Abstract
Expanded CAG·CTG trinucleotide repeat tracts are associated with several human inherited diseases, including Huntington's disease, myotonic dystrophy, and spinocerebellar ataxias. Here we describe a new model system to investigate repeat instability in the Escherichia coli chromosome. Using this system, we reveal patterns of deletion instability consistent with secondary structure formation in vivo and address the molecular basis of orientation-dependent instability. We demonstrate that the orientation dependence of CAG·CTG trinucleotide repeat deletion is determined by the proofreading subunit of DNA polymerase III (DnaQ) in the presence of the hairpin nuclease SbcCD (Rad50/Mre11). Our results suggest that, although initiation of slippage can occur independently of CAG·CTG orientation, the folding of the intermediate affects its processing and this results in orientation dependence. We propose that proofreading is inefficient on the CTG-containing strand because of its ability to misfold and that SbcCD contributes to processing in a manner that is dependent on proofreading and repeat tract orientation. Furthermore, we demonstrate that transcription and recombination do not influence instability in this system.
CAG·CTG repeat expansion is associated with human hereditary neurodegenerative diseases such as Huntington's disease, myotonic dystrophy, and spinocerebellar ataxias (Cummings and Zoghbi 2000; Sinden et al. 2002). The mechanism(s) involved in trinucleotide repeat (TNR) instability (expansion and deletion) has been studied in a number of model systems, including Escherichia coli, yeast, mouse, and cultured cells (Maurer et al. 1996; Freudenreich et al. 1997; Kaytor et al. 1997; Miret et al. 1998; Sarkar et al. 1998; Iyer and Wells 1999; Kovtun and McMurray 2001; McMurray and Kortun 2003; Pelletier et al. 2005). Instability is affected by both cis- and trans-acting factors. Cis-acting factors include the length and purity of the repeat array. In rapidly dividing cells, such as E. coli and yeast, instability is orientation dependent with respect to replication, suggesting a central role for DNA synthesis in instability. Trans-acting factors include the replication machinery and the repair and recombination proteins of the organism. Furthermore, mutations in replication genes in yeast—Polα, Polδ, PCNA, Fen1/Rad27, Dna2 helicase, and primase—have profound effects on repeat instability (Schweitzer and Livingston 1999; Callahan et al. 2003). On the other hand, studies of instability in mice and in cultured cells have revealed evidence for replication-independent expansion of repeat arrays (Takano et al. 1996; Hashida et al. 1997; Kovtun and McMurray 2001). This is consistent with expansion of repeats in human tissues, such as the brain where cells seldom replicate. However, even these replication-independent expansion events are likely to be dependent on DNA synthesis at sites of DNA repair. It is therefore essential to understand how the machinery required to copy DNA interacts with these repetitive sequences.
E. coli provides an attractive model system in which to study the basic properties of DNA replication, recombination, and genetic instability because of the detailed understanding of its genetic and biochemical pathways, its rapid growth, and sophisticated biotechnology. Despite these advantages, all previous studies have considered instability on bacterial plasmid-borne repeats apart from one recently reported study on chromosomal trinucleotide repeat instability (Kim et al. 2006). The studies based on plasmid systems have included investigations of the effects of transcription (Bowater et al. 1997; Schumacher et al. 2001), mismatch repair (Jaworski et al. 1995; Schmidt et al. 2000), nucleotide excision repair (Oussatcheva et al. 2001), proofreading (Iyer et al. 2000), and recombination (Jakupciak and Wells 1999, 2000a,b; Hashem et al. 2004). This body of work has revealed many different effects, and contradictory conclusions have sometimes been reached. It is likely that aspects of plasmid biology such as copy number, size, and specialized processing pathways have contributed to the differences observed.
Orientation dependence of TNR instability has previously been seen for E. coli plasmids and in yeast. This has been explained on the basis of lagging-strand replication dynamics coupled to the greater thermodynamic stability of CTG repeat hairpins relative to CAG repeat hairpins (Gacy et al. 1995; Kang et al. 1995; Rosche et al. 1995; Maurer et al. 1996; Freudenreich et al. 1997; Miret et al. 1998). The model suggests that CTG repeat hairpins formed on the lagging-strand template lead to deletions while CTG repeat hairpins formed on the nascent lagging strand lead to expansions. Since the E. coli system is biased toward deletions and not many expansions are observed, even when CTG repeats are present on the nascent lagging strand, this model is at best an incomplete explanation of orientation dependence of repeat instability in E. coli.
Trinucleotide repeats, when single stranded, can fold into hairpins in vitro (Gacy et al. 1995; Mitas et al. 1995a; Smith et al. 1995; Yu et al. 1995a,b; Petruska et al. 1996), which can be attacked by SbcCD (Connelly et al. 1999). The SbcCD nuclease of E. coli is the homolog of the Mre11/Rad50 nuclease in eukaryotes (Sharples and Leach 1995). The enzyme has double-strand exonuclease and hairpin endonuclease activities and acts on a variety of substrates, including DNA hairpins (Connelly and Leach 1996; Connelly et al. 1997, 1999). It has also been shown to affect the frequency and nature of deletions between 101-bp direct repeats flanking an inverted repeat sequence and can affect the nature of a deletion event between 101-bp direct repeats, even in the absence of an inverted repeat sequence, in a manner consistent with nucleolytic processing of the slippage intermediate (Bzymek and Lovett 2001a,b). The observations of CTG and CAG hairpins (Gacy et al. 1995; Mitas et al. 1995b; Smith et al. 1995; Yu et al. 1995a; Petruska et al. 1996) and of slipped mispairing structures in vitro (Pearson et al. 1998; Sinden et al. 2002) have given rise to the hypothesis that misfolded structures play a role in instability. Preformed slipped mispairing structures are repaired in vitro in human cell extracts to delete, retain, or shorten the looped-out repeat containing strand, demonstrating the presence of activities capable of processing misfolded structures (Panigrahi et al. 2005). The hypothesis that secondary structures may be involved in the processing of CAG·CTG repeats in vivo has received some support from observations implicating the SbcCD and Rad50–Mre11 complexes in E. coli and yeast. In E. coli, it has been reported that a multiply mutant strain (SURE) shows dramatic expansion of CAG·CTG repeats in a plasmid that is prevented by SbcCD (Sarkar et al. 1998). In yeast, a reduction in CAG repeat expansions associated with double-strand break repair in mre11Δ strains was suppressed by overexpressing the Rad50–Mre11 complex, suggesting that it may cleave hairpin structures (Richard et al. 2000). Furthermore, CTG repeat-induced spontaneous double-strand breaks were reduced in a rad50 mutant (Freudenreich et al. 1998). Arguments against the involvement of secondary structures come from the inability to drive a structural transition in vitro in supercoiled templates (Bacolla et al. 1997) and the lack of effect of the sbcCD genotype on CAG·CTG repeat instability in a plasmid system different from that of Sarker et al. (1998; Schmidt et al. 2000). Furthermore, the small changes in repeat length observed in plasmid substrates show no bias to even-numbered patterns of deletion products (except as a consequence of mismatch repair, which eliminates +1 and −1 repeat changes; see Schmidt et al. 2000) despite the existence of preferred folding patterns comprising even numbers of trinucleotide repeats in vitro (Petruska et al. 1998) and in vivo (Darlow and Leach 1995). Relevant to many of these arguments is the observation of an SbcCD effect on strand slippage in the absence of a hairpin-forming substrate (Bzymek and Lovett 2001a,b), which weakens any argument for DNA structures based on SbcCD or Rad50/MRE11 effects. It is clear that despite the strong evidence that CAG·CTG repeats can form secondary structures in vitro that are substrates for enzymatic processing, the question of whether secondary structure plays a role in instability in vivo has been more difficult to determine experimentally and the hypothesis remains controversial.
In E. coli, proofreading during DNA replication is performed by the 3′–5′ exonucleolytic ɛ-subunit of DNA polymerase III, which is encoded by the dnaQ gene. During replication, the proofreading function prevents slipped-strand pairing events that can lead to instability in repeated sequences. A mutation in the proofreading function of DNA polymerase III, dnaQ49ts, was shown to enhance instability of CAG·CTG trinucleotide repeats (Iyer et al. 2000). Another mutation, mutD5, along with dnaQ49ts, was shown to enhance instability of tandem repeat sequences (Saveson and Lovett 1997; Bzymek et al. 1999).
Here, we describe a study of trinucleotide repeat instability carried out in the E. coli chromosome. We demonstrate that instability is length and orientation dependent. Longer repeat arrays are more unstable than shorter repeat arrays and CTG repeats on the lagging-strand template are more unstable than on the leading-strand template. Furthermore, for both orientations of CAG·CTG trinucleotide repeat tracts, the distributions of deletion lengths are skewed in a way that is consistent with deletions stimulated by hairpin structures. This is direct evidence that secondary structure plays a role in CAG·CTG repeat instability in vivo. We also demonstrate that mutation of the gene encoding the proofreading subunit of DNA polymerase III (DnaQ) destabilizes CAG·CTG trinucleotide repeat tracts. Furthermore, orientation dependence of instability is lost in the dnaQ mutant, and SbcCD, whose homolog in eukaryotes is Rad50/Mre11, modulates the effect of DnaQ. These data argue that intermediates in the replicative pathway leading to trinucleotide repeat instability are detected and processed by both proofreading and the SbcCD (Rad50/Mre11) complex. Furthermore, we demonstrate that this replicative instability is not caused by transcription or recombination.
MATERIALS AND METHODS
Construction of pLacD1 and pLacD2:
The plasmid pLacD1, a derivative of pTOF24 (Merlin et al. 2002), was created to insert TNR arrays into the beginning of lacZ in the E. coli genome using a region of lacZ homology. The 800-bp lacZ DNA fragment was constructed using crossover PCR. Using CSH100 (DL844) as template DNA, primers Lac1F (AAA AAC TGC AGT TGG TGC GGA TAT CTC GGT AGT GG) and Lac1R (GAA GAC GCA ATT GGA GAC CAT GGT CAT AGC TGT TTC CTG TG) were used to create one homology arm, while primers Lac2F (TTT TTC AGC TGA ATA ATT CGC GTC TGG CCT TCC TG) and Lac2R (GGT CTC CAA TTG CGT CTT CGT CGT TTT ACA ACG TCG TGA CTG) were used to create the other. Primers Lac1F and Lac2F were used to amplify the fusion of the two PCR products. CSH100 contains the L8 mutation in the CRP-binding site upstream of the lacZ start codon, which was introduced into the lac homology arms during cloning. The three restriction enzyme sites, BsaI, MfeI, and BbsI, were introduced 10 bp downstream from the start codon of lacZ without altering the reading frame, and the restriction sites for these enzymes in the plasmid pTOF24 were removed using site-directed mutagenesis (SDM) before inserting the 800 bp of lacZ homology between PstI and SalI restriction sites. MfeI was removed using the primers MfeI_SDM_F (CAT CTC AAC TGG TCT AGG TGA TTT TAA TCA CTA TAC CAA CTG AGA TGG G) and MfeI_SDM_R (CCC ATC TCA GTT GGT ATA GTG ATT AAA ATC ACC TAG ACC AGT TGA GAT G); BsaI was removed with the primers BsaI_SDM_F (GTC TAT TGC TGG TAT CGG TAC CCG ACC TGC AGG) and BsaI_SDM_R (CCT GCA GGT CGG GTA CCG ATA CCA GCA ATA GAC); and BbsI was removed with the primers BbsI_SDM_F (CGA CTC CTG CAT CCC TTT CAT CTT CGA ATA AAT ACC) and BbsI_SDM_R (GGT ATT TAT TCG AAG ATG AAA GGG ATG CAG GAG TCG). All alterations to pTOF24 were confirmed via restriction enzyme digestion and DNA sequencing. After rounds of site-directed mutagenesis, the 800-bp lacZ fragment was cloned using PstI and SalI. This modified plasmid was named pLacD1.
Plasmid pLacD2 was derived from pLacD1. pLacD1 had an extra site for BbsI in one lac homology arm, which was removed by changing A to G using primers SDM_BbsI_F [5′-GGG ATA CGA CGA TAC CGA GGA CAG CTC ATG-3′ (underlined sequences define the position of the BbsI site and the boldface G is the base that has been changed from A by SDM)] and SDM_BbsI_R (5′-CAT CAG CTG TCC TCG GTA TCG TCG TAT CCC-3′) by site-directed mutagenesis, resulting in the plasmid pLacD2. A list of plasmids is provided in Table 1.
Building of long repeat arrays:
Repeat arrays were generated in the plasmid pLacD2, which was further used to integrate the repeats in chromosomes. (CAG)5 and (CTG)5 repeats were introduced between the lac homology arms of pLacD2 by site-directed mutagenesis using primer pairs ExCAG-01 (5′-CTA TGA CCA TGG TCT CGC AGC AGC AGC AGC AGG TCT TCG TCG TTT TAC-3′), ExCTG-01 (5′-GTA AAA CGA CGA AGA CCT GCT GCT GCT GCT GCG AGA CCA TGG TCA TAG-3′), ExCAG-02 (5′-CTA TGA CCA TGG TCT CGC TGC TGC TGC TGC TGG TCT TCG TCG TTT TAC-3′), and ExCTG-02 (5′-GTA AAA CGA CGA AGA CCA GCA GCA GCA GCA GCG AGA CCA TGG TCA TAG-3′), removing the MfeI site. Another unique restriction site, HindIII, was used to perform double digestions. The pLacD2 plasmid containing the repeats was digested by BsaI and HindIII and by BbsI and HindIII, giving two fragments of 2774 and 3702 bp. The fragments containing the repeats were extracted from gels and ligated together to increase the repeat number.
Integration into chromosome:
Repeat sequences were integrated into the chromosome in both CAG and CTG orientations in the 5′ part of the lacZ gene using the pKO3 integration strategy (Link et al. 1997). Figure 1A shows the structure of the CAG-leading (CAG) orientation where CAG repeats are on the leading-strand template and CTG repeats are on the lagging-strand template. Following integration, the presence of the repeat tract and the absence of the lacL8 mutation were checked by PCR and sequencing. A list of bacterial strains is provided in Table 2.
Figure 1.—
(A) The location of repeats integrated into the chromosome. Repeats were integrated at the 5′-end of the lacZ coding sequence to generate in-frame insertions of CAG or CTG codons. The construct shows the CAG orientation where CAG repeats are on the leading-strand template and CTG repeats are on the lagging-strand template. (B) Map of plasmid pLacD2, showing the restriction sites (BsaI and BbsI) between lac1 and lac2 homology arms, where repeats were introduced by site-directed mutagenesis, and HindIII, which was used for double digestions. (C–E) Examples showing the data output of GeneMapper. (C) The arrow points to a 373-bp peak, which corresponds to the repeat length of 75 (373 bp = 3 × 75 bp + 148 bp) as the PCR product size without repeats is 148 bp. The inset shows a magnification of the region around the 373-bp peak to illustrate the “stutter” bands more clearly. (D) The arrow points to a peak of 230 bp, which is a deletion of (CAG)75 to (CAG)27. (E) An example of a mixed colony showing a parental length of (CAG)75 with a 373-bp peak (arrow on the right) along with a deletion peak of 205 bp (arrow on the left), which corresponds to (CAG)19.
Instability assay:
Twelve parental colonies were tested in each assay. Each colony was grown overnight in LB with and without IPTG (2 mm) at 37° with shaking. The cultures were diluted 106-fold in LB and 100 μl was plated onto LB plates. The plates were incubated at 37° overnight. For each parental colony, 10 sibling colonies were selected for analysis from IPTG and no-IPTG cultures. PCR was carried out to check the length of the repeat tract. No significant differences were observed in any of the mutants between instabilities in the presence or absence of IPTG, and the data, plus or minus IPTG, were pooled.
GeneMapper analysis of repeats:
Repeat tracts were amplified using primers Ex-test-F (5′-TTA TGC TTC CGG CTC GTA TG-3′) and Ex-test-R (5′-GGC GAT TAA GTT GGG TAA CG-3′). Primer Ex-test-F was labeled with the fluorescence tag 6-Fam (Metabion). A size standard (GeneScan-500 LIZ from ABI) was added to the fluorescent PCR product(s), and fragments were resolved by capillary electrophoresis on a polyacrylamide medium in an ABI 3730 genetic analyzer. The results were analyzed by using GeneMapper software version 4. Characteristic result outputs are shown in Figure 1, C–E. Here it can be seen that, in addition to the main peaks characteristic of the repeat array lengths, several “stutter” peaks are observed. These represent deletions and expansions that have arisen during the PCR reaction and not in vivo.
The instability proportion was defined as the proportion of sibling colonies that had a repeat length changed from the parental length. To avoid counting deletions that had arisen on the plates, mixed colonies (containing cells with parental and new lengths) were classified as parental in the instability proportion. However, these lengths were included in the analysis of deletion length distributions. Rare sibling colonies, derived from one parental colony containing the same length of deletion, were counted only once on the assumption that they were sister clones. Rare expansions of the repeat array were detected but have not been included in this analysis. Logistic regression models were fitted to the CAG and CTG arrays separately, using Genstat 8th edition, to compare the instability proportions in the different arrays. Approximate 95% confidence intervals were calculated for each estimated instability proportion as the mean ± 2 × standard error.
RESULTS
Strategy for the construction and analysis of expanded CAG·CTG trinucleotide repeat tracts in the E. coli chromosome:
A strategy was developed to generate a set of uninterrupted repeat tracts of various lengths in both CAG-leading and CTG-leading orientations. CAG leading is defined here as the orientation where the CAG repeat tract is on leading-strand template while CTG leading is defined as the orientation where the CTG repeat tract is on leading-strand template. For simplicity, CAG leading and CTG leading will be described as “CAG” and “CTG,” respectively, from now onward in this article.
Both CAG and CTG repeats of length 5 were introduced between BsaI and BbsI restriction sites of plasmid pLacD2 (Figure 1B) by site-directed mutagenesis. This was followed by rounds of DNA restriction and ligation to construct longer repeat lengths. The recognition sites of BsaI and BbsI direct cleavage inside the repeat sequence (Figure 2B), so in every restriction and ligation round, there was a doubling of the repeat array length coupled to the loss of two repeats. This method follows the formula nx = 2nx−1 − 2, where n is the number of repeat units in the repeat tract and x is the round of restriction and ligation.
Figure 2.—
Schematic of the strategy for building long repeat arrays. (A) (CAG)5 and (CTG)5 were introduced between BsaI and BbsI restriction sites by site-directed mutagenesis. Double digestions of plasmid DNA were carried out using BbsI plus HindIII or BsaI plus HindIII. The bands were separated on a 1% agarose gel. The fragments containing repeats (A and B) were extracted from a gel and ligated to obtain a longer repeat tract length than in the original plasmid. (B) The restriction sites of BsaI and BbsI direct cleavage inside the repeat sequence so every new repeat tract length will be twice the original tract length minus 2 repeats. As shown, after cleavage by BsaI and BbsI and ligation, (CAG)5 gives rise to (CAG)8.
A schematic of the strategy is shown in Figure 2A (for details, see experimental procedures). This strategy is similar to one used previously (Grabczyk and Usdin 1999; Krasilnikova and Mirkin 2004). However, it is simpler in that it requires the ligation of only two fragments (as opposed to three) and all the products of ligation carry the expanded repeat array without any requirement for multiple rounds of ligation or dephosphorylation.
The following strategy was used to measure the instability of the repeat arrays. In every instability assay, 12 parental colonies were taken and grown in the presence and absence of IPTG (2 mm). For each parental colony, 10 sibling colonies were analyzed. PCR was carried out across the repeat array and the length of the repeat tract was determined by running the PCR products on an ABI 3730 genetic analyzer (Applied Biosystems, Foster City, CA), which automatically detects and determines the sizes of DNA fragments based on electrophoretic separation. The data collected from the ABI 3730 Genetic Analyzer were analyzed using GeneMapper software version 4.0. The fragments were visualized as peaks on a graph as displayed in Figure 1.
Instability increases with increasing repeat length and depends on repeat orientation with respect to the direction of replication:
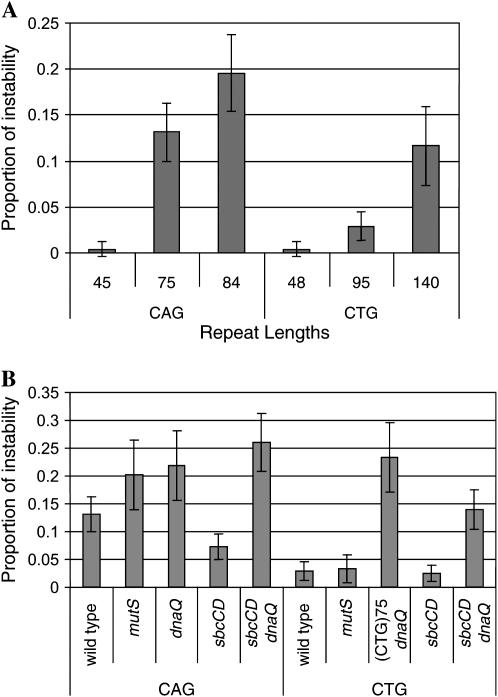
Three different lengths of the two orientations of CAG·CTG repeats were initially studied in wild-type cells. In both orientations, instability was found to be dependent on repeat tract length as the proportion of instability increased with the length of the repeat tract. The instability proportion represents the frequency of sibling colonies that had a repeat length changed (expanded or contracted) from the parental length (see experimental procedures for a more detailed description of the instability proportion). Figure 3A shows the instability proportions of all repeat lengths studied. The instability proportion for (CAG)84 was 31-fold higher than that for (CAG)45. Similarly, (CTG)140 had a 28-fold higher instability proportion than (CTG)48. Notably, the highest repeat length studied in the CAG orientation (CAG)84 had an instability proportion 1.7-fold higher than that for the highest length of the CTG orientation i.e., (CTG)140. It is the orientation where CAG repeats lie on the leading-strand template that is more unstable than the opposite orientation, where CTG repeats lie on the leading-strand template. The same orientation dependence with respect to the direction of replication was observed for CAG·CTG repeats inserted at the λ attB site (data not shown).
Figure 3.—
(A) Instability proportion of different repeat lengths for CAG and CTG orientations. The instability proportion is the frequency of sibling colonies that had a repeat length changed from the parental length. Each bar represents the pooled data of two independent assays and corresponds to the individual analysis of 480 clones by capillary electrophoresis and GeneMapper. The error bars show 95% confidence intervals. (B) Instability proportions for dnaQ, mutS, sbcCD, and sbcCD dnaQ mutants of CAG·CTG repeats compared with wild-type cells. The CAG repeat tract length studied in wild-type and all mutants was 75 and the CTG repeat tract length was 95 except in the dnaQ mutant where it was 75. Each bar represents the proportion of instability (pooled data of two independent assays of 240 clones each). The error bars show 95% confidence intervals.
CAG·CTG repeat arrays are destabilized in a dnaQ mutant and orientation dependence is lost:
As can be seen in Figure 3B, instability was increased for both repeat orientations, with a more pronounced effect on the CTG orientation in a PolIII (dnaQ) proofreading mutant. The instability proportion for (CTG)75 (the repeat length was deleted down to 75 during the construction of mutant strain) was increased 8-fold in a dnaQ mutant compared with that of (CTG)95 in wild type while (CAG)75 had an instability proportion only 1.6-fold higher than (CAG)75 in wild type (P = 0.008). Similar increases in instability were not observed in a mutS mutant (deficient in mismatch repair pathway) in the CTG orientation (Figure 3B), suggesting that the instability observed in the dnaQ mutant is likely to be attributable to its proofreading defect and not to a nonspecific effect of elevated mutagenesis. A small increase in instability proportion was observed for the mutS mutant in the CAG orientation (P = 0.050). Most interestingly, the orientation dependence of instability was lost in the dnaQ mutant but not in the mutS mutant. This result indicates that orientation dependence could be a direct consequence of different efficiencies of proofreading of CAG and CTG templates.
CAG repeat instability is reduced in an sbcCD mutant and CTG repeat instability is reduced in an sbcCD dnaQ double mutant:
The effect of SbcCD was investigated for both CAG and CTG repeat orientations. The instability proportion for the (CAG)75 repeat array was 1.8-fold lower in the sbcCD mutant (P = 0.027). The stabilization observed in an sbcCD mutant was lost in an sbcCD dnaQ double mutant (Figure 3B).
The CTG orientation was too stable at the length studied to obtain sufficient data permitting a statistical distinction between wild-type and sbcCD mutants (Figure 3B). However, an effect of sbcCD could be measured in an sbcCD dnaQ mutant where a significant decrease in the instability proportion was observed relative to that in dnaQ, even though the repeat length was shorter in the dnaQ mutant (P = 0.004). In contrast to the dnaQ mutant, orientation dependence was retained in sbcCD dnaQ (P < 0.001), suggesting that intermediates in the CTG orientation pathway can escape deletion in the absence of SbcCD and proofreading more easily than intermediates in the CAG orientation pathway.
Large deletions predominate over small deletions in sbcCD and sbcCD dnaQ mutants in the CAG orientation and in a dnaQ mutant in both repeat orientations:
To see the sizes of deletions obtained in CAG·CTG repeats in wild-type and mutant cells, all observed deletions were plotted as a function of the percentage of deletion size against the number of events (Figure 4). Both CAG and CTG repeat deletion distributions are negatively skewed as seen from the long tails toward the left in Figure 4. The median (midpoint of the distribution) for (CAG)75 comes at 65% and for (CAG)84 at 70%. The (CTG)95 deletion distribution has a median at 58% while, for (CTG)140, it can be seen at 61%. These distributions suggest that large deletions are more frequent than small deletions, consistent with the existence of intermediates comprising many repeats as would be predicted if large hairpins could form. Given the low frequency of deletions and their origins in populations grown from single cells carrying the original length of repeat array, the vast majority of deletions will have arisen in single events from arrays of parental length. The distributions of deletion lengths in CAG repeat tracts are not influenced substantially by mutations in dnaQ or sbcCD (Figure 4), suggesting that these genes do not affect the nature of the primary intermediate in the pathway but instead the frequency of its processing to a product with a new repeat length. In the CTG repeat orientation, the number of events observed make a comparison most meaningful between the dnaQ and dnaQ sbcCD mutants. Here, the negatively skewed distribution observed in dnaQ (median 63%) disappears in dnaQ sbcCD, giving a flat distribution with a median of 51% (Figure 4). This suggests that, contrary to the CAG orientation, the presence of SbcCD (in the absence of proofreading) favors the formation of large deletions.
Figure 4.—
Distributions of deletion sizes in cells containing CAG·CTG repeats. The deletions observed are plotted as the percentage of the tract deleted against the number of times that the particular deletions were observed.
Mutations in recombination genes recA, recB, and recF do not influence CAG·CTG repeat instability:
It can be seen in Figure 5 that mutations in the recombination genes recA, recB, and recF do not affect the proportions of instability for (CAG)75 and (CTG)95 repeat tracts. This suggests that recombination does not contribute to the instability of CAG·CTG repeats in the E. coli chromosome at the lengths studied in this work.
Figure 5.—
Instability proportions of recA, recB, and recF mutants containing CAG·CTG repeats The length studied for CTG repeats is 95 in wild type and mutants. For CAG repeats, it is 75 for all mutants except in recF, where it is 80. Each bar represents the instability proportion calculated from the data of two independent experiments (480 clones). Error bars represent 95% confidence intervals.
Transcription does not affect CAG·CTG repeat instability:
To analyze the influence of transcription on the instability of CAG·CTG repeats, the repeats were integrated in the 5′-end of the lacZ gene in the wild-type strain that also bears a lacIq repressor gene. Under uninduced conditions (the absence of inducer IPTG), transcription from the lacZ promoter is repressed by the LacI repressor, while growing the cells in IPTG induces transcription. In the CTG orientation, the transcribed strand is the CTG strand, which is also the leading-strand template, while in the CAG orientation it is the CAG repeat-containing strand that acts as leading-strand template and the transcribed strand. The repeat lengths studied in both orientations showed no difference in the proportion of instability in the presence or absence of IPTG (Figure 6). To obtain further evidence for or against a potential effect of transcription and to specifically test whether stalling of transcription complexes might affect instability, the effect of the transcription-coupled repair factor Mfd was investigated. As shown in Figure 7, mfd mutants do not show a difference in proportion of instability as a function of induction of transcription with IPTG or as compared to their corresponding wild-type levels in either repeat orientation, demonstrating that in this system rescue of stalled RNA polymerases by Mfd protein is not involved in CAG·CTG repeat instability.
Figure 6.—
Proportions of instability for CAG·CTG repeats in the presence or absence of IPTG. The cells were grown overnight in the presence and absence of IPTG (2 mm). The x-axis shows the repeat lengths for CAG and CTG repeat orientations. The bars represent the data from two independent assays and correspond to the individual analyses of 240 clones. Error bars represent 95% confidence intervals.
Figure 7.—
Instability proportions of (CAG)75 and (CTG)95 repeats in mfd mutants in the presence or absence of IPTG. Each bar represents the pooled data of two independent experiments (240 clones). Error bars represent 95% confidence intervals.
DISCUSSION
CAG·CTG trinucleotide repeat instability is known to be length and orientation dependent in E. coli. Here, we have devised a polymerization-independent strategy for the expansion of repeat arrays in vitro and have applied this to the generation and insertion of CAG·CTG repeats in the E. coli chromosome (Figure 2). Using this new model system, we have investigated the basis of the orientation dependence of instability.
CAG·CTG trinucleotide repeats show length- and orientation-dependent instability in the E. coli chromosome:
We show that CAG·CTG repeat instability in the E. coli chromosome increases with increasing repeat tract length and that instability is orientation dependent (Figure 3A). In humans, length dependence of repeat array mutation underlies the phenomenon of dynamic mutation where expanded repeat arrays have an increased probability of further expansion leading to anticipation in the inheritance of disease phenotypes. The orientation in which elevated instability is observed is that where the CTG repeat lies on the template for the lagging strand of the replication fork. This is the same orientation dependence with respect to replication as observed in previous studies using bacterial plasmids and yeast (Kang et al. 1995; Rosche et al. 1995; Maurer et al. 1996; Freudenreich et al. 1997; Miret et al. 1998), but differs from a recent study of repeat instability in the E. coli chromosome (Kim et al. 2006) where the instability observed shows variable length and orientation dependence. The authors have used the same assay in both plasmid and chromosomal contexts and observed no length or orientation dependence in the plasmid. The system of Kim et al. (2006) also differs from ours in that it detects only a subset of deletion events that gives rise to chloramphenicol resistance and complete deletion of the repeat array is a common outcome. Since we do not observe complete deletion of the repeat array, it seems that the two assays are detecting different molecular events.
In both E. coli plasmids and yeast, instability is replicative and is strongly biased toward deletions, but in mice instability can occur in nondividing cells (Takano et al. 1996; Hashida et al. 1997; Kovtun and McMurray 2001) and can be influenced by the position of the transgenic insert (Monckton et al. 1997; Seznec et al. 2000). This correlates with the expansion patterns observed in humans (Seznec et al. 2000). However, the observation of nonreplicative instability in a mammalian system does not exclude replicative sources of instability or the importance of replicative stability in mammals. Furthermore, the nonreplicative instability itself is likely to involve DNA synthesis. This is particularly clear in the case of nonreplicative expansion where expansion in the absence of accompanying contraction implies net DNA synthesis.
The proofreading subunit of DNA polymerase III (DnaQ) determines orientation dependence of replicative instability in cells with active SbcCD nuclease:
We demonstrate that a mutation in dnaQ destabilizes CAG·CTG repeat arrays and that orientation dependence of instability is lost in this mutant. This is a specific effect of dnaQ mutation since a mutation in mutS does not have a corresponding effect (Figure 3B). Previous studies suggested that the orientation dependence of CAG·CTG repeat instability is caused by the dynamics of lagging-strand DNA synthesis accompanied by the greater thermodynamic stability of CTG repeat hairpins compared to CAG repeat hairpins (Maurer et al. 1996; Freudenreich et al. 1997; Miret et al. 1998). However, it has not previously been shown how this process is mediated. Here, we suggest that proofreading is inefficient on the CTG repeat template of the lagging strand (CAG orientation), leading to orientation-dependent instability. It is the more foldable CTG repeat strand that behaves as if it is more refractory to proofreading. Although we favor the interpretation that the dnaQ mutation destabilizes the repeat tract because of its effect on proofreading, we cannot exclude the possibility that other indirect effects of the dnaQ mutation contribute or are responsible.
Our work is consistent with the previous observation that the dnaQ49ts mutation destabilizes CTG repeats in bacterial plasmids (Iyer et al. 2000) but is in divergence with experiments demonstrating that proofreading mutants of DNA polymerases δ and ɛ do not destabilize these repeats in yeast (Schweitzer and Livingston 1999). The yeast results are interesting, given that homo and dinucleotide repeats are destabilized in these mutants (Strand et al. 1993; Tran et al. 1997), suggesting some particular resistance to proofreading of CAG·CTG triplet repeats by polymerases δ and ɛ. In this context, it should be noted that DnaQ has been shown to share sequence homology with human DNA editing enzyme DNase III (Hoss et al. 1999). This enzyme is present in equal amounts in nondividing and proliferating cells, which suggests that it is involved in repair processes as well as in replication. An alternative is that eukaryotic cells might correct trinucleotide repeat slippage by sharing proofreading activities between polymerases (Pavlov et al. 2006). So, it is plausible that proofreading during replication and repair in human cells may contribute to repeat stability.
A number of studies have documented the stabilizing and destabilizing effects of MutS and its homologs on CAG·CTG repeat instability in E. coli, yeast, and mouse (Jaworski et al. 1995; Schumacher et al. 1998; Manley et al. 1999; Schmidt et al. 2000). In this system, we observe a small destabilizing effect of the mutS mutation in one orientation (CAG on leading-strand template) that lies on the border of significance and no significant effect in the other orientation (CTG on leading-strand template). The absence of a substantial effect of mutS on the frequency of deletion formation is consistent with our observation here that deletions are distributed over a wide range of sizes, while mismatch repair can correct only small insertion/deletion loops of up to three nucleotides.
The SbcCD nuclease increases CAG repeat instability when proofreading is active and CTG repeat instability when proofreading is inactive:
We show that CAG·CTG repeat instability is reduced in an sbcCD mutant when the CTG-containing strand is the template for the lagging strand of the replication fork (CAG orientation). This stabilizing effect of an sbcCD mutation is lost in a dnaQ sbcCD double mutant. This result argues for antagonistic action of SbcCD and the proofreading subunit of DNA polymerase III. It is consistent with the existence of an SbcCD-dependent pathway of deletion formation for the CAG orientation that is significant only in the presence of proofreading. This may be because the action of SbcCD is antagonistic to proofreading through removal of the structure signaling the need to proofread.
In the CTG orientation (CAG on the lagging-strand template), SbcCD plays an active role in stimulating deletions in the absence of proofreading. This is evidenced by the small but significant decrease in instability in an sbcCD dnaQ strain compared to dnaQ and the shift from a skewed distribution of deletion sizes (in dnaQ) to a flat distribution (in sbcCD dnaQ) for the CTG orientation. Furthermore, the observation of orientation dependence in sbcCD dnaQ (but not in dnaQ) implies a role of SbcCD in removing orientation dependence in the absence of proofreading. These observations are consistent with SbcCD having access to an intermediate in the CTG deletion pathway (CAG on the lagging-strand template) in the absence of dnaQ and stimulating its conversion to a deletion product rather than its return to a parental template. It has recently been shown that fluorescently tagged fusions of Bacillus subtilis SbcC localized with a pattern similar to that of the replication factory, consistent with action of SbcCD at the site of DNA replication (Meile et al. 2006). A similar colocalization of fluorescently tagged SbcC with a replication factory protein has also been observed in E. coli (E. Darmon, personal communication).
CAG·CTG repeat instability in the chromosome is not caused by recombination:
We demonstrate that, at the lengths studied here, CAG·CTG repeat instability in the E. coli chromosome is not affected by mutations in recombination genes recA, recB, and recF. These findings are interesting since recombination has been reported to influence instability of repeats in plasmids (Jakupciak and Wells 1999, 2000a,b; Napierala et al. 2002; Pluciennik et al. 2002; Hashem et al. 2004; Hebert et al. 2004). Further work is required to reconcile these observations.
Transcription does not influence CAG·CTG instability in the E. coli chromosome:
We report another observation that contrasts with several previous plasmid studies. We show here that, at the repeat lengths studied, instability in the chromosome is not affected by transcription. Previously, transcription was reported to affect plasmid-borne CAG·CTG repeat instability (Bowater et al. 1997; Schumacher et al. 2001; Mochmann and Wells 2004) although one study did not detect such an effect (Schmidt et al. 2000). As we observed no effect of transcription, we reasoned that the absence of effect might be caused by the enzymatic removal of stalled transcription complexes before they were able to affect instability. We therefore tested the effect of an mfd mutant on instability. Mfd protein, a transcription-repair coupling factor, ensures the repair of DNA damage in transcribed strands of active genes. It can bind DNA, RNA polymerase, and the UvrA protein, removes RNA polymerase from the DNA, and recruits the excision repair apparatus to the damaged site. It is also required in the removal of stalled transcription complexes (Park et al. 2002). A mutation in the mfd gene did not significantly change the proportion of instability in the CAG or CTG repeat orientations, suggesting no role of the transcription-repair coupling factor Mfd in repeat instability at the lengths studied. This result lends no support to the hypothesis that stalled transcription complexes influence CAG·CTG repeat instability in E. coli (Kim et al. 2006).
Evidence for the influence of secondary structures on instability in vivo:
Several of our observations lend weight to the hypothesis that secondary structures do influence the instability of CAG·CTG repeat arrays. First, in the CAG orientation (CTG on the lagging-strand template), the distribution of sizes of deletion products is negatively skewed, consistent with a preference for large deletions comprising enough repeats to form stable hairpins. This is independent of any of the genotypes tested here and argues for the formation of hairpins stable enough to influence the spectrum of deletion products irrespective of the presence of SbcCD. Second, we observe an effect of SbcCD on the frequency of instability in the orientation predicted to form the more stable CTG repeat hairpins, and we know that SbcCD is a hairpin nuclease that has been shown to cleave CTG repeat hairpins (Connelly et al. 1999). Third, we observe a negatively skewed distribution of deletion products for the CTG orientation (CAG on the lagging-strand template) in the presence of SbcCD and the absence of proofreading, suggesting the existence of less stable secondary structures in this orientation that require the presence of SbcCD to manifest themselves as deletions. Longer repeat arrays give rise to longer deletion products, arguing against the formation of a specific structure composed of a set number of repeats. Instead, it would appear that larger secondary structures are free to form within longer repeat arrays. The strongest evidence for secondary structures comes from the skewed distributions of deletion products as these reflect the nature of the slippage intermediates. Any arguments based on the action of SbcCD as a hairpin nuclease must be moderated by the observation that SbcCD can affect the nature of a deletion event between 101-bp direct repeats, even in the absence of an inverted repeat sequence (Bzymek and Lovett 2001a,b).
A model for replicative instability of CAG·CTG repeats:
Combining the results of sbcCD and dnaQ mutations, we propose a model to explain the orientation dependence of CAG·CTG repeat instability during replication (Figure 8). Orientation dependence is determined by proofreading of slippage intermediates formed during replication of the lagging strand. We suggest that the CTG repeat template for the lagging strand is partially refractory to proofreading, leading to elevated frequencies of deletions in wild-type cells. Intermediates in the slippage reaction in this orientation of the repeat array are accessible to the SbcCD nuclease, which can increase instability by digesting the strands that signal the presence of a substrate for proofreading. In the absence of proofreading, SbcCD can no longer affect instability and we suggest that this is because its effect is to divert intermediates from effective proofreading. An alternative possibility is that cleavage with SbcCD is not possible in a proofreading mutant. The latter hypothesis is made less likely with the observation of an effect of SbcCD on instability in a dnaQ mutant when CAG repeats are the template for the lagging strand. In this orientation, we hypothesize that more unstable intermediates are formed and that the effect of SbcCD is to divert them from a proofreading-independent pathway of return to parental length. The model is explained in detail in the legend of Figure 8.
Figure 8.—
Model for orientation-dependent replicative instability DNA synthesis arrests within a CAG or CTG repeat array (see DNA structure 1) and strand slippage occurs either accompanied (b) or not (c) with stable secondary structure formation (a). A template containing CAG repeats will form less stable secondary structures (see DNA structure 2) and so primarily will adopt “deletion pathway A” whereas a template with CTG repeats will form more stable secondary structures (see DNA structure 3 and DNA structure 4) and so primarily will adopt “deletion pathway B.” Some interconversion between well-folded and less well-folded structures (and between differently folded structures, not shown) may also be possible (interchange d). The observation that orientation dependence of repeat instability is lost in a dnaQ mutant implies that the initiation of slippage is independent of whether the template contains CAG or CTG repeats. This has the further implication that initiation of slippage occurs independently of the potential of the strand to form stable secondary structures. The requirement for the presence of SbcCD for loss of orientation dependence in a dnaQ mutant implies that SbcCD ensures the efficient processing of slippage intermediates initiated on both CAG and CTG repeats. Orientation dependence is generated by poor proofreading of the CTG template strand. In the model, this is envisaged to be because 3′–5′ exonucleolytic proofreading does not remove a secondary structure if it is present (compare f with e) and so new synthesis has the potential to slip again on such a template (DNA structure 3 to DNA structure 4). In the absence of proofreading, intermediates with stable structures are committed to deletion (whether or not SbcCD is present) while intermediates with less stable secondary structures can escape through an inefficient disassembly pathway (g) that is significant only in the absence of SbcCD (and of proofreading).
Conclusion:
CAG·CTG repeat instability in the E. coli chromosome shows length- and orientation-dependent behavior consistent with preferential deletion when CTG repeats are the template for the lagging strand. Deletion patterns are consistent with the formation of hairpins that are sufficiently stable to influence the spectrum of products for this unstable orientation irrespective of the gene products investigated here. In the more stable orientation, the spectrum of deletion products is also consistent with secondary structure formation, but this is revealed only in the presence of the hairpin nuclease SbcCD (Rad50/Mre11). The observed patterns of CAG·CTG instability provide direct evidence for the formation of secondary structures in vivo. These structures must be large and their sizes determined by the lengths of the repeat arrays. In cells containing the active SbcCD nuclease, orientation dependence of replicative CAG·CTG repeat instability is determined by DnaQ, the proofreading subunit of DNA polymerase III. These results demonstrate an interaction between the proofreading of slippage intermediates and their processing by the SbcCD nuclease that affects replicative instability of repeated sequences. Whether similar processing reactions are important in the replicative or nonreplicative pathways of trinucleotide repeat instability in human cells remains to be determined.
TABLE 1.
Plasmids used in the study
| Plasmid | Characteristics | Source | DL no. |
|---|---|---|---|
| pTOF24 | repAts sacB Cmr; used for SalI–PstI cloning | Millicent Masters | 1605 |
| pLacD1 | pTOF24 derivative; contains BbsI, MfeI, and BsaI sites in center of two 400-bp lac homology arms, lacL8 | This study | 1823 |
| pLacD2 | pLacD1 derivative; BbsI site in lacZ homology arm removed | This study | 2911 |
| pLacD2 (CAG)5 | pLacD2 derivative; (CAG)5 in place of MfeI site, | This study | 1816 |
| pLacD2 (CAG)8 | pLacD2 derivative; (CAG)8 in place of MfeI site | This study | 1892 |
| pLacD2 (CTG)8 | pLacD2 derivative; (CTG)8 in place of MfeI site | This study | 1893 |
| pLacD2 (CAG)14 | pLacD2 derivative; (CAG)14 in place of MfeI site | This study | 1899 |
| pLacD2 (CAG)26 | pLacD2 derivative; (CAG)26 in place of MfeI site | This study | 1894 |
| pLacD2 (CTG)26 | pLacD2 derivative; (CTG)26 in place of MfeI site | This study | 1895 |
| pLacD2 (CAG)28 | pLacD2 derivative; (CAG)28 in place of MfeI site | This study | 1900 |
| pLacD2 (CTG)28 | pLacD2 derivative; (CTG)28 in place of MfeI site | This study | 1901 |
| pLacD2 (CAG)50 | pLacD2 derivative; (CAG)50 in place of MfeI site | This study | 1911 |
| pLacD2 (CTG)50 | pLacD2 derivative; (CTG)50 in place of MfeI site | This study | 1912 |
| pLacD2 (CAG)98 | pLacD2 derivative; (CAG)98 in place of MfeI site | This study | 2912 |
| pLacD2 (CTG)98 | pLacD2 derivative; (CTG)98 in place of MfeI site | This study | 2913 |
| pTOF24-mfd | pTOF24 derivative to integrate mfd deletion | This study | 2519 |
“DL” indicates strains that are constructed in the Leach Lab.
TABLE 2.
E. coli strains
| Strain | Genotype | Source, reference, or construction |
|---|---|---|
| DL732 | F−thr-1 leuB6 proA2 his4 thi1argE3 lacY1 galK2 rpsL supE44 ara-14 xyl-15 mtl-1, txs-33) sbcCD∷Km | Leach Lab |
| CSH115 | ara Δ(gpt-lac)5 rpsL mutS∷mini-Tn10 | Cold Spring Harbor Lab |
| DB1318 | recD1014 hsdR2 zjj-202∷Tn10 recA∷Cm | Wertman et al. (1986) |
| RM6972 | dnaQ∷mini-Tn10 | Geneviève Maenhaut-Michel |
| JJC450 | recF400∷Tn5 (KmR) | Bénédicte Michel |
| JJC1086 | recB∷Km | Bénédicte Michel |
| DL1786 | MG1655 lacZχ− lacIq ZeoRχ+ | John Eykelenboom |
| DL1994 | DL1786 lacZ∷(CTG)48 | This study |
| DL1995 | DL1786 lacZ∷(CAG)75 | This study |
| DL2009 | DL1786 lacZ∷(CTG)95 | This study |
| DL2079 | DL2009 recA∷Cm | This study (P1 from DB1318) |
| DL2080 | DL2009 recB∷Km | This study (P1 from JJC1086) |
| DL2081 | DL2009 recF400∷Km | This study (P1 from JJC450) |
| DL2104 | DL2009 sbcCD∷Km | This study (P1 from DL732) |
| DL2250 | DL1786 lacZ∷(CAG)45lacL8 | This study |
| DL2300 | DL2009 mutS∷Tc | This study (P1 from CSH115) |
| DL2301 | DL1995 dnQ∷Tc | This study (P1 from RM6972) |
| DL2302 | DL1995 mutS∷Tc | This study (P1 from CSH115) |
| DL2303 | DL1995 sbcCD∷Km | This study (P1 from DL732) |
| DL2304 | DL1995 recA∷Cm | This study (P1 from DB1318) |
| DL2305 | DL2009 expansion to (CTG)140 | This study |
| DL2437 | DL1995 recB∷Km | This study (P1 from JJC1086) |
| DL2445 | DL1786 lacZ∷(CTG)75dnaQ∷Tc | This study (P1 from RM6972) |
| DL2639 | DL1786 lacZ∷(CAG)84 | This study |
| DL2831 | DL1995 mfd− | This study (using pDL2519) |
| DL2915 | DL2009 mfd− | This study (using pDL2519) |
| DL2976 | DL2303 dnaQ∷Tc | This study (P1 from RM6972) |
| DL3046 | DL2104 dnaQ∷Tc | This study (P1 from RM6972) |
| DL3138 | DL1786 lacZ∷(CAG)80recF400∷Km | This study (P1 from JJC450) |
P1 represents construction by P1 transduction. DL strains are strains constructed in the Leach Lab.
Acknowledgments
We thank Camelia Mihaescu and Siarhei Mankou (past lab members) who obtained initial results demonstrating orientation dependence and the importance of proofreading in chromosomal instability using a set of CAG·CTG repeats they constructed and analyzed at the λ attB site. In addition, we thank Ewa Okely for technical support, Elise Darmon for critical reading of the manuscript, John Eykelenboom for providing the strain DL1786, and the School of Biological Sciences sequencing service for DNA fragment analysis. R.Z. holds a Ph.D. studentship funded by the Commonwealth Scholarship Commission, UK. The work is supported by a grant from the Medical Research Council to D.R.F.L.
References
- Bacolla, A., R. Gellibolian, M. Shimizu, S. Amirhaeri, S. Kang et al., 1997. Flexible DNA: genetically unstable CTG.CAG and CGG.CCG from human hereditary neuromuscular disease genes. J. Biol. Chem. 272: 16783–16792. [DOI] [PubMed] [Google Scholar]
- Bowater, R. P., A. Jaworski, J. E. Larson, P. Parniewski and R. D. Wells, 1997. Transcription increases the deletion frequency of long CTG.CAG triplet repeats from plasmids in Escherichia coli. Nucleic Acids Res. 25: 2861–2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bzymek, M., and S. T. Lovett, 2001. a Evidence for two mechanisms of palindrome-stimulated deletion in Escherichia coli: single-strand annealing and replication slipped mispairing. Genetics 158: 527–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bzymek, M., and S. T. Lovett, 2001. b Instability of repetitive DNA sequences: the role of replication in multiple mechanisms. Proc. Natl. Acad. Sci. USA 98: 8319–8325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bzymek, M., C. J. Saveson, V. V. Feschenko and S. T. Lovett, 1999. Slipped misalignment mechanisms of deletion formation: in vivo susceptibility to nucleases. J. Bacteriol. 181: 477–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan, J. L., K. J. Andrews, V. A. Zakian and C. H. Freudenreich, 2003. Mutations in yeast replication proteins that increase CAG/CTG expansions also increase repeat fragility. Mol. Cell. Biol. 23: 7849–7860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connelly, J. C., and D. R. Leach, 1996. The sbcC and sbcD genes of Escherichia coli encode a nuclease involved in palindrome inviability and genetic recombination. Genes Cells 1: 285–291. [DOI] [PubMed] [Google Scholar]
- Connelly, J. C., E. S. de Leau, E. A. Okely and D. R. Leach, 1997. Overexpression, purification, and characterization of the SbcCD protein from Escherichia coli. J. Biol. Chem. 272: 19819–19826. [DOI] [PubMed] [Google Scholar]
- Connelly, J. C., E. S. de Leau and D. R. Leach, 1999. DNA cleavage and degradation by the SbcCD protein complex from Escherichia coli. Nucleic Acids Res. 27: 1039–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings, C. J., and H. Y. Zoghbi, 2000. Fourteen and counting: unraveling trinucleotide repeat diseases. Hum. Mol. Genet. 9: 909–916. [DOI] [PubMed] [Google Scholar]
- Darlow, J. M., and D. R. Leach, 1995. The effects of trinucleotide repeats found in human inherited disorders on palindrome inviability in Escherichia coli suggest hairpin folding preferences in vivo. Genetics 141: 825–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freudenreich, C. H., J. B. Stavenhagen and V. A. Zakian, 1997. Stability of a CTG/CAG trinucleotide repeat in yeast is dependent on its orientation in the genome. Mol. Cell. Biol. 17: 2090–2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freudenreich, C. H., S. M. Kantrow and V. A. Zakian, 1998. Expansion and length-dependent fragility of CTG repeats in yeast. Science 279: 853–856. [DOI] [PubMed] [Google Scholar]
- Gacy, A. M., G. Goellner, N. Juranic, S. Macura and C. T. McMurray, 1995. Trinucleotide repeats that expand in human disease form hairpin structures in vitro. Cell 81: 533–540. [DOI] [PubMed] [Google Scholar]
- Grabczyk, E., and K. Usdin, 1999. Generation of microgram quantities of trinucleotide repeat tracts of defined length, interspersion pattern, and orientation. Anal. Biochem. 267: 241–243. [DOI] [PubMed] [Google Scholar]
- Hashem, V. I., W. A. Rosche and R. R. Sinden, 2004. Genetic recombination destabilizes (CTG)n.(CAG)n repeats in E. coli. Mutat. Res. 554: 95–109. [DOI] [PubMed] [Google Scholar]
- Hashida, H., J. Goto, H. Kurisaki, H. Mizusawa and I. Kanazawa, 1997. Brain regional differences in the expansion of a CAG repeat in the spinocerebellar ataxias: dentatorubral-pallidoluysian atrophy, Machado-Joseph disease, and spinocerebellar ataxia type 1. Ann. Neurol. 41: 505–511. [DOI] [PubMed] [Google Scholar]
- Hebert, M. L., L. A. Spitz and R. D. Wells, 2004. DNA double-strand breaks induce deletion of CTG.CAG repeats in an orientation-dependent manner in Escherichia coli. J. Mol. Biol. 336: 655–672. [DOI] [PubMed] [Google Scholar]
- Hoss, M., P. Robins, T. J. Naven, D. J. Pappin, J. Sgouros et al., 1999. A human DNA editing enzyme homologous to the Escherichia coli DnaQ/MutD protein. EMBO J. 18: 3868–3875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer, R. R., and R. D. Wells, 1999. Expansion and deletion of triplet repeat sequences in Escherichia coli occur on the leading strand of DNA replication. J. Biol. Chem. 274: 3865–3877. [DOI] [PubMed] [Google Scholar]
- Iyer, R. R., A. Pluciennik, W. A. Rosche, R. R. Sinden and R. D. Wells, 2000. DNA polymerase III proofreading mutants enhance the expansion and deletion of triplet repeat sequences in Escherichia coli. J. Biol. Chem. 275: 2174–2184. [DOI] [PubMed] [Google Scholar]
- Jakupciak, J. P., and R. D. Wells, 1999. Genetic instabilities in (CTG.CAG) repeats occur by recombination. J. Biol. Chem. 274: 23468–23479. [DOI] [PubMed] [Google Scholar]
- Jakupciak, J. P., and R. D. Wells, 2000. a Gene conversion (recombination) mediates expansions of CTG[middle dot]CAG repeats. J. Biol. Chem. 275: 40003–40013. [DOI] [PubMed] [Google Scholar]
- Jakupciak, J. P., and R. D. Wells, 2000. b Genetic instabilities of triplet repeat sequences by recombination. IUBMB Life 50: 355–359. [DOI] [PubMed] [Google Scholar]
- Jaworski, A., W. A. Rosche, R. Gellibolian, S. Kang, M. Shimizu et al., 1995. Mismatch repair in Escherichia coli enhances instability of (CTG)n triplet repeats from human hereditary diseases. Proc. Natl. Acad. Sci. USA 92: 11019–11023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang, S., A. Jaworski, K. Ohshima and R. D. Wells, 1995. Expansion and deletion of CTG repeats from human disease genes are determined by the direction of replication in E. coli. Nat. Genet. 10: 213–218. [DOI] [PubMed] [Google Scholar]
- Kaytor, M. D., E. N. Burright, L. A. Duvick, H. Y. Zoghbi and H. T. Orr, 1997. Increased trinucleotide repeat instability with advanced maternal age. Hum. Mol. Genet. 6: 2135–2139. [DOI] [PubMed] [Google Scholar]
- Kim, S. H., M. J. Pytlos, W. A. Rosche and R. R. Sinden, 2006. (CAG)*(CTG) repeats associated with neurodegenerative diseases are stable in the Escherichia coli chromosome. J. Biol. Chem. 281: 27950–27955. [DOI] [PubMed] [Google Scholar]
- Kovtun, I. V., and C. T. McMurray, 2001. Trinucleotide expansion in haploid germ cells by gap repair. Nat. Genet. 27: 407–411. [DOI] [PubMed] [Google Scholar]
- Krasilnikova, M. M., and S. M. Mirkin, 2004. Replication stalling at Friedreich's ataxia (GAA)n repeats in vivo. Mol. Cell. Biol. 24: 2286–2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link, A. J., D. Phillips and G. M. Church, 1997. Methods for generating precise deletions and insertions in the genome of wild-type Escherichia coli: application to open reading frame characterization. J. Bacteriol. 179: 6228–6237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manley, K., T. L. Shirley, L. Flaherty and A. Messer, 1999. Msh2 deficiency prevents in vivo somatic instability of the CAG repeat in Huntington disease transgenic mice. Nat. Genet. 23: 471–473. [DOI] [PubMed] [Google Scholar]
- Maurer, D. J., B. L. O'Callaghan and D. M. Livingston, 1996. Orientation dependence of trinucleotide CAG repeat instability in Saccharomyces cerevisiae. Mol. Cell. Biol. 16: 6617–6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurray, C. T., and I. V. Kortun, 2003. Repair in haploid male germ cells occurs late in differentiation as chromatin is condensing. Chromosoma 111: 505–508. [DOI] [PubMed] [Google Scholar]
- Meile, J. C., L. J. Wu, S. D. Ehrlich, J. Errington and P. Noirot, 2006. Systematic localisation of proteins fused to the green fluorescent protein in Bacillus subtilis: identification of new proteins at the DNA replication factory. Proteomics 6: 2135–2146. [DOI] [PubMed] [Google Scholar]
- Merlin, C., S. McAteer and M. Masters, 2002. Tools for characterization of Escherichia coli genes of unknown function. J. Bacteriol. 184: 4573–4581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miret, J. J., L. Pessoa-Brandao and R. S. Lahue, 1998. Orientation-dependent and sequence-specific expansions of CTG/CAG trinucleotide repeats in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 95: 12438–12443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitas, M., A. Yu, J. Dill and I. S. Haworth, 1995. a The trinucleotide repeat sequence d(CGG)15 forms a heat-stable hairpin containing Gsyn.Ganti base pairs. Biochemistry 34: 12803–12811. [DOI] [PubMed] [Google Scholar]
- Mitas, M., A. Yu, J. Dill, T. J. Kamp, E. J. Chambers et al., 1995. b Hairpin properties of single-stranded DNA containing a GC-rich triplet repeat: (CTG)15. Nucleic Acids Res. 23: 1050–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochmann, L. H., and R. D. Wells, 2004. Transcription influences the types of deletion and expansion products in an orientation-dependent manner from GAC*GTC repeats. Nucleic Acids Res. 32: 4469–4479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monckton, D. G., M. I. Coolbaugh, K. T. Ashizawa, M. J. Siciliano and C. T. Caskey, 1997. Hypermutable myotonic dystrophy CTG repeats in transgenic mice. Nat. Genet. 15: 193–196. [DOI] [PubMed] [Google Scholar]
- Napierala, M., P. Parniewski, A. Pluciennik and R. D. Wells, 2002. Long CTG.CAG repeat sequences markedly stimulate intramolecular recombination. J. Biol. Chem. 277: 34087–34100. [DOI] [PubMed] [Google Scholar]
- Oussatcheva, E. A., V. I. Hashem, Y. Zou, R. R. Sinden and V. N. Potaman, 2001. Involvement of the nucleotide excision repair protein UvrA in instability of CAG*CTG repeat sequences in Escherichia coli. J. Biol. Chem. 276: 30878–30884. [DOI] [PubMed] [Google Scholar]
- Panigrahi, G. B., R. Lau, S. E. Montgomery, M. R. Leonard and C. E. Pearson, 2005. Slipped (CTG)*(CAG) repeats can be correctly repaired, escape repair or undergo error-prone repair. Nat. Struct. Mol. Biol. 12: 654–662. [DOI] [PubMed] [Google Scholar]
- Park, J. S., M. T. Marr and J. W. Roberts, 2002. E. coli transcription repair coupling factor (Mfd protein) rescues arrested complexes by promoting forward translocation. Cell 109: 757–767. [DOI] [PubMed] [Google Scholar]
- Pavlov, Y. I., C. Frahm, N. McElhinny, A. Niimi, M. Suzuki et al., 2006. Evidence that errors made by DNA polymerase alpha are corrected by DNA polymerase delta. Curr. Biol. 16: 202–207. [DOI] [PubMed] [Google Scholar]
- Pearson, C. E., Y. H. Wang, J. D. Griffith and R. R. Sinden, 1998. Structural analysis of slipped-strand DNA (S-DNA) formed in (CTG)n. (CAG)n repeats from the myotonic dystrophy locus. Nucleic Acids Res. 26: 816–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier, R., B. T. Farrell, J. J. Miret and R. S. Lahue, 2005. Mechanistic features of CAG*CTG repeat contractions in cultured cells revealed by a novel genetic assay. Nucleic Acids Res. 33: 5667–5676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petruska, J., N. Arnheim and M. F. Goodman, 1996. Stability of intrastrand hairpin structures formed by the CAG/CTG class of DNA triplet repeats associated with neurological diseases. Nucleic Acids Res. 24: 1992–1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petruska, J., M. J. Hartenstine and M. F. Goodman, 1998. Analysis of strand slippage in DNA polymerase expansions of CAG/CTG triplet repeats associated with neurodegenerative disease. J. Biol. Chem. 273: 5204–5210. [DOI] [PubMed] [Google Scholar]
- Pluciennik, A., R. R. Iyer, M. Napierala, J. E. Larson, M. Filutowicz et al., 2002. Long CTG.CAG repeats from myotonic dystrophy are preferred sites for intermolecular recombination. J. Biol. Chem. 277: 34074–34086. [DOI] [PubMed] [Google Scholar]
- Richard, G. F., G. M. Goellner, C. T. McMurray and J. E. Haber, 2000. Recombination-induced CAG trinucleotide repeat expansions in yeast involve the MRE11–RAD50–XRS2 complex. EMBO J. 19: 2381–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosche, W. A., T. Q Trinh and R. R. Sinden, 1995. Differential DNA secondary structure-mediated deletion mutation in the leading and lagging strands. J. Bacteriol. 177: 4385–4391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar, P. S., H. C. Chang, F. B. Boudi and S. Reddy, 1998. CTG repeats show bimodal amplification in E. coli. Cell 95: 531–540. [DOI] [PubMed] [Google Scholar]
- Saveson, C. J., and S. T. Lovett, 1997. Enhanced deletion formation by aberrant DNA replication in Escherichia coli. Genetics 146: 457–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt, K. H., C. M. Abbott and D. R. Leach, 2000. Two opposing effects of mismatch repair on CTG repeat instability in Escherichia coli. Mol. Microbiol. 35: 463–471. [DOI] [PubMed] [Google Scholar]
- Schumacher, S., R. P. Fuchs and M. Bichara, 1998. Expansion of CTG repeats from human disease genes is dependent upon replication mechanisms in Escherichia coli: the effect of long patch mismatch repair revisited. J. Mol. Biol. 279: 1101–1110. [DOI] [PubMed] [Google Scholar]
- Schumacher, S., I. Pinet and M. Bichara, 2001. Modulation of transcription reveals a new mechanism of triplet repeat instability in Escherichia coli. J. Mol. Biol. 307: 39–49. [DOI] [PubMed] [Google Scholar]
- Schweitzer, J. K., and D. M. Livingston, 1999. The effect of DNA replication mutations on CAG tract stability in yeast. Genetics 152: 953–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seznec, H., A. S. Lia-Baldini, C. Duros, C. Fouquet, C. Lacroix et al., 2000. Transgenic mice carrying large human genomic sequences with expanded CTG repeat mimic closely the DM CTG repeat intergenerational and somatic instability. Hum. Mol. Genet. 9: 1185–1194. [DOI] [PubMed] [Google Scholar]
- Sharples, G. J., and D. R. Leach, 1995. Structural and functional similarities between the SbcCD proteins of Escherichia coli and the RAD50 and MRE11 (RAD32) recombination and repair proteins of yeast. Mol. Microbiol. 17: 1215–1217. [DOI] [PubMed] [Google Scholar]
- Sinden, R. R., V. N. Potaman, E. A. Oussatcheva, C. E. Pearson, Y. L. Lyubchenko et al., 2002. Triplet repeat DNA structures and human genetic disease: dynamic mutations from dynamic DNA. J. Biosci. 27: 53–65. [DOI] [PubMed] [Google Scholar]
- Smith, G. K., J. Jie, G. E. Fox and X. Gao, 1995. DNA CTG triplet repeats involved in dynamic mutations of neurologically related gene sequences form stable duplexes. Nucleic Acids Res. 23: 4303–4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand, M., T. A. Prolla, R. M. Liskay and T. D. Petes, 1993. Destabilization of tracts of simple repetitive DNA in yeast by mutations affecting DNA mismatch repair. Nature 365: 274–276. [DOI] [PubMed] [Google Scholar]
- Takano, H., O. Onodera, H. Takahashi, S. Igarashi, M. Yamada et al., 1996. Somatic mosaicism of expanded CAG repeats in brains of patients with dentatorubral-pallidoluysian atrophy: cellular population-dependent dynamics of mitotic instability. Am. J. Hum. Genet. 58: 1212–1222. [PMC free article] [PubMed] [Google Scholar]
- Tran, H. T., J. D. Keen, M. Kricker, M. A. Resnick and D. A. Gordenin, 1997. Hypermutability of homonucleotide runs in mismatch repair and DNA polymerase proofreading yeast mutants. Mol. Cell. Biol. 17: 2859–2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wertman, K. F., A. R. Wyman and D. Botstein, 1986. Host/vector interactions which affect the viability of recombinant phage lambda clones. Gene 49: 253–262. [DOI] [PubMed] [Google Scholar]
- Yu, A., J. Dill and M. Mitas, 1995. a The purine-rich trinucleotide repeat sequences d(CAG)15 and d(GAC)15 form hairpins. Nucleic Acids Res. 23: 4055–4057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, A., J. Dill, S. S. Wirth, G. Huang, V. H. Lee et al., 1995. b The trinucleotide repeat sequence d(GTC)15 adopts a hairpin conformation. Nucleic Acids Res. 23: 2706–2714. [DOI] [PMC free article] [PubMed] [Google Scholar]