We also observed that most epistatic QTL for the nine traits were distributed in the same intervals or in regions adjacent to the significant additive QTL (Table 4). These hot-spot regions of QTL could carry genetic information about accumulation and remobilization of SWSC and grain filling of wheat. Colocation of QTL associated with different traits can result from four alternative scenarios: (1) two tightly linked genes modulating the expression of separate traits; (2) one gene with a single function leading to a sequence of causally related events; (3) one gene with an effect on two or more independent traits; and (4) two tightly linked genes with effects on the same two or more traits (Lebreton et al. 1995).
QTL × environment interactions:
The ability of a genotype to modify phenotypic expression in response to different environmental conditions is referred to as phenotypic plasticity (Ungerer et al. 2003). Phenotypic plasticity of quantitative traits arises in nature from interactions between QTL and environments at molecular levels. Several examples of QEIs for agronomic traits showed that the expression of particular chromosome regions differs across environments (Crossa et al. 1999; Hemamalini et al. 2000; Asíns 2002; Campbell et al. 2003). In this study, in addition to identifying the additive and epistatic QTL, A-QEIs and E-QEIs for all nine traits were also detected. In contrast, traits associated with SWSC were involved in more A-QEIs and E-QEIs than traits related to TGW. Most phenotypic variations explained by A-QEIs and E-QEIs were much higher than those explained by the corresponding additive QTL or epistatic QTL (Tables 3 and 4). In addition, 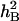 of traits associated with SWSC showed wide fluctuations between the different water conditions (Table 1). From the above-mentioned characteristics, the inheritance of WSC and its associated traits in wheat could be dominated by polygenes that are sensitive to the environment. Likewise, the QTL for WSC content in rice leaf sheaths and culms in different years (Nagata et al. 2002), as well as for WSC content in fresh leaves of barley under different water stress conditions, were located on different chromosomes without sharing regions (Teulat et al. 2001). These results suggested that WSC in crop plants was very sensitive to environments, and the sensitivity consequently brought on phenotypic plasticity.
of traits associated with SWSC showed wide fluctuations between the different water conditions (Table 1). From the above-mentioned characteristics, the inheritance of WSC and its associated traits in wheat could be dominated by polygenes that are sensitive to the environment. Likewise, the QTL for WSC content in rice leaf sheaths and culms in different years (Nagata et al. 2002), as well as for WSC content in fresh leaves of barley under different water stress conditions, were located on different chromosomes without sharing regions (Teulat et al. 2001). These results suggested that WSC in crop plants was very sensitive to environments, and the sensitivity consequently brought on phenotypic plasticity.
In this study, 7 of 10 significantly additive QTL identified for SWSC interacted with environment. QSwscf.cgb-4B.1 was detected only by its A-QEI effect. The contributions of the corresponding A-QEIs (from 3.88 to 12.43% of phenotypic variation) were approximately two- to fourfold higher than those of the independently additive QTL (1.87–5.60%), and a majority of A-QEIs (five of eight) were associated with DS, indicating that DS more greatly enhanced the effects of these loci for SWSC content. This seemed to more appropriately explain why SWSC content under DS was more significantly affected than under WW. In contrast, the number of E-QEIs (only six) was less than that of the A-QEIs and equally distributed between DS and WW (Table 4). However, the majority of epistatic QTL (19 among 24 pairs) were not involved in E-QEIs, indicating that these interacting pairs of loci were less affected by environments than the significantly additive QTL (Table 4). QEIs appeared to be very complex, and QTL for SWSC could have different expression patterns at different growth stages or under different environments. Similar results were obtained for highly heritable traits such as plant height and maturity in rice (Li et al. 2003).
The inheritances of accumulation and remobilization of SWSC and grain filling were also quantitative. The expressions of QTL for AESWC, RESWC, TGWG, TGWM, GFEE, and GFEL were similar to those of SWSC content. Interestingly, A-QEIs and E-QEIs for AEWSC, and A-QEIs for TGWG, occurred only under DS conditions. This could be a further important reason for improving TGW under DS conditions, in addition to the contribution of the additive and epistatic effects. As opposed to early grain filling, the later grain-filling aspects seemed more closely associated with DS, because more A-QEIs effects occurred with DS, and even individual A-QEIs explained up to 19.43% of the phenotypic variation. Li et al. (2003) indicated that this phenomenon might occur in any of the following situations: (1) a QTL expressed in one environment but not in another, as reflected by inconsistent detection of QTL across environments; (2) a QTL expressed strongly in one environment but weakly in another, as indicated by variation in its effects across environments; and (3) a QTL expressed very differently and with opposite effects in different environments.
Acknowledgments
We thank Timothy L. Setter (Crop Improvement Institute, Department of Agriculture, Western Australia) for kindly providing the technical advice for measuring SWSC content in wheat and Robert A. McIntosh (Plant Breeding Institute, University of Sydney, New South Wales, Australia) for revising the manuscript. This work was supported by the Hi-Tech Research and Development (863) Program (2006AA100201) and the State Key Basic Research and Development Plan (2004CB117202) of the People's Republic of China.
References
- Asíns, M.J., 2002. Present and future of quantitative trait locus analysis in plant breeding. Plant Breed. 121: 281–291. [Google Scholar]
- Bancal, P., and E. Triboi, 1993. Temperature effects on fructan oligomer contents and fructan related enzyme activities in stems of wheat (Triticum aestivum L.) during grain filling. New Phytol. 123: 247–253. [Google Scholar]
- Bidinger, F., R. B. Musgrave and R. A. Fisher, 1977. Contribution of stored pre-anthesis assimilate to grain yield in wheat and barley. Nature 270: 431–433. [Google Scholar]
- Blum, A., 1996. Improving wheat grain filling under stress by stem reserve utilization, pp. 135–142 in Wheat: Prospects for Global Improvement, edited by H. J. Braun, F. Altay, W. E. Kronstad, S. P. S. Beniwal and A. McNab, Proceedings of the 5th International Wheat Conference, Ankara, Turkey. Kluwer Academic, Dordrecht, The Netherlands.
- Börner, A., E. Schumann, A. Fürste, H. Cöster, B. Leithold et al., 2002. Mapping of quantitative trait loci determining agronomic important characters in hexaploid wheat (Triticum aestivum L.). Theor. Appl. Genet. 105: 921–936. [DOI] [PubMed] [Google Scholar]
- Campbell, B. T., P. S. Baenziger, K. S. Gill, K. M. Eskridge, H. Budak et al., 2003. Identification of QTLs and environmental interactions associated with agronomic traits on chromosome 3A of wheat. Crop Sci. 43: 1493–1505. [Google Scholar]
- Ceccarelli, S., E. Acevedo and S. Grando, 1991. Breeding for yield stability in unpredictable environments: single traits, interaction between traits, and architecture of genotypes. Euphytica 56: 169–185. [Google Scholar]
- Crossa, J., M. Vargas, F. A. van Eeuwijk, C. Jiang, G. O. Edmeades et al., 1999. Interpreting genotype environment interaction in tropical maize using linked molecular markers and environmental covariables. Theor. Appl. Genet. 99: 611–625. [DOI] [PubMed] [Google Scholar]
- Cruz-Aguado, J. A., R. Rodés, I. P. Pérez and M. Doradoa, 2000. Morphological characteristics and yield components associated with accumulation and loss of dry mass in the internodes of wheat. Field Crops Res. 66: 129–139. [Google Scholar]
- Diab, A. A., B. Teulat-Merah, D. This, N. Z. Ozturk, D. Benscher et al., 2004. Identification of drought-inducible genes and differentially expressed sequence tags in barley. Theor. Appl. Genet. 109: 1417–1425. [DOI] [PubMed] [Google Scholar]
- Dilbirligi, M., M. Erayman, B. T. Campbell, H. S. Randhawa, P. S. Baenziger et al., 2006. High-density mapping and comparative analysis of agronomically important traits on wheat chromosome 3A. Genomics 88: 74–87. [DOI] [PubMed] [Google Scholar]
- Ehdaie, B., and J. G. Waines, 1989. Adaptation of landrace and improved spring wheat genotypes to stress environments. J. Genet. Breed. 43: 151–156. [Google Scholar]
- Ehdaie, B., J. G. Waines and A. E. Hall, 1988. Differential responses of landrace and improved spring bread wheat genotypes to stress environments. Crop Sci. 28: 838–842. [Google Scholar]
- Ehdaie, B., G. A. Alloush, M. A. Madore and J. G. Waines, 2006. Genotypic variation for stem reserves and mobilization in wheat: I. Postanthesis changes in internode dry matter. Crop Sci. 46: 735–746. [Google Scholar]
- Evans, L. T., and I. F. Wardlaw, 1996 Wheat, pp. 501–518 in Photoassimilate Distribution in Plants and Crops: Source-Sink Relationships, edited by E. Zamski and A. A. Schaffer. Marcel Dekker, New York.
- Galiba, G., I. Kerepesi, J. W. Snape and J. Sutka, 1997. Location of a gene regulating cold-induced carbohydrate production on chromosome 5A of wheat. Theor. Appl. Genet. 95: 265–270. [Google Scholar]
- Hao, Z. F., X. P. Chang, X. J. Guo, R. L. Jing, R. Z. Li et al., 2003. QTL mapping for drought tolerance at stages of germination and seedling in wheat (Triticum aestivum L.) using a DH population. Agri. Sci. China 2: 943–949. [Google Scholar]
- Hemamalini, G. S., H. E. S. Shashidhar and S. Hittalmani, 2000. Molecular marker assisted tagging of morphological and physiological traits under two contrasting moisture regimes at peak vegetative stage in rice (Oryza sativa L.). Euphytica 112: 69–78. [Google Scholar]
- Jing, R. L., X. P. Chang, J. Z. Jia and R. H. Hu, 1999. Establishing wheat doubled haploid population for genetic mapping by anther culture. Biotechnology 9: 4–8. [Google Scholar]
- Kato, K., H. Miura and S. Sawada, 2000. Mapping QTLs controlling grain yield and its components on chromosome 5A of wheat. Theor. Appl. Genet. 101: 1114–1121. [Google Scholar]
- Kerepesi, I., and G. Galiba, 2000. Osmotic and salt stress-induced alteration in soluble carbohydrate content in wheat seedlings. Crop Sci. 40: 482–487. [Google Scholar]
- Lebreton, C., V. Lazic-Jancic, A. Steed, S. Pekic and S. A. Quarrie, 1995. Identification of QTL for drought responses in maize and their use in testing causal relationships between traits. J. Exp. Bot. 46: 853–865. [Google Scholar]
- Li, Z. K., S. B. Yu, H. R. Lafitte, N. Huang, B. Courtois et al., 2003. QTL × environment interactions in rice. I. Heading date and plant height. Theor. Appl. Genet. 108: 141–153. [DOI] [PubMed] [Google Scholar]
- Marza, F., G. H. Bai, B. F. Carver and W. C. Zhou, 2006. Quantitative trait loci for yield and related traits in the wheat population Ning7840×Clark. Theor. Appl. Genet. 112: 688–698. [DOI] [PubMed] [Google Scholar]
- McCullough, D. E., and L. A. Hunt, 1989. Respiration and dry matter accumulation around the time of anthesis in field stands of winter wheat (Triticum aestivum). Ann. Bot. 63: 321–329. [Google Scholar]
- McIntosh, R. A., G. E. Hart, K. M. Devos and W. J. Rogers, 1999. Catalogue of Gene Symbols for Wheat. http://grain.jouy.inra.fr/ggpages/wgc.
- Nagata, K., H. Shimizu and T. Terao, 2002. Quantitative trait loci for nonstuctural carbohydrate accumulation in leaf sheaths and culms of rice (Oryza sativa L.) and their effects on grain filling. Breed. Sci. 52: 275–283. [Google Scholar]
- Plaut, Z., B. J. Butow, C. S. Blumenthal and C. W. Wrigley, 2004. Transport of dry matter into developing wheat kernels and its contribution to grain yield under post-anthesis water deficit and elevated temperature. Field Crops Res. 86: 185–198. [Google Scholar]
- Quarrie, S. A., A. Steed, C. Calestani, A. Semikhodskii, C. Lebreton et al., 2005. A high-density genetic map of hexaploid wheat (Triticum aestivum L.) from the cross Chinese Spring × SQ1 and its use to compare QTLs for grain yield across a range of environments. Theor. Appl. Genet. 110: 865–880. [DOI] [PubMed] [Google Scholar]
- Rawson, H. M., J. H. Hindmarsh, R. A. Fisher and Y. M. Stockman, 1983. Changes in leaf photosynthesis with plant ontogeny and relationships with yield per ear in wheat cultivars and 120 progeny. Aust. J. Plant Physiol. 10: 503–514. [Google Scholar]
- Setter, T. L., W. K. Anderson, S. Asseng and I. Barclay, 1998. Review of the impact of high shoot carbohydrate concentrations on maintenance of high yields in wheat exposed to environmental stress during grain filling, pp. 237–255 in Wheat Research Needs Beyond 2000 AD, edited by S. Nagarajan, G. Singh and B. S. Tyagi. Narosa, New Delhi/London.
- Slafer, G. A., J. L. Araus, C. Royo and L. F. G. Del Moral, 2005. Promising eco-physiological traits for genetic improvement of cereal yields in Mediterranean environments. Ann. Appl. Biol. 146: 61–70. [Google Scholar]
- Takai, T., Y. Fukuta, T. Shiraiwa and T. Horie, 2005. Time-related mapping of quantitative trait loci controlling grain-filling in rice (Oryza sativa L.). J. Exp. Bot. 56: 2107–2118. [DOI] [PubMed] [Google Scholar]
- Tanksley, S. D., 1993. Mapping polygenes. Annu. Rev. Genet. 27: 205–233. [DOI] [PubMed] [Google Scholar]
- Teulat, B., C. Borries and D. This, 2001. New QTLs identified for plant water status, water-soluble carbohydrate and osmotic adjustment in a barley population grown in a growth-chamber under two water regimes. Theor. Appl. Genet. 103: 161–170. [Google Scholar]
- Teulat, B., O. Merah, X. Sirault, C. Borries, R. Waugh et al., 2002. QTLs for grain carbon isotope discrimination in field-grown barley. Theor. Appl. Genet. 106: 118–126. [DOI] [PubMed] [Google Scholar]
- Thévenot, C., E. Simond-Côte, A. Reyss, D. Manicacci, J. Trouverie et al., 2005. QTLs for enzyme activities and soluble carbohydrates involved in starch accumulation during grain filling in maize. J. Exp. Bot. 56: 945–958. [DOI] [PubMed] [Google Scholar]
- Ungerer, M. C., S. S. Halldorsdottir, M. D. Purugganan and T. F. C. Mackay, 2003. Genotype-environment interactions at quantitative trait loci affecting inflorescence development in Arabidopsis thaliana. Genetics 165: 353–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Herwaarden, A., R. Richards and J. Angus, 2006. Water-Soluble Carbohydrates and Yield in Wheat. The Australian Society of Agronomy. Proceedings of 13th Agronomy Conference (http://www.regional.org.au/au/asa/2003/c/6/vanherwaarden.htm).
- Verma, V., M. J. Foulkes, A. J. Worland, R. Sylvester-Bradley and P. D. S. Caligari, 2004. Mapping quantitative trait loci for flag leaf senescence as a yield determinant in winter wheat under optimal and drought-stressed environment. Euphytica 135: 255–263. [Google Scholar]
- Virgona, J. M., and E. W. R. Barlow, 1991. Drought stress induced changes in the non-structural carbohydrate composition of wheat stem. Aust. J. Plant Physiol. 18: 239–247. [Google Scholar]
- Wang, D. L., J. Zhu, Z. K. Li and A. H. Paterson, 1999. Mapping QTL with epistatic effects and QTL × environment interaction by mixed linear model approaches. Theor. Appl. Genet. 99: 1255–1264. [Google Scholar]
- Wardlaw, I. F., and J. Willenbrink, 1994. Carbohydrate storage and mobilization by the culm of wheat between heading and grain maturity: the relation to sucrose synthase and sucrose-phosphate synthase. Aust. J. Plant Physiol. 21: 255–271. [Google Scholar]
- Yang, J., and J. Zhang, 2006. Grain filling of cereals under soil drying. New Phytol. 169: 223–236. [DOI] [PubMed] [Google Scholar]
- Yang, J., J. Zhang, Z. Huang, Q. Zhu and L. Wang, 2000. Remobilization of carbon reserves is improved by controlled soil-drying during grain filling of wheat. Crop Sci. 40: 1645–1655. [Google Scholar]
- Yang, J., J. Zhang, Z. Wang, Q. Zhu and L. Liu, 2001. Water deficit-induced senescence and its relationship to the remobilization of pre-stored carbon in wheat during grain filling. Agron. J. 93: 196–206. [Google Scholar]
- Yang, J., J. Zhang, L. Liu, Z. Wang and Q. Zhu, 2002. Carbon remobilization and grain filling in japonica/indica hybrid rice subjected to postanthesis water deficits. Agron. J. 94: 102–109. [Google Scholar]
- Yemm, E. W., and A. J. Willis, 1954. The estimation of carbohydrates in plant extracts by anthrone. Biochem. J. 57: 508–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin, X., P. Stam, C. J. Dourleijn and M. J. Kropff, 1999. AFLP mapping of quantitative trait loci for yield-determining physiological characters in spring barley. Theor. Appl. Genet. 99: 244–253. [Google Scholar]
- Zhou, X. G., R. L. Jing, Z. F. Hao, X. P. Chang and Z. B. Zhang, 2005. Mapping QTL for seedling root traits in common wheat. Sci. Agri. Sin. 38: 1951–1957. [Google Scholar]