Abstract
Sphingolipid C4 hydroxylase catalyzes the conversion of dihydrosphingosine to phytosphingosine. In Saccharomyces cerevisiae, Sur2 is essential for sphingolipid C4 hydroxylation activity but not essential for normal growth. Here we demonstrate that the Aspergillus nidulans Sur2 homolog BasA is also required for phytosphingosine biosynthesis but is also essential for viability. We previously reported that a point missense mutation in basA resulted in aberrant cell wall thickening. Here our data suggest that accumulation of dihydrosphingosine is responsible for this phenotype. In addition, two different mutations in basA consistently accelerated the transition from asexual development to sexual development compared to the wild-type strain. The phenotype could be suppressed by exogenous addition of phytosphingosine. Northern analysis suggests that faster sexual development in the basA mutant might be due to a higher transcription level of ppoA and steA, genes demonstrated to coordinate a balance between asexual and sexual development in A. nidulans. Consistent with these findings, mutations in the ceramide-synthase-encoding genes barA and lagA also caused faster transition from asexual to sexual development, supporting the involvement of sphingolipid metabolism in fungal morphogenesis.
SPHINGOLIPIDS are a group of lipids, derived from long chain bases (also known as sphingoid bases), which are N-acylated and linked with various polar ligands. Sphingolipids are ubiquitous components of membranes in eukaryotic species, forming, together with sterols, membrane microdomains (also known as lipid rafts). These lipid rafts serve as platforms for signal transduction and membrane trafficking (Simons and Ikonen 1997). In addition, the intermediates in the sphingolipid synthesis pathway are also signaling molecules that mediate cell-to-cell recognition, differentiation, apoptosis, and modulation of the immune response in mammalian cells (Merrill et al. 1993; Vaux and Korsmeyer 1999; Warnecke and Heinz 2003; Helms and Zurzolo 2004). Sphingolipid synthesis is essential for cell viability in mammal cells (Hanada et al. 2000), in the yeast Saccharomyces cerevisiae (Pinto et al. 1992), and in the filamentous fungus Aspergillus nidulans (Cheng et al. 2001).
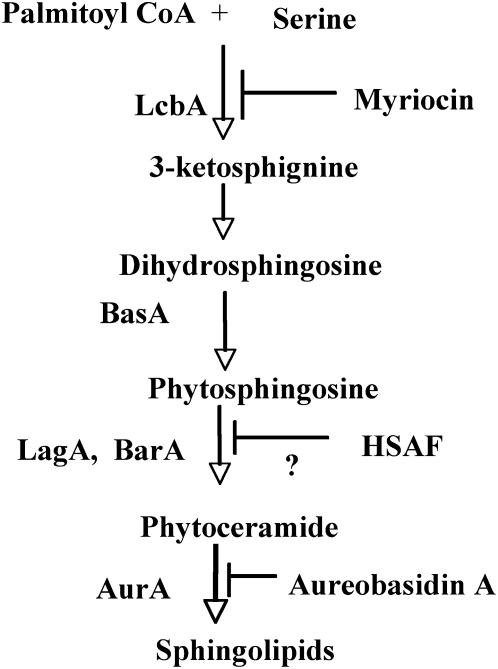
The budding yeast S. cerevisiae has been a model for studies on sphingolipid biosynthesis, thereby permitting the identification of most genes required for sphingolipid synthesis in A. nidulans (summarized in Figure 1). De novo synthesis of sphingolipids in S. cerevisiae starts with the condensation of serine and palmitoyl Coenzyme A (CoA) to yield 3-ketosphinganine, which is catalyzed by a serine palmitoyltransferase (Hanada 2003). The lcbA gene is required for this step in A. nidulans (Cheng et al. 2001). 3-Ketosphinganine is then reduced to form sphingoid base dihydrosphingosine (DHS) in yeast (Beeler et al. 1998). The corresponding gene for this step has not been reported in A. nidulans. DHS is hydroxylated on the C-4 to form the sphingoid base phytosphingosine (PHS) in yeast and further converted to phytoceramide by the condensation of acyl-CoA with PHS (Merrill 2002). Two functionally nonoverlapping ceramide synthases, BarA and LagA, are responsible for this step in A. nidulans (Li et al. 2006). Phytoceramides are further converted to inositol phosphorylceramide (IPC) by the addition of myo-inositol phosphate in yeast (Merrill and Wang 1986). The aurA gene is required for IPC synthesis in A. nidulans (Cheng et al. 2001). A number of sphingolipid synthesis inhibitors are well characterized. Among them, myriocin and aureobasidin A (AbA), with inhibitory activity against serine palmitoyltransferase (Ikushiro et al. 2004) and IPC synthase (Zhong et al. 2000), respectively, are widely used to investigate the physiological functions of sphingolipid synthesis (Cheng et al. 2001; Gopee and Sharma 2003; Jahnson et al. 2004).
Figure 1.—
A simplified scheme for the sphingolipid synthesis pathway in A. nidulans. Enzymes and inhibitors for the corresponding reaction steps are listed on the left and right, respectively.
In yeast, phytosphingosine synthesis is catalyzed by Sur2 (identical to SYR2), a sphingolipid C4-hydroxylase that converts dihydrosphingosine to phytosphingosine (Grilley et al. 1998). Genes encoding Sur2 homologs in Pichia ciferrii (Bae et al. 2004) and in Arabidopsis thaliana (Sperling et al. 2001) are also required for DHS C4 hydroxylation, suggesting that the biochemical activity of Sur2 homologs is highly conserved in a wide range of species. Other than yeast, the physiological role of Sur2 homologs has not been investigated in other fungi. Predicted Sur2 homologs are widely found in fungal species but their functions are still unknown in filamentous fungi. Recently, we identified an A. nidulans mutant with a missense point mutation (TGG → TGC; W44 → C) in the Sur2 homolog (47% identity over 328 amino acids) encoded by the basA gene. This mutant, basA1, displays hypersensitivity to an antibiotic [heat stable antifungal factor (HSAF)] produced by the biocontrol agent Lysobacter enzymogenesis strain C3 (Li et al. 2006). basA1 also displays other defects, including hyperbranching, cell wall thickening, and growth arrest at restrictive temperature (Li et al. 2006). In contrast, deletion of yeast SUR2 does not cause growth defects (Grilley et al. 1998).
Cell wall thickening is a striking phenotypic caused by the basA1 mutation and other perturbations of ceramide synthesis (Li et al. 2006). Similar to our observation in A. nidulans, dramatic alterations in cell wall structure were also observed in other fungi in response to defects in sphingolipid metabolism. For example, the cell wall is drastically thicker in S. cerevisiae when the paralogous ceramide synthases Lag1 and Lac1 are simultaneously deleted (Barz and Walter 1999). Cell wall thickening was also observed in a Schizosaccharomyces pombe temperature-sensitive mutant defective in sphingolipid hydrolytic activity (Feoktistova et al. 2001). All these observations suggest that cell wall thickening might be a general response to the disruption of sphingolipid metabolism. However, the mechanism for cell wall thickening in these studies was not investigated.
In addition, sphingolipids and their intermediates serve as secondary messengers mediating differentiation in mammal cells (Schwarz et al. 1995). The role of sphingolipids or their intermediates in fungal differentiation has not been described until now. Differentiation from vegetative hyphal growth into asexual or sexual spores is essential for fungal dissemination, survivability, and pathogenecity for many filamentous fungal species (Adams et al. 1998; Deising et al. 2000; D'Souza and Heitman 2001; Calvo et al. 2002). A. nidulans has been a useful model system for studies of both asexual and sexual differentiation in filamentous fungi (Adams et al. 1998; Calvo et al. 2002). Morphological differentiation is triggered by specific environmental factors such as light, air, and nutrients. For example, light suppresses sexual sporulation and promotes asexual sporulation (Mooney and Yager 1990; Yager 1992). Responding to environmental cues, the expression of genes required for sexual or asexual development is activated through complex signal transduction networks. Several developmental genes have been identified. For example, brlA, which encodes a zinc (Zn)-finger protein that plays an essential role in the initiation of asexual sporulation (Adams et al. 1988; Mirabito et al. 1989), or the regulatory gene steA, which encodes a homeodomain-C2/H2-Zn+2 finger transcription factor required for development of ascogenous tissue and cleistothecia (Vallim et al. 2000). The delicate balance between asexual and sexual differentiation is regulated by multiple mechanisms. The veA gene is a major regulator controlling the asexual/sexual ratio (Yager 1992; Kim et al. 2002; Kato et al. 2003). Deletion of veA completely suppresses sexual development and promotes asexual sporulation (Kim et al. 2002). In addition, lipogenic signal molecules, known as precocious sexual inducers (psi factors), also play an important role in governing asexual and sexual differentiation (Champe et al. 1987; Champe and el-Zayat 1989; Mazur et al. 1991). Psi factors are composed of hydroxylated oleic (18:1) and linoleic (18:2) moieties called psiβ and psiα, respectively (Calvo et al. 2001). The position of the hydroxyl groups on the fatty acid backbone further defines the psi compounds as psiB (8′-hydroxy-), psiC (5′,8′-dihydroxy-), and psiA with a lactone ring at the 5′ position of psiC (Mazur et al. 1991). psiBα and psiCα promote sexual development and suppress asexual development (Champe and el-Zayat 1989) while psiAα has the opposite effect (Champe et al. 1987). PpoA, a putative fatty acid dioxygenase required for biosynthesis of psiBα, is known to be required for sexual development. On the other hand, overexpression of ppoA reduced asexual sporulation and increased sexual sporulation (Tsitsigiannis et al. 2004a). Psi factors communicate with transcriptional factors regulating fungal differentiation. For example, expression of brlA was elevated in the ppoA deletion mutant while ppoA expression was reduced in the brlA deletion mutant (Tsitsigiannis et al. 2004a). ppoA is also regulated by veA. Expression of ppoA was completely suppressed in the veA deletion mutant (Tsitsigiannis et al. 2004b).
In this study, we demonstrate that BasA, essential for hyphal growth in A. nidulans, is required for phytosphingosine synthesis. Our results here indicate that accumulation of DHS might be the cause of the cell wall thickening in the basA1 mutant. Furthermore, we found that BasA has a role in A. nidulans morphogenesis, regulating the transition from asexual and to sexual development via its effects on the expression of the oxilipin gene ppoA and steA.
MATERIALS AND METHODS
Fungal strains and media:
The strains used in this study are described in Table 1. Media used for growing A. nidulans include minimal medium MNV (1% glucose, nitrate salts, vitamins, trace elements, pH 6.5), MNVTF (0.1 m threonine, 0.2% fructose, nitrate salts, vitamins, trace elements, and vitamins, pH 6.5), MAG (2% malt extract, 2% glucose, 0.2% peptone, trace elements, and vitamins), YGV (2% dextrose, 0.5% yeast extracts, and vitamins), and YGT (2% dextrose, 0.5% yeast extracts, and 1 ml/liter trace elements). Trace elements, vitamins, and nitrate salts are described in the Appendix to Käfer (1977). Media were solidified using 1.5% agar. Uridine (5 mm) and uracil (10 mm) were added as needed. Strains were grown at 28° unless otherwise indicated. For temperature-sensitive (Ts) strains, permissive temperature was 28° and restrictive temperature was 42°.
TABLE 1.
Strain list
| Strain | Pertinent genotype | Source |
|---|---|---|
| A28 | pabaA, biA; veA1 | FGSCa |
| GR5 | pyrG89; wA3; pyroA4; veA1 | FGSCa |
| Ree1 | pyroA4; veA1 | Stinnett et al. (2006) |
| A773 | pyrG89; wA3; pyroA4; veA1 | FGSCa |
| 8-145 | pabaA, biA; basA1, veA1 | Li et al. (2006) |
| TSA2 | pabaA, biA, pyrG89; basA∷ pyrG; basA1, veA1 | Li et al. (2006) |
| FGSC4 | veA+ | FGCSa |
| UV13 | pabaA6; biA1; barA1, veA1 | Li et al. (2006) |
| UV13p | pyrG89; wA3; pyroA4; barA1, veA1 | This study |
| ASL5 | pyrG89; wA3; pyroA4; alcA(p)∷basA∷pyr-4, veA1 | This study |
| ASL6 | pyrG89; wA3; pyroA4; alcA(p)∷basA∷pyr-4, veA1 | This study |
| ASL10 | alcA(p)∷lagA∷pyr-4; wA3; pyrG89; veA1 | Li et al. (2006) |
Fungal Genetic Stock Center, Department of Microbiology, University of Kansas Medical Center, Kansas City, Kansas 66160-7420.
Culture chemical treatments and preparation of samples for microscopy studies:
Phytosphingosine (Avanti Polar Lipids, Alabaster, AL), C2 phytoceramide (Sigma-Aldrich, St. Louis), Aba (Takara, Madison, WI), and myriocin (Sigma-Aldrich) were dissolved in methanol at 1 mg/ml, 10 mm, 1 mg/ml, and 80 mg/ml, respectively, and stored at −20°. For chemical treatment, chemicals were added into media with 0.05% Tergitol NP-40 (Sigma-Aldrich). For treatments in liquid media, conidiospores (∼104 conidia/ml) were incubated in YGV statically at 28° for 12 hr, allowing them to fully germinated. Coverslips attached with germlings were then transferred to YGV supplemented with chemicals and incubated at 42° for the indicated time. Germlings were fixed and stained with Calcofluor before microscopic observation (as described in Harris et al. 1994).
Construction of the alcA(p)∷basA strain:
A 635-bp fragment starting from the predicted initiation codon of basA was amplified with the primers Kpn0640 (ACTGGTACCATGGCTACAAACACAACTTTG; KpnI site underlined) and Pac0640R (ACC TTAATTAAGCACGACACAGATACCCCAGGTGAAC; PacI site underlined). The amplified fragment was cloned into pMCB17apx (Efimov 2003) using the KpnI and PacI cloning sites. This construct was transformed in wild-type strain GR5. Homologous integration of this construct generates a single full-length copy of basA regulated by alcA(p), plus a truncated version (51% of the encoding region was truncated off) controlled by the native promoter. Accordingly, transformants were propagated under alcA(p)-inducing conditions (0.1 m threonine and 0.2% fructose) and then grown under repressing conditions (1% glucose) to characterize the mutant phenotypes (Oakley and Osmani 1993).
Studies of sexual and asexual development:
To calculate the production of conidiospores, Hülle cells, and ascospores, a plug (12.5 mm2) of fungal growth on agar surface was harvested with a borer and homogenized in 0.2 ml distilled water. Conidiospores, Hülle cells, and ascospores were counted with a Bright-Line hemacytomer (Hausser Scientific, Horsham, PA) under a light microscope. The mean of conidiospores, Hülle cells, and ascospores per square millimeter from three replicate growth plugs was used to represent the asexual or sexual reproductive levels on each plate. Three independent experiments were conducted.
mRNA studies:
Mycelium was harvested at various developmental stages (as indicated in each case) and lyophilized. RNA was exacted using Trizol as described by the supplier (Invitrogen, San Diego). Approximately 20 μg of total RNA was used for RNA blot analysis. Probes for steA and brlA were generated according to our previous description (Kato et al. 2003). Other probe templates were made by PCR amplifying from A. nidulans genomic DNA with the following primer pairs: for fksA, 5′-AGGAATTGACCACCGACA-3′ and 5′-GGAATCCAGGGTGAGCA′; for chsB, 5′-AGCGTGACGTTGATGGA-3′ and 5′-ACCAGGCAACACACTGA-3′; for ppoA, 5′-CCTGGTGTTGTTGTGGAA-3′ and 5′-CTGGGAGACCACTATCCA-3′; and for basA, 5′-CCTTCGCTTGTTGATGGA-3′ and 5′-TGAAAGCTGCCAGAGACA-3′.
RESULTS
BasA is required for phytosphingosine synthesis and essential for hyphal growth:
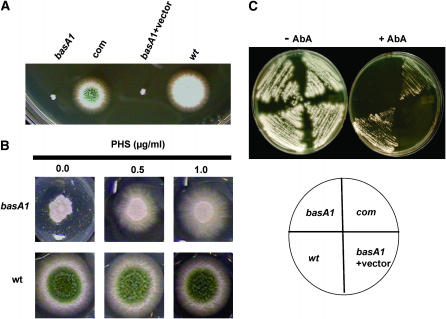
To identify the possible antifungal mechanism of a dihydromaltophilin-like antibiotic HSAF produced by L. enzymogenes strain C3 (a bacterial strain used for biocontrol), 1156 temperature-sensitive mutants were generated by 4-nitroquinoline l-oxide (Harris et al. 1994). One mutant, 8-145, displayed hypersensitivity to HSAF. Genetic analysis revealed that HSAF hypersensitivity and the Ts growth were caused by a single recessive mutation. The corresponding gene and mutation were annotated as basA and basA1, respectively. Sequence analysis of the basA1 allele showed that it is caused by a missense point mutation (TGG → TGC; W44C). Complementation of the basA1 mutation with the wild-type allele resulted in normal growth (Figure 2A). BLAST searches revealed that BasA is 47% identical to the yeast SUR2 dihydrosphingosine hydroxylase over 328 amino acids (Li et al. 2006).
Figure 2.—
Functional characterization of basA. (A) Complementation of basA1 mutant. basA1 mutant, a complemented strain cotransformed with a basA PCR product, and plasmid pRG3-AMA1, a basA1 strain transformed with vector pRG3-AMA1 and wild-type strain (GR5), were grown at 42° for 3 days. (B) Restoration of wild-type growth of basA1 mutant with exogenous phytosphingosine on solid medium. Wild-type strain A28 or basA1 mutant conidiophores were inoculated on MAG with PHS at the indicated dosages and incubated at 42° for 3 days. (C) Sensitivity of basA1 mutant to AbA. Strains were streaked onto MAG plates with or without 0.25 μg/ml AbA and incubated at 28° for 3 days.
Dihydrosphingosine hydroxylase is required for PHS synthesis. To determine whether BasA is functionally similar to Sur2, we assessed colony growth of the basA1 mutant on agar medium with various concentrations of PHS. Our results show that addition of PHS dramatically improves the growth of the basA1 strain at restrictive temperature, whereas the concentrations used (0.5–1.0 μg/ml) have no effect on wild-type growth (Figure 2B). Furthermore, the basA1 mutant displayed hypersensitivity to the inositol phosphorylceramide synthase inhibitor AbA at permissive temperature (Figure 2C). These data, together with the hypersensitivity of the basA1 mutant to the putative ceramide synthesis inhibitor HSAF (Li et al. 2006), strongly suggest that BasA is required for sphingolipid synthesis and is functionally equivalent to its homolog gene Sur2.
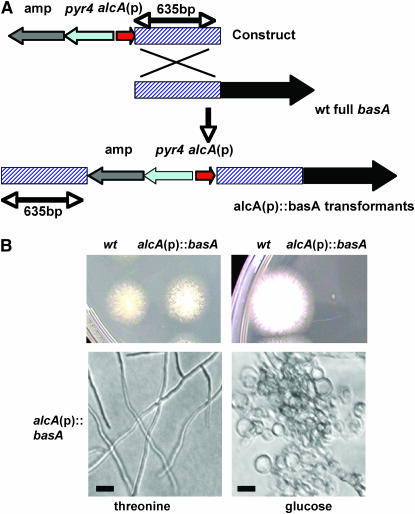
In yeast, sphingolipid synthesis is required for survival, but SUR2 is not essential for viability (Haak et al. 1997). To determine whether BasA is essential for growth (as opposed to a mere requirement for growth only at high temperatures), we generated a conditional disruption mutant following the strategy detailed in Figure 3A and in Li et al. (2006) in which full-length basA was controlled by an alcohol-dependent promoter while an additional basA copy with a 49% truncated coding region was controlled by the natural promoter (Figure 3A). When grown on minimal medium with alcA(p)-inducing carbon sources such as 100 mm threonine, 1% glycerol, or 2% ethanol, these strains grew as well as wild-type controls and displayed normal hyphal morphology (Figure 3B). However, on repressing medium, the wild-type colony size was similar to that on inducing medium, whereas the alcA(p)∷basA mutant strain fails to form visible colonies even at permissive temperature, as in the case of the basA1 mutant. These results, together with the fact that the basA1 growth phenotype could be remediated by complementation with the wild-type allele (Figure 2A), indicated that the colony growth defect was due to repression of basA expression. Conidiospores from the alcA(p)∷basA disruption mutant inoculated on the agar surface of repressing medium exhibited excessive swelling and formed short, fat germ tubes (Figure 3B), similar to basA1 germlings grown at restrictive temperature (Li et al. 2006). These observations demonstrate that BasA is crucial for viability in A. nidulans.
Figure 3.—
Essential role of basA in growth. (A). Schematic of the generation of alcA(p)-controlled strains. (B) Phenotypic characterization of the alcA(p)∷basA strain. Wild-type strain A28 and alcA (p)∷basA strain ASL6 conidiospores were inoculated on alcA(p)-inducing medium MNVTF (threonine) or repressing medium MNV (glucose) and incubated at 28° for 3 days. Microscopic images were captured under a light microscope with a ×10 objective. Bar, 10 μm.
Balanced sphingolipid synthesis is required for normal cell wall organization:
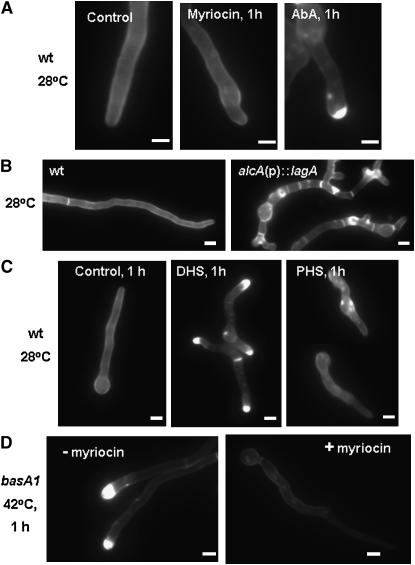
The basA1 mutant displayed aberrant deposition of cell wall materials at restrictive temperature (Li et al. 2006). To test whether cell wall thickening is a general response to the disruption of sphingolipid synthesis, we examined cell walls of wild-type hyphae grown in the presence of myriocin or AbA. AbA blocks the synthesis of complex sphingolipids, but does not prevent the accumulation of their precursor sphingoid bases and ceramides. Exposure of wild-type hyphae to AbA caused the accumulation of cell wall material (Figure 4A). In contrast, myriocin, which blocks the synthesis of all sphingolipids, including sphingoid bases and ceramide, did not trigger the formation of cell wall thickenings (Figure 4A). Therefore, the accumulation of sphingoid bases rather than the depletion of sphingolipids is most likely responsible for the aberrant accumulation of cell wall material in basA1 mutants.
Figure 4.—
Effects of sphingolipid synthesis disruption on cell wall structure. (A and C) Wild-type strain A28 conidia were germinated at 28° for 12 hr and then shifted to a drug-free YGV (control) or to YGV supplemented with 0.25 μg/ml AbA, 20 μg/ml of myriocin, 1 μg/ml dihydrosphingosine, or 1 μg/ml phytosphingosine and incubated for an additional 1 hr. (B) Wild-type strain A28 and alcA(p)∷lagA strain ASL10 conidia were grown in alcA(p)-repressing medium YGV at 42° for 13 hr. (D) basA1 mutant conidiospores were germinated in YGV at 28° for 12 hr and shifted to fresh YGV with or without 20 μg/ml of myriocin for an additional 1 hr of incubation at 42°. Hyphae were fixed and stained with Calcofluor. Bar, 3 μm.
Chemical or genetic disruption of ceramide synthase activity also causes cell wall thickening in A. nidulans (i.e., the alcA(p)∷lagA incubated under repressing conditions; Figure 4B). Because this effect could not be remediated by supplementation with C2-ceramide (10 μm/ml; data not shown), it is presumably caused by the accumulation of upstream intermediate(s) such as the sphingoid bases. To identify which intermediate is able to induce cell wall thickening, we treated wild-type hyphae with either PHS or DHS. DHS treatment (1.0 μg/ml) caused the formation of thick cell wall at hyphal tips in 89% of germlings (n = 143) within 1 hr (Figure 4C). Prolonged treatment (4 hr) resulted in the formation of multiple thick cell wall patches distributed throughout the hyphae (supplemental Figure 1 at http://www.genetics.org/supplemental/). At the same dosage, PHS did not cause the accumulation of cell wall material (Figure 4C). Although thickened cell wall patches were ultimately observed in germlings subjected to prolonged treatment with PHS, the effects were milder than those induced by DHS in terms of the intensity of Calcofluor staining (supplemental Figure 1 at http://www.genetics.org/supplemental/). Therefore, accumulation of DHS is likely responsible for cell wall thickening in response to basA1 mutation and other perturbations of sphingolipid synthesis. In support of this possibility, exposure of the basA1 mutant to 20 μg/ml myriocin at 42° largely suppressed the accumulation of cell wall material (Figure 4D).
A. nidulans has five chitin synthase genes, among which chsB is essential for normal hyphal growth (Borgia et al. 1996). Our Northern analysis showed that chsB transcript accumulation was higher at 28° than after the heat treatment (42°) in both wild type and the basA1 mutant. chsB expression was similar in the basA1 mutant and in the wild-type strain (supplemental Figure 2 at http://www.genetics.org/supplemental/). The transcription of other chitin synthesis genes, including chsA, chsC, and chsD, was also examined. However, the transcriptional levels of these genes were too low to make a comparison between wild type and mutant (data not shown). Accumulation of fksA, the only glucan synthase gene in A. nidulans, was also higher at 28° than at 42° in both wild type and the basA1 mutant (supplemental Figure 2 at http://www.genetics.org/supplemental/). Most of the mannoprotein-encoding genes remain uncharacterized in A. nidulans, with the exception of mnpA. However, the mnpA mutant did not show any phenotypic differences with respect to wild type (Jeong et al. 2003). For this reason, we did not examine the transcriptional response of these genes in the basA1 mutation background.
Normal developmental pattern requires an intact phytosphingosine synthesis:
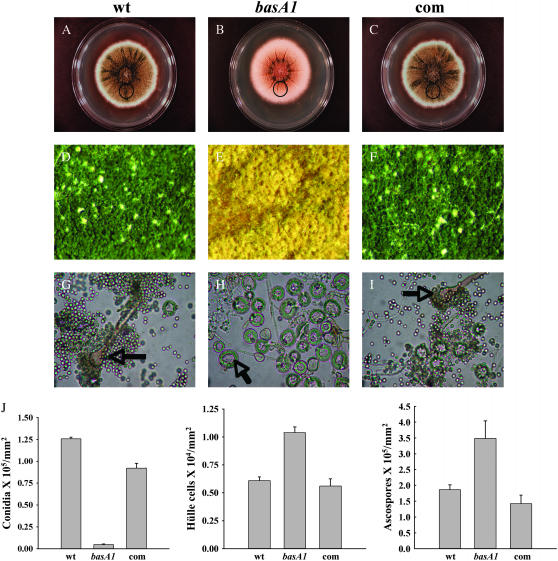
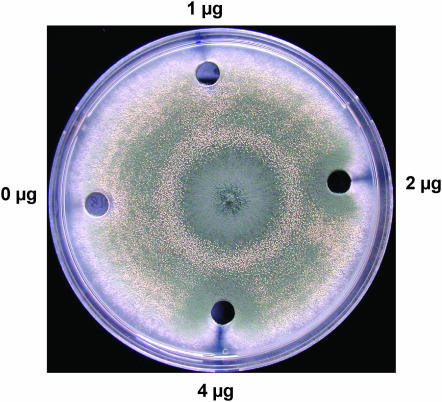
In cultures growing in the dark, sexual sporulation is the predominant mode of reproductive development in strains with a wild-type veA+ genetic background, while conidiation dominates in strains with a veA1 mutation (encoding a truncated VeA protein missing the first 36 amino acids) (Käfer 1965; Kim et al. 2002). Interestingly, the basA1 strain in the veA1 background displayed increased sexual reproduction in the dark. At permissive temperature, when conidia were inoculated at the center of the plates and incubated in the dark, the control strain A28 and a complemented basA1 mutant produced a high number of conidiophores on YGT media during a 7-day incubation period. In contrast, the basA1 mutant developed sexually under the same experimental conditions (Figure 5). The golden nursing tissue composed of Hülle cells surrounding the cleistothecial primordia could be seen under the microscope (Figure 5, E and H), as well as a higher ascospore production in older cultures (Figure 5J) . Similar observations were also made with the alcA(A)∷basA mutant growing on alcA(p)-repressing solid medium. When mycelium grown in liquid alcA(p)-inducible medium was transferred onto repressing solid medium, the yield of conidia was 76% lower than that of the wild type. In contrast, the production of Hülle cells in the alcA(A)∷basA mutant was 39-fold higher than that of the wild type (supplemental Figure 3 at http://www.genetics.org/supplemental/). This induction of sexual development observed in alcA(A)∷basA resulted in high production of ascospores. Supplementation of PHS restored a wild-type sporulation pattern to the basA1 mutant in a concentration-dependent manner. As shown in Figure 6, sexual development in the basA1 mutant was suppressed and conidiation was restored around wells where 2–4 μg of PHS was added.
Figure 5.—
Comparison of asexual and sexual differentiation between wild type and the basA1 mutant. Conidiospores of A28 (wt), basA1 mutant, or complementation strain (com) were inoculated at the center of YGT plates and incubated at 28° in constant dark for 7 days. (A–C) Images of full colonies. (D–F) Images of growth in the area 3.5 cm away from the inoculation origin (circled area in A–C) under a dissecting microscope with a ×6 objective. Conidiophores (in G and I) and Hülle nursing cells (in H) are labeled with arrows, respectively. (G–I) Samples were collected, crushed, and fixed. Images in G–I were captured under a light microscope with a ×40 objective. (J) Quantitative comparison of conidiospore, Hülle cell, and ascospore production among wild type, basA1 mutant, and complementation strain. Quantitative comparison of ascospore production among wild-type, basA1 mutant, and complementation strain was done 10 days after inoculation. Samples were harvested from circled regions in A–C.
Figure 6.—
Supplementation of PHS suppresses sexual sporulation in the basA1 mutant. Conidiospores were inoculated at the center of the MAG plates, and wells (7-mm diameter) were made 3 cm away from the inoculation point. A total of 50 μl of YGV with the indicated doses of PHS was added to the wells.
Expression of basA correlates with Aspergillus development:
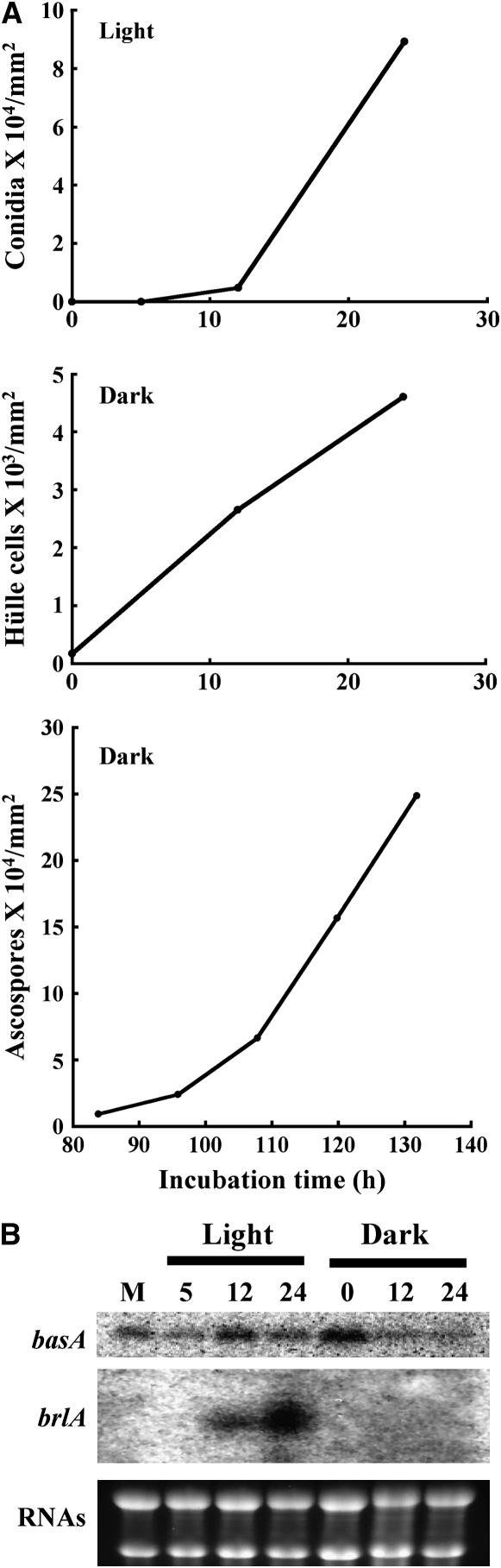
Mutation of basA altered the sporulation pattern. Does this suggest that asexual development requires high levels of basA expression compared to those required for sexual development? To test this hypothesis, we compared transcription levels of basA in mycelia, as well as during asexual development and sexual development, using a veA+ wild-type strain that facilitates the induction of either asexual or sexual sporulation (Käfer 1965). In a shift experiment where the mycelium was initially grown in liquid medium and then transferred onto solid medium, conidiation was induced 12–24 hr after the shift in cultures exposed to light, whereas sexual development was induced after 20 hr of incubation in the dark. Northern blot analysis showed that basA was expressed in all developmental stages (Figure 7). However, the highest transcript levels were observed mostly at the early stages of asexual or sexual development. For example, during asexual development, basA transcript levels were highest during conidiophore formation (12 hr) and then decreased after that time (12–24 hr). During sexual development, basA transcripts reached the highest levels when Hülle cells started to form (after 20 hr of sexual induction) and then gradually decreased to a level similar to that of mycelial growth. Accumulation of brlA transcripts coincided with the highest level of expression of basA during asexual development, at 12 hr, and continued to accumulate overtime. No obvious differences in basA transcription levels between asexual and sexual development stages were observed. These data suggest that high levels of basA expression are correlated with the initiation of both asexual and sexual development.
Figure 7.—
Correlation of basA transcription with fungal development. Conidiospores from wild-type strain FGSC4 were germinated in YGV liquid medium at 28° for 18 hr. Mycelium was harvested and then transferred onto YGV solid medium. Asexual development was induced by exposing plates to constant light, and sexual development was induced by sealing plates with parafilm and incubating in the dark for 20 hr. Samples were taken at various time points during asexual development (5–24 hr after shifting to solid medium) and sexual development (0–24 hr after 20 hr sexual induction) for RNA analysis. (A) Quantitative analysis of conidia, Hülle cells, and ascospores after induction of asexual and sexual development, respectively. (B) Northern analysis of basA transcripts. rRNAs stained with ethidium bromide were shown as an indicator for equal loading of RNA.
basA is required for normal transcription pattern of ppoA and steA:
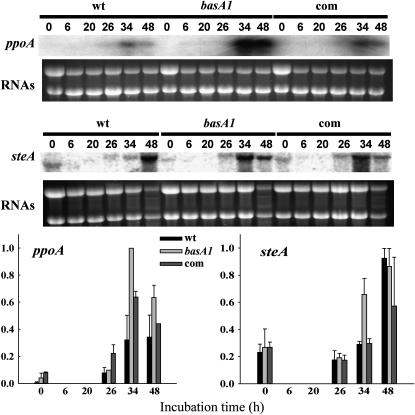
To investigate whether basA interacts with known regulatory factors directing the morphological development of the fungus, the transcription levels of ppoA and steA were compared between the basA1 mutant and both the wild-type and complemented strain. When mycelium grown in liquid medium was transferred onto solid medium and incubated under conditions that induce sexual development, asexual and sexual sporulation were observed in both wild type and basA1 mutant. However, sexual sporulation was more abundant than asexual sporulation in the basA1 mutant compared to the control (data not shown). Correlated with these observations, transcript levels of ppoA and steA were increased in the basA1 mutant compared to the wild-type and complementation strain after induction of sexual development by reducing aeration and dark conditions (Figure 8). These observations suggest that the effects of the basA1 mutation on sexual development could be caused by increased expression of ppoA, leading to the activation of steA.
Figure 8.—
Effects of the basA mutation on the transcription of developmental genes in A. nidulans. The wild-type strain A28, the basA1 mutant 8-145, and the complementation strain were cultured in shaken conditions in YGT liquid medium for 20 hr and then shifted onto solid YGT medium. At that time, plates were sealed with parafilm and incubated at 28° in the dark to induce sexual development for 20 hr. Total RNA corresponding to wild type and basA1 strains was isolated at the time of the shift (t = 0), at 6 and 20 hr (during sexual development induction), and at 26, 34, and 48 hr after the shift (6, 14, and 28 hr after sexual development induction, respectively). Transcriptional levels of ppoA and steA were examined by Northern analysis. rRNAs stained with ethidium bromide are shown to indicate RNA loading. The experiment was repeated twice with similar results (average values are shown). Relative transcript levels were quantified by densitometry using Scion Image Beta 4.0.2.
Ceramide synthase genes barA and lagA are required for normal sporulation pattern:
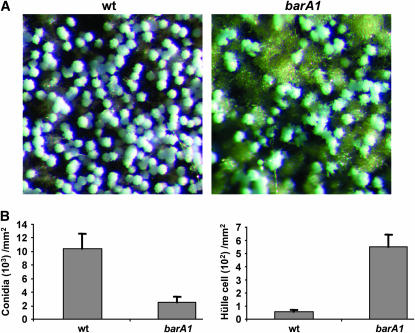
The data above suggest that insufficient PHS synthesis might induce sexual development. To see whether PHS is the sole sphingolipid intermediate involved in Aspergillus development, we also examined the role of the ceramide synthases BarA and LagA. In the barA1 mutant, in which a truncated nonfunctional BarA protein was expressed (Li et al. 2006), the sporulation pattern is similar to that of the wild-type strain when the plates were not sealed with parafilm (data not shown). However, if the plates were sealed (sexual-inducing conditions), the yield of conidia was 76% less than that of wild type, while sexual fruiting structures were more abundant than in the wild-type strain and the yield of Hülle cells was 8.3-fold higher than wild type (Figure 9). Although there was notable induction of the initial steps of sexual development in the barA1, maturation of cleistothecial primordia was delayed in this case and ascospores were first seen after 14 days of incubation (data not known). Similarly, when the alcA(p)∷lagA strain was grown on repressing media (corresponding to a disruption phenotype), conidial production was 66% lower than in the control strain, while Hülle cell production was 10.9-fold higher compared to the control strain. As in the case of barA1, but different from that of basA1, maturation of cleistothecia was also decreased (data not shown). Under these conditions, conidial production in the alcA(p)∷lagA strain was higher than in the alcA(p)∷basA strain and Hülle cell production was lower.
Figure 9.—
Role of BarA in sporulation. Conidiospores from wild-type strain A773 and the barA1 mutant UV13p were inoculated at the center of the MAG plates. Plates were sealed with parafilm and incubated at 28° in the dark for 7 days. (A) Image of the colony surface 1 cm away from the growth front. The image was captured with a Spot Insight Color camera on a Leica MZ75 dissecting microscope with a ×5 objective. (B) Quantitative comparison of conidial and Hülle cell production in the imaged area.
These data indicate that mutations in basA, barA, and lagA affect A. nidulans asexual/sexual developmental balance, although barA and lagA seemed also to be critical for proper maturation of the forming cleistothecia. Taken together, these observations suggest that, in addition to PHS, other sphingolipid intermediates are also important for Aspergillus development. Alternatively, alteration of the sporulation pattern could be a general response to the disruption of sphingolipid synthesis.
DISCUSSION
In this study, we demonstrated that the A. nidulans Sur2 homolog BasA is also required for the synthesis of PHS by supplementation studies. The functional similarity of Sur2 homologs in a broad range of species such as S. cerevisiae (Haak et al. 1997), P. ciferrii (Bae et al. 2004), A. nidulans (this study), and even in plants, as described in A. thaliana (Sperling et al. 2001), strongly suggest that this protein group has a conserved enzymatic activity in various eukaryotic systems.
In S. cerevisiae, the BasA homolog Sur2 is essential for C4 hydroxylation on sphingoid bases, but is not required for growth (Haak et al. 1997; Grilley et al. 1998). Therefore, C4 hydroxylation of sphingoid bases is not essential for growth in yeast, which can survive by utilizing dihydrosphongosine as a substrate to form dihydroceramide and sphingolipids (Haak et al. 1997). By contrast, in the model filamentous fungus A. nidulans, we have demonstrated that the synthesis of phytosphingosine is essential for hyphal growth. Furthermore, the growth defect of the basA1 mutant is at least partially due to insufficient supply of substrate for sphingolipid synthesis. Therefore, sphingolipids that have undergone C4 hydroxylation are presumably required for essential biological functions in A. nidulans. This might reflect an inability of the fungus to use dihydrosphingosine as substrate to form ceramide and sphingolipids.
Cell wall thickening has been previously observed in three different fungal species with defects in sphingolipid metabolism (Barz and Walter 1999; Feoktistova et al. 2001; Li et al. 2006), suggesting a possible role for sphingolipids in cell wall construction. Here, we provide additional evidence supporting such a role by a systemic study of the cell wall response to the disruption of sphingolipid synthesis. Our results strongly suggest that the cell wall thickening caused by various perturbations of sphingolipid synthesis is due to the accumulation of sphingoid bases such as DHS or PHS. PHS in vitro is able to activate the yeast protein kinases Pkh1 and Pkh2 (Friant et al. 2001; Liu et al. 2005). The well-characterized downstream substrate of the Pkh kinases is the protein kinase Pkc1, which is an essential component of the cell wall integrity pathway (de Nobel et al. 2000; Levin 2005). In yeast, the expression of ∼20 genes involved in cell wall synthesis is upregulated by the PKC1–MAP signaling pathway (Jung and Levin 1999). The entire Pkc1–MAP kinase signaling pathway is conserved in filamentous fungi, including A. nidulans (de Nobel et al. 2000). Notably, MpkA, the A. nidulans homolog of the terminal MAP kinase Mpk1, is required for cell wall integrity (Bussink and Osmani 1999). This information suggests that the sphingoid-base-dependent signal transduction pathway may also be present in A. nidulans. Therefore, the increased deposition of cell wall materials in response to the disruption of sphingolipid synthesis might reflect the activation of a sphingoid-base-dependent signaling pathway. Potential targets of this pathway in A. nidulans may include the genes involved in chitin synthesis (i.e., chsB) or the glucan synthase encoded by the fksA gene. However, the expression of these genes was not elevated in the basA1 mutant. This suggests that the downstream targets of the sphingoid-base-regulated cell wall integrity pathway might vary in different fungal species or that other additional mechanism(s) might trigger cell wall thickening in the basA1 mutant. PHS is the only identified sphingolipid intermediate that is involved in cell wall synthesis. The role of DHS in cell wall synthesis or deposition was not investigated. However, we found that DHS induced cell wall thickening more quickly than PHS, suggesting that DHS is likely a more effective signaling sphingoid base compared to PHS in regulating cell wall construction in A. nidulans. The pathway involved in the activation of cell wall synthesis by DHS will be the subject of future research.
In fungi, sphingolipids and their intermediates regulate multiple physiological processes, including the responses to heat (Dickson 1998; Jenkins 2003) and the loss of cell wall integrity (Friant et al. 2001), as well as hyphal polarity (Cheng et al. 2001; Li et al. 2006). Here, we present the first evidence that sphingolipids regulate the normal developmental pattern in fungal species. The consistent developmental phenotypes caused by mutations affecting the synthesis of either phytosphingosine or ceramide indicate that the normal formation of sphingolipids is required for proper fungal differentiation. Transcription of basA was highest during the initiation stage of both conidiation and sexual development, suggesting that a burst of PHS or sphingolipid synthesis may be required for the initiation of both asexual and sexual sporulation. However, mutations of basA or the ceramide synthase genes decreased asexual sporulation but enhanced initiation of sexual development, suggesting that initiation of asexual sporulation might require higher levels of sphingolipid synthesis compared to those needed for sexual sporulation.
Although the transcription of the Aspergillus developmental gene brlA was not affected in the basA1 mutant, transcription of the oxilipin gene, ppoA, and the sexual development transcription factor steA were earlier and slightly elevated compared to wild type. Overexpression of ppoA has been demonstrated to increase the ratio of sexual sporulation to asexual sporulation (Tsitsigiannis et al. 2004a). Therefore the enhancement in sexual development and reduction of asexual development observed in the basA1 mutant might be a consequence of the increase in ppoA transcript levels that would lead to activation of sexual developmental genes such as steA and the consequent increase in sexual development. Considering that sphingolipids and psi factor synthesis share the same upstream precursor substrate, acetyl-CoA, and share an overlapping pathway corresponding to fatty acid synthesis, communication or interaction might exist between these two biosynthetic pathways. In this case, it is also possible that PHS or other sphingolipid intermediates might affect ppoA transcription indirectly through interactions between the sphingolipid and the psi factor biosynthetic pathways.
In conclusion, in this study we have demonstrated the role of BasA in hyphal growth and showed that sphingolipid synthesis is required for normal cell wall organization. Furthermore, our study revealed for the first time in fungi that sphingolipid synthesis also regulates the complex balance between sexual and asexual morphological differentiation in the model filamentous fungus A. nidulans.
Acknowledgments
This work was supported by Northern Illinois University and by an award from the Nebraska Research Initiative.
References
- Adams, T. H., M. T. Boylan and W. E. Timberlake, 1988. brlA is necessary and sufficient to direct conidiophore development in Aspergillus nidulans. Cell 54: 353–362. [DOI] [PubMed] [Google Scholar]
- Adams, T. H., J. K. Wieser and J. H. Yu, 1998. Asexual sporulation in Aspergillus nidulans. Microbiol. Mol. Biol. Rev. 62: 35–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae, J. H., J. H. Sohn, C. S. Park, J. S. Rhee and E. S. Choi, 2004. Cloning and functional characterization of the SUR2/SYR2 gene encoding sphinganine hydroxylase in Pichia ciferrii. Yeast 21: 437–443. [DOI] [PubMed] [Google Scholar]
- Barz, W. P., and P. Walter, 1999. Two endoplasmic reticulum (ER) membrane proteins that facilitate ER-to-Golgi transport of glycosylphosphatidylinositol-anchored proteins. Mol. Biol. Cell 10: 1043–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeler, T., D. Bacikova, K. Gable, L. Hopkins, C. Johnson et al., 1998. The Saccharomyces cerevisiae TSC10/YBR265w gene encoding 3-ketosphinganine reductase is identified in a screen for temperature-sensitive suppressors of the Ca2+-sensitive csg2Delta mutant. J. Biol. Chem. 273: 30688–30694. [DOI] [PubMed] [Google Scholar]
- Borgia, P. T., N. Iartchouk, P. J. Riggle, K. R. Winter, Y. Koltin et al., 1996. The chsB gene of Aspergillus nidulans is necessary for normal hyphal growth and development. Fungal Genet. Biol. 20: 193–203. [DOI] [PubMed] [Google Scholar]
- Bussink, H. J., and S. A. Osmani, 1999. A mitogen-activated protein kinase (MPKA) is involved in polarized growth in the filamentous fungus, Aspergillus nidulans. FEMS Microbiol. Lett. 173: 117–125. [DOI] [PubMed] [Google Scholar]
- Calvo, A. M., H. W. Gardner and N. P. Keller, 2001. Genetic connection between fatty acid metabolism and sporulation in Aspergillus nidulans. J. Biol. Chem. 276: 25766–25774. [DOI] [PubMed] [Google Scholar]
- Calvo, A. M., R. A. Wilson, J. W. Bok and N. P. Keller, 2002. Relationship between secondary metabolism and fungal development. Microbiol. Mol. Biol. Rev. 66: 447–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champe, S. P., and A. A. el-Zayat, 1989. Isolation of a sexual sporulation hormone from Aspergillus nidulans. J. Bacteriol. 171: 3982–3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champe, S. P., P. Rao and A. Chang, 1987. An endogenous inducer of sexual development in Aspergillus nidulans. J. Gen. Microbiol. 133: 1383–1387. [DOI] [PubMed] [Google Scholar]
- Cheng, J., T. S. Park, A. S. Fischl and X. S. Ye 2001. Cell cycle progression and cell polarity require sphingolipid biosynthesis in Aspergillus nidulans. Mol. Cell. Biol. 21: 6198–6209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deising, H. B., S. Werner and M. Wernitz, 2000. The role of fungal appressoria in plant infection. Microbes Infect. 2: 1631–1641. [DOI] [PubMed] [Google Scholar]
- De Nobel, H., H. van Ende and F. M. Klis, 2000. Cell wall maintenance in fungi. Trends Microbiol. 8: 344–345. [DOI] [PubMed] [Google Scholar]
- Dickson, R. C., 1998. Sphingolipid functions in Saccharomyces cerevisiae: comparison to mammals. Annu. Rev. Biochem. 67: 27–48. [DOI] [PubMed] [Google Scholar]
- D'Souza, C. A., and J. Heitman, 2001. Conserved cAMP signaling cascades regulate fungal development and virulence. FEMS Microbiol. Rev. 25: 349–364. [DOI] [PubMed] [Google Scholar]
- Efimov, V. P., 2003. Roles of NUDE and NUDF proteins of Aspergillus nidulans: insights from intracellular localization and overexpression effects. Mol. Biol. Cell 14: 871–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feoktistova, A., P. Magnelli, C. Abeijon, P. Perez, R. L. Lester et al., 2001. Coordination between fission yeast glucan formation and growth requires a sphingolipase activity. Genetics 158: 1397–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friant, S., R. Lombardi, T. Schmelzle, M. N. Hall and H. Riezman, 2001. Sphingoid base signaling via Pkh kinases is required for endocytosis in yeast. EMBO J. 20: 6783–6792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopee, N. V., and R. P. Sharma, 2003. Sphingoid bases and their phosphates: transient activation and delayed repression of protein kinase C isoforms and their possible involvement in fumonisin B1 cytotoxicity. Toxicology 187: 239–250. [DOI] [PubMed] [Google Scholar]
- Grilley, M. M., S. D. Stock, R. C. Dickson, R. L. Lester and J. Y. Takemoto, 1998. Syringomycin action gene SYR2 is essential for sphingolipid 4-hydroxylation in Saccharomyces cerevisiae. J. Biol. Chem. 273: 11062–11068. [DOI] [PubMed] [Google Scholar]
- Haak, D., K. Gable, T. Beeler and T. Dunn, 1997. Hydroxylation of Saccharomyces cerevisiae ceramides requires Sur2p and Scs7p. J. Biol. Chem. 272: 29704–29710. [DOI] [PubMed] [Google Scholar]
- Hanada, K., 2003. Serine palmitoyltransferase, a key enzyme of sphingolipid metabolism. Biochim. Biophys. Acta 1632: 16–30. [DOI] [PubMed] [Google Scholar]
- Hanada, K., M. Nishijima, T. Fujita and S. Kobayashi S. 2000. Specificity of inhibitors of serine palmitoyltransferase (SPT), a key enzyme in sphingolipid biosynthesis, in intact cells. A novel evaluation system using an SPT-defective mammalian cell mutant. Biochem. Pharmacol. 59: 1211–1216. [DOI] [PubMed] [Google Scholar]
- Harris, S. D., J. L. Morrel and J. H. Hamer, 1994. Identification and characterization of Aspergillus nidulans mutants defective in cytokinesis. Genetics 136: 517–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms, J. B., and C. Zurzolo, 2004. Lipids as targeting signals: lipid rafts and intracellular trafficking. Traffic 5: 247–254. [DOI] [PubMed] [Google Scholar]
- Ikushiro, H., H. Hayashi and H. Kagamiyama, 2004. Reactions of serine palmitoyltransferase with serine and molecular mechanisms of the actions of serine derivatives as inhibitors. Biochemistry 43: 1082–1092. [DOI] [PubMed] [Google Scholar]
- Jahnson, V. J., Q. He, M. F. Osuchowski and R. P. Sharma, 2004. Disruption of sphingolipid homeostasis by myriocin, a mycotoxin, reduces thymic and splenic T-lymphocyte populations. Toxicology 201: 67–75. [DOI] [PubMed] [Google Scholar]
- Jenkins, G. M., 2003. The emerging role for sphingolipids in the eukaryotic heat shock response. Cell. Mol. Life Sci. 60: 701–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong, H. Y., H. Kim, D. M. Han, K. Y. Jahng and K. S. Chae, 2003. Expression of the mnpA gene that encodes the mannoprotein of Aspergillus nidulans is dependent on fadA and flbA as well as veA. Fungal Genet. Biol. 38: 228–236. [DOI] [PubMed] [Google Scholar]
- Jung, U. S., and D. E. Levin, 1999. Genome-wide analysis of gene expression regulated by the yeast cell wall integrity signalling pathway. Mol. Microbiol. 34: 1049–1057. [DOI] [PubMed] [Google Scholar]
- Käfer, E., 1977. Meiotic and mitotic recombination in Aspergillus and its chromosomal aberrations. Adv. Genet. 19: 33–131. [DOI] [PubMed] [Google Scholar]
- Kato, N., W. Brooks and A. M. Calvo, 2003. The expression of sterigmatocystin and penicillin genes in Aspergillus nidulans is controlled by veA, a gene required for sexual development. Eukaryot. Cell 2: 1178–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, H., K. Han, K. Kim, D. Han, K. Jahng et al., 2002. The veA gene activates sexual development in Aspergillus nidulans. Fungal Genet. Biol. 37: 72–80. [DOI] [PubMed] [Google Scholar]
- Levin, D. E., 2005. Cell wall integrity signaling in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 69: 262–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, S., L. Du, G. Yuen and S. D. Harris, 2006. Distinct ceramide synthases regulate polarized growth in the filamentous fungus Aspergillus nidulans. Mol. Biol. Cell 17: 1218–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, K., X. Zhang, R. L. Lester and R. C. Dickson, 2005. The sphingoid long chain base phytosphingosine activates AGC-type protein kinases in Saccharomyces cerevisiae including Ypk1, Ypk2, and Sch9. J. Biol. Chem. 280: 22679–22687. [DOI] [PubMed] [Google Scholar]
- Mazur, P., K. Nakanishi, A. A. E. El-Zayat and S. P. Champe, 1991. Structure and synthesis of sporogenic psi factors from Aspergillus nidulans. J. Chem. Soc. Chem. Commun. 20: 1486–1487. [Google Scholar]
- Merrill, A. H., Jr., 2002. De novo sphingolipid biosynthesis: a necessary, but dangerous pathway. J. Biol. Chem. 277: 25843–25846. [DOI] [PubMed] [Google Scholar]
- Merrill, A. H., Jr., and E. Wang, 1986. Biosynthesis of long-chain (sphingoid) bases from serine by LM cells. Evidence for introduction of the 4-trans-double bond after de novo biosynthesis of N-acylsphinganine(s). J. Biol. Chem. 261: 3764–3769. [PubMed] [Google Scholar]
- Merrill, A. H., Jr., Y. A. Hannun and R. M. Bell, 1993. Introduction: sphingolipids and their metabolites in cell regulation. Adv. Lipid Res. 25: 1–24. [PubMed] [Google Scholar]
- Mirabito, P. M., T. H. Adams and W. E. Timberlake, 1989. Interactions of three sequentially expressed genes control temporal and spatial specificity in Aspergillus development. Cell 57: 859–868. [DOI] [PubMed] [Google Scholar]
- Mooney, J. L., and L. N. Yager, 1990. Light is required for conidiation in Aspergillus nidulans. Genes Dev. 4: 1473–1482. [DOI] [PubMed] [Google Scholar]
- Oakley, B. R., and S. A. Osmani, 1993. Cell cycle analysis using the filamentous fungus Aspergillus nidulans, pp. 127–142 in The Cell Cycle: Practical Approach, edited by P. Fantes and R. Brooks. Oxford University Press, Oxford.
- Pinto, W. J., B. Srinivasan, S. Shepherd, A. Schmidt, R. C. Dickson et al., 1992. Sphingolipid long-chain-base auxotrophs of Saccharomyces cerevisiae: genetics, physiology and a method for their selection. J. Bacteriol. 174: 2565–2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz, A., E. Rapaport, K. Hirschberg and A. H. Futerman, 1995. A regulatory role for sphingolipids in neuronal growth. Inhibition of sphingolipid synthesis and degradation have opposite effects on axonal branching. J. Biol. Chem. 270: 10990–10998. [DOI] [PubMed] [Google Scholar]
- Semighini, C., M. Savoldi, G. H. Goldman and S. D. Harris, 2006. Functional characterization of the putative Aspergillus nidulans poly(ADP-ribose) polymerase homologue PrpA. Genetics 173: 87–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons, K., and E. Ikonen 1997. Functional rafts in cell membranes. Nature 387: 569–572. [DOI] [PubMed] [Google Scholar]
- Sperling, P., P. Ternes, H. Moll, S. Franke, U. Zahringer et al., 2001. Functional characterization of sphingolipid C4-hydroxylase genes from Arabidopsis thaliana. FEBS Lett. 494: 90–94. [DOI] [PubMed] [Google Scholar]
- Stinnett, S. M., E. A. Espeso, L. Cobeno, L. Araujo-Bazan and A. M. Calvo, 2006. Aspergillus nidulans VeA subcellular localization is dependent on the importin alpha carrier and on light. Mol. Microbiol. 63: 242–255. [DOI] [PubMed] [Google Scholar]
- Tsitsigiannis, D. I., R. Zarnowski and N. P. Keller, 2004. a The lipid body protein, PpoA, coordinates sexual and asexual sporulation in Aspergillus nidulans. J. Biol. Chem. 279: 11344–11353. [DOI] [PubMed] [Google Scholar]
- Tsitsigiannis, D. I., T. M. Kowieski, R. Zarnowski and N. P. Keller, 2004. b Endogenous lipogenic regulators of spore balance in Aspergillus nidulans. Eukaryot. Cell 3: 1398–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallim, M. A., K. Y. Miller and B. L. Miller, 2000. Aspergillus SteA (sterile12-like) is a homeodomain-C2/H2-Zn+2 finger transcription factor required for sexual reproduction. Mol. Microbiol. 36: 290–301. [DOI] [PubMed] [Google Scholar]
- Vaux, D. L., and S. J. Korsmeyer, 1999. Cell death in development. Cell 96: 245–254. [DOI] [PubMed] [Google Scholar]
- Warnecke, D., and E. Heinz, 2003. Recently discovered functions of glucosylceramides in plants and fungi. Cell. Mol. Life Sci. 60: 919–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yager, L. N., 1992. Early developmental events during asexual and sexual sporulation in Aspergillus nidulans. Biotechnology 23: 19–41. [PubMed] [Google Scholar]
- Zhong, W., M. W. Jeffries and N. H. Georgopapadakou, 2000. Inhibition of inositol phosphorylceramide synthase by Aba in Candida and Aspergillus species. Antimicrob. Agents Chemother. 44: 651–653. [DOI] [PMC free article] [PubMed] [Google Scholar]