Abstract
We have previously shown that CD8 T cells from IFN-γ gene knockout (GKO) donors induces more severe lethal graft-vs.-host disease (GVHD) compared to CD8 T cells from wild type (WT) donors in fully MHC-mismatched strain combinations. In this study, we investigated the mechanisms by which IFN-γ inhibits GVHD in a parent→F1 (B6→;B6D2F1) allogeneic hematopoietic cell transplantation (allo-HCT) model. IFN-γ was strongly protective against GVHD in this parent→;F1 haplotype-mismatched allo-HCT model. Irradiated B6D2F1 mice that received GKO B6 CD4-depleted splenocytes develop lethal GVHD with severe lung and liver injury, whereas those receiving a similar cell population from WT B6 donors survived long-term. Donor CD8 cells showed rapid activation, accelerated cell division and reduced/delayed activation-induced cell death in allogeneic recipients in which donor cells were incapable of producing IFN-γ. Consequently, the numbers of activated/effector (i.e., CD25+, CD62L− and CD44high) donor CD8 T cells in the recipients of GKO allo-HCT significantly exceeded those in mice receiving WT allo-HCT. These data show that IFN-γ negatively regulates the CD8 T cell response by inhibiting cell division and promoting cell death, and suggest that blockade of IFN-γ could augment the severity of GVHD in allo-HCT recipients.
INTRODUCTION
IFN-γ is a potent proinflammatory cytokine that plays important and complex roles in both innate and adaptive immune responses. The compromised immunity to multiple intracellular pathogens in IFN-γ- and IFN-γ receptor-deficient mice suggests an important role for IFN-γ in the induction of cellular immune responses [1,2]. A recent study using IFN-γ receptor-deficient mice shows that IFN-γ acts directly on CD8 T cells to stimulate the development of CTL responses after LCMV infection [3]. However, increasing evidence demonstrates that IFN-γ may also downregulate immune responses. IFN-γ plays an important role in the maintenance of T cell homeostasis and eliminates activated CD4 [4–8] and CD8 [8–10] T cells by inducing apoptosis. It has been shown that IFN-γ promotes cell death of activated T cells [11-14]. IFN-γ has also been reported to facilitate induction of long-term allograft survival by blockade of T cell costimulation pathways [15]. Furthermore, recent studies showed that IFN-γ is required for the function of alloantigen-specific regulatory T cells [16], and may inhibit CD8 memory T cell generation [17]. Although the mechanisms remain largely unknown, these studies indicate that IFN-γ has a complex role in the regulation of immune responses.
Activated T cells produce IFN-γ and the level of IFN-γ in patients receiving allo-HCT may reflect ongoing GVH alloresponses [18-21]. Such a correlation between high levels of IFN-γ and severe GVHD has led to a suggestion that IFN-γ may be involved in the pathogenesis of GVHD. Of note, this cytokine has been reported to contribute to gut injury [22,23] and lymphoid hypoplasia [24,25] in allo-HCT recipients. However, studies using anti-IFN-γ antibody and IFN-γ gene knockout (GKO) mice demonstrated that this cytokine can inhibit the development of acute GVHD by promoting apoptosis of alloreactive CD4 T cells [26–29].
We have recently observed that IFN-γ-deficient CD8 T cells induce more severe GVHD than wild-type (WT) cells in fully MHC- plus minor antigen-mismatched allogeneic recipients [30]. In the present study, we explore the mechanisms of the regulation of alloreactive CD8 T cells by IFN-γ using a clinically relevant, parent→F1, allo-HCT model. We observed that IFN-γ plays a critical role in controlling donor CD8 T cell activation, proliferation, and survival in allo-HCT recipients. In the absence of IFN-γ, activation and expansion of alloreactive donor CD8 T cells was significantly augmented and apoptotic cell death of such cells was markedly reduced, resulting in increased accumulation of highly divided donor CD8 T cells. Furthermore, IFN-γdeficient, but not WT, donor CD8 T cells induced lethal GVHD characterized by severe damage to non-lymphoid target tissues.
MATERIALS AND METHODS
Animals
Female wild-type (WT) C57BL/6 mice (B6; H-2b), IFN-γ gene knockout (GKO) mice on the B6 background (B6.129S7-Ifngtm1Ts; H-2b) and B6D2F1 (H-2b×d) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Anti-Ld 2C TCR transgenic mice on the B6 background were generously provided by Dr. Dennis Y. Loh [31]. IFN-γ-deficient 2C TCR transgenic (GKO 2C) mice on the B6 background were generated as previously described [30]. Mice were housed in a specific pathogen-free microisolator environment, and fed autoclaved feed and autoclaved, acidified water. Protocols involving animals were approved by the Massachusetts General Hospital Subcommittee on Research Animal Care.
Allo-HCT and induction of GVHD
Recipient B6D2F1 mice were lethally irradiated (9.75 Gy) and reconstituted within 4-8 hours with 7×106 T cell (i.e., CD4 and CD8)-depleted (TCD) bone marrow cells (BMC) and 3×107 CD4-depleted spleen cells from either WT or GKO B6 mice, or with 5×106 BMC and 1.5×107 spleen cells from 2C or GKO 2C B6 mice. Mice that received TCD BMC and TCD splenocytes from allogeneic donors were used as non-GVHD controls. CD4- and CD8-depleted cells were prepared with anti-CD4 or CD8α microbeads followed by negative selection using the MACS separation system according to the manufacturer’s protocol (Miltenyi Biotec, CA).
Histopathology
GVHD target tissues (liver, lungs and colon) were harvested at various times after HCT and fixed in Bouin’s solution or 10% formalin. Paraffin sections were prepared and stained with hematoxylin and eosin (H&E). Pathological GVHD was evaluated by a pathologist (R. Bronson) without knowledge of the treatment the animals received based on the parameters described in Table 1. Each parameter was scored using a score system ranging from 0 to 4: 0, normal; 0.5, focal and rare; 1, focal and mild; 2, diffuse and mild; 3, diffuse and moderate; and 4, diffuse and severe.
Table 1.
Pathological GVHD criteria
| Colon | Lung | Liver |
|---|---|---|
| a). Crypt epithelial cell regeneration, apoptosis | a). Perivascular cell infiltrate | a). Portal tract inflammatory cell infiltrate |
| b). Crypt loss | b). Peribronchiolar cell infiltrate | b). Bile duct lymphocytic infiltrate |
| c). Surface colonocyte attenuation | c). Vascular endothelialitis | |
| d). Inflammatory cell infiltrate | d). Parenchymal apoptosis | |
| e). Mucosal ulceration | e). Parenchymal microabscesses | |
| f). Thickness of mucosa | f). Parenchymal mitotic figures |
Flow cytometric (FCM) analysis
To determine T cell activation, single cell suspensions were prepared from spleen and lymph nodes, and stained with fluorescent (FITC or PE)-conjugated mAb specific for activation/memory markers (CD25, CD44, and CD62L) and anti-CD8 mAb. NKT cells and subsets were identified by staining the cells with FITC-conjugated anti-CD3, PE-conjugated anti-NK1.1, plus APC-conjugated anti-CD4 and/or CD8. Regulatory T (T-reg) cells were determined by an APC-conjugated anti-Foxp3 mAb (eBioscience, San Diego, CA). In allo-HCT recipients, donor cells were identified by staining with biotinylated anti-H-2Dd mAb 34-2-12 [32] developed with phycoerythrin-streptavidin or streptavidin-allophycocyanin. Non-specific FcγR binding was blocked with anti-mouse FcγR mAb 2.4G2. Exclusion of dead cells was performed by propidium iodine (PI) staining and live gating on PI-negative cells. Non-reactive FITC-conjugated and biotinylated mouse IgG2a mAb HOPC-1, PE- and allophycocyanin-labeled rat IgG2a mAbs were used as negative controls. In proliferation studies, donor spleen cells were incubated with 1 μM carboxyfluorescein diacetate succinimidyl ester (CFSE; Molecular Probes, Eugene, OR) at 50×106 cells/mL for 15 minutes at 37°C in the dark. Cold medium with 2% FCS was added to stop the labeling reaction. Cells were then washed twice with fresh serum-free medium and resuspended at desired concentrations prior to transplant. In apoptotic studies, cells were stained with allophycocyanin-conjugated Annexin-V and 7-AAD. Apoptotic cells were defined by Annexin-V positive and 7-AAD negative population. Unless otherwise indicated, all antibodies used were purchased from BD PharMingen (San Diego, CA).
Statistical analysis
Statistical analysis of survival data was performed with the log-rank test. The student’s t test was used to determine the level of significance of differences in group means. A p value of <0.05 was considered significant in both types of analysis.
RESULTS
IFN-γ deficient CD8 T cells induce acute lethal GVHD
To determine the role of IFN-γ in CD8-mediated GVHD, B6D2F1 mice were lethally irradiated and reconstituted with 7×106 T-cell depleted BMC and 30×106 CD4-depleted spleen cells from WT or GKO B6 mice via the tail vein. As shown in Figure 1A, allo-HCT from GKO donors induced severe lethal GVHD, with over 50% (10 of 19) mortality by 3 weeks and 100% mortality by 6 weeks (p<0.01 compared to the recipients of WT allo-HCT). These mice developed severe clinical signs of acute GVHD, including massive weight loss (Figure B), ruffled fur, hunched posture, and diarrhea before death. In contrast, most mice (12 of 15) receiving a similar HCT inoculum from WT B6 donors survived long-term. The recipients of WT allo-HCT showed only moderate (~10%) weight loss during the first week and gradually gained the weight back (Figure 1B). Control mice that received TCD allogeneic cells from either WT or GKO B6 mice appeared healthy throughout the experiment (Figure 1). These results demonstrate that IFN-γ mediates significant protection against GVHD induced by CD8 T cells in F1 recipients of allo-HCT from parental donors, consistent with previous observations in fully MHC-mismatched strain combinations [30].
Figure 1. IFN-γ inhibits GVHD induced by donor CD8 T cells.
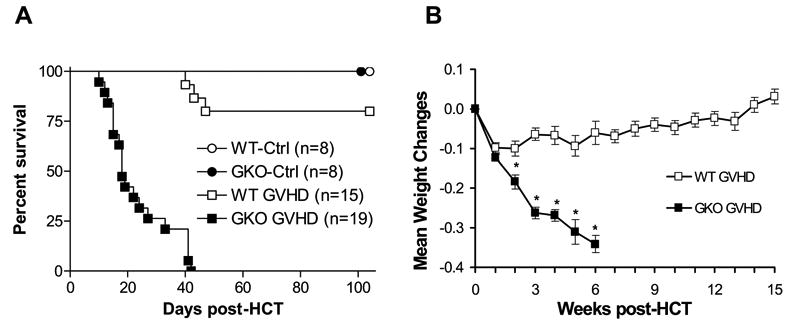
Recipient B6D2F1 mice were lethally irradiated and reconstituted with 7×106 TCD BMC and 3×107 CD4-depleted spleen cells from WT (WT GVHD; n=15) or GKO (GKO GVHD; n=19) B6 donors. Non-GVHD controls were lethally-irradiated B6D2F1 mice that received TCD BMC alone or along with TCD splenocytes from WT (WT-Ctrl; n=8) or GKO (GKO-Ctrl; n=8) donors. Data from 3 independent experiments are combined. The percentages of CD8+ cells in WT and GKO allo-HCT inocula (i.e., CD4-depleted splenocytes) used in these experiments were 17.9% and 17.7%, 16.1% and 15.7%, and 21% and 23%, respectively. Shown are percent survival (A) and mean body weight changes (mean±SDs) (B) of the allo-HCT recipients. Mean weight changes = [(current weight/weight at day 0) – 1]. *, p<0.05.
Increased Pathological GVHD in recipients of GKO allo-HCT
Histological examination was performed on tissues prepared from the recipient mice at days 2, 4, 5, 7, and 11 post-HCT, and pathological changes were scored blindly. Both WT and GKO allo-HCT groups showed minimal pathological changes in lungs and liver at days 2 (not shown) and 4 (Figures 2A-B). By day 7 post-HCT, severe inflammatory infiltrates were seen in both the lungs and liver in the recipients of GKO allo-HCT, whereas significant inflammatory infiltrates were not detected in the same tissues from the recipients of WT allo-HCT until day 11 post-HCT. Pathological GVHD scores for both tissues harvested at days 5 and 7 were significantly greater in the recipients of GKO allo-HCT than in those receiving WT allo-HCT (Figures 2A-B). Furthermore, the recipients of GKO allo-HCT showed markedly increased infiltration by CD8 T cells in the liver compared to mice receiving WT allo-HCT (Figure 2D).
Figure 2. GKO allo-HCT induces more severe GVHD pathology.

A–C. Shown are pathological GVHD scores (mean±SDs, n=3-6/group) and representative H&E photomicrographs of the lungs (A), liver (B) and colon (C) from the recipients of WT (closed columns) or GKO allo-HCT (open columns). *, p<0.05. D. Immunofluorescent staining of liver sections prepared from allo-HCT recipients at day 7 with anti-CD8. Three mice from each group were analyzed and representative photomicrographs are shown.
During the acute phase of GVHD progression, pathological changes in the intestine include crypt destruction and mucosal alterations [33]. As shown in Figure 2C, by day 5 post-HCT, the recipients of GKO allo-HCT developed severe colonic GVHD, as demonstrated by massive inflammatory cell infiltrates, crypt regeneration, epithelial cell apoptosis, surface colonocyte attenuation and thickening of mucosa. Colonic GVHD in these recipients decreased after day 5 and subsided by day 11. Although a similar pathological pattern was also seen in the recipients of WT allo-HCT, its development was delayed compared to that in recipients of GKO allo-HCT.
Augmented activation and expansion of donor CD8 T cells in recipients of GKO allo-HCT
To determine the mechanisms by which IFN-γ deficiency augments GVHD, we compared the activation and expansion of donor CD8 T cells in the spleens of lethally-irradiated B6D2F1 mice that received TCD BMC plus CD4-depleted splenocytes from WT or GKO B6 donors. The numbers of total donor CD8 T cells in the spleens of the recipients of GKO allo-HCT were significantly greater at days 4 and 5, but became similar or lower at day 7 than that in recipients of WT allo-HCT (Table 2). Because T cells gain CD25 expression and lose CD62L expression upon activation, we also compared the expression of these molecules on donor CD8 T cells. As shown in Figure 3A-B, the percentages of CD25+ and CD62- donor CD8 T cells in the recipients of GKO allo-HCT were markedly greater than those in recipients of WT allo-HCT, suggesting an increased activation in the absence of IFN-γ. Furthermore, GKO donor CD8 T cells acquired a “memory” phenotype (i.e., CD44high and CD45RBlow) more rapidly than WT donor CD8 T cells in allo-HCT recipients (Fig. 3A-B, and data not shown). Since nearly all GKO donor CD8 T cells lost expression of CD62L, a secondary lymphoid organ homing receptor [34], by day 5, and increased CD8 T cell infiltration was detected in the GVHD target tissues in the GKO allo-HCT group (Figure 2D), the reduction in donor CD8 T cells detected at day 7 in mice receiving GKO allo-HCT (Table 2) might be in part due to more rapid trafficking of activated CD8 T cells to GVHD target tissues.
Table 2.
Numbers of donor CD8 T cells in the spleen of allo-HCT recipients at the indicated times
| Day 4
|
Day 5
|
Day 7
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| WT | GKO | p | WT | GKO | p | WT | GKO | p | |
| Exp. 1 | 1.0 ± 0.2 | 1.4 ± 0.2 | >0.05 | 3.8 ± 0.8 | 11.8 ± 2.5 | <0.05 | 28.4 ± 1.7 | 26.4 ± 1.3 | >0.05 |
| Exp. 2 | 0.7 ± 0.04 | 1.4 ± 0.1 | <0.05 | 3.9 ± 0.2 | 18.1 ± 1.3 | <0.001 | N/A | N/A | N/A |
| Exp. 3 | 2.0 ± 0.4 | 7.1 ± 0.7 | <0.01 | 6.2 ± 1.0 | 11.9 ± 2.7 | >0.05 | 15.0 ± 0.7 | 7.1 ± 1.3 | <0.01 |
The number of donor CD8 T cells per spleen was determined by the product of the percentage of donor CD8 T cells measured by FCM analysis and the total number of spleen cells harvested from each individual animal, and data are presented as group means ± SDs (x106) per spleen. N=3 per group in all 3 experiments. N/A, not available
Figure 3. Augmented activation and expansion of donor CD8 T cells in the recipients of GKO allo-HCT.
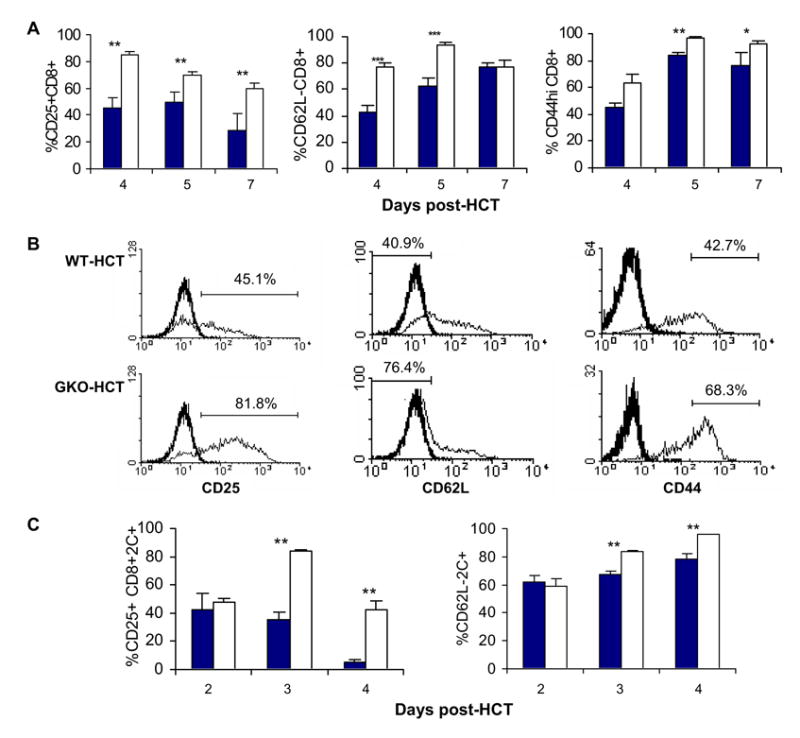
A–B: Expression of activation markers on donor CD8 T cells in the spleens of lethally-irradiated B6D2F1 mice receiving allo-HCT from WT or GKO B6 donors (n=6 per group). A. Percentages of donor CD25+, CD62L-, and CD44high CD8 T cells at the indicated time points. WT and GKO donor CD8 T cells are presented as closed and open bars, respectively. B. Representative FCM profiles showing the expression of CD25, CD62L and CD44 on gated donor CD8 T cells at day 4. Thick lines are the isotype staining controls. Analysis gates were set based on staining profiles of appropriate isotype control mAs and naïve splenocytes stained with the same mAbs at the same time. C. Spleen cells were prepared from B6D2F1 recipients of allo-HCT from WT (closed bars) or GKO (open bars) 2C donors at the indicated time points, and the percentages of CD25+ and CD62L- 2C T cells were analyzed by FCM analysis. Data are shown as mean ± SDs (n=3 per group). *, p<0.05; **, p<0.01.
We also performed a similar analysis in a 2C TCR transgenic allo-HCT model. The 2C cytotoxic T cell clone was derived from mice with H-2b that were immunized with H-2d cells [35]. Since the majority of T cells in 2C TCR transgenic mice are CD8+2C TCR+ cells that specifically recognize H-2Ld, administration of 2C T cells into H-2Ld+ mice allows the study of graft-versus-host reactivity of CD8 T cells. Lethally irradiated (9.75 Gy) B6D2F1 mice received 5×106 T cell-depleted BMCs and 15×106 spleen cells from either WT 2C or GKO 2C TCR transgenic B6 mice. The percentages of CD25+ and CD62L− 2C CD8 T cells were significantly higher in the B6D2F1 mice receiving GKO 2C cells than in those receiving WT 2C cells (Figure 3C). Together, these results indicate that the activation and expansion of GVH-reactive CD8 T cells are significantly increased in allo-HCT recipients when the donor cells are deficient in IFN-γ production.
Role of IFN-γ in apoptosis and cell division of alloreactive donor CD8 T cells
The total number of activated donor CD8 T cells could be determined by the combined effects of activation induced cell death and cell division. When we assessed apoptosis of donor CD8 T cells in the recipient spleens in both the B6→B6D2F1 (Fig. 4A) and 2C→B6D2F1 (Fig. 4B) models, we observed that donor CD8 T cell apoptosis was markedly reduced/delayed in the recipients of GKO allo-HCT compared to those receiving WT allo-HCT. In the B6→B6D2F1 model, GKO donor CD8 T cell apoptosis was significantly reduced at earlier times, at days 4 and 5, but increased by day 7 (Fig. 4A). The later increase in apoptosis of GKO donor CD8 T cells may also contribute to the reduction in donor CD8 T cells in the spleen detected at day 7 in mice receiving GKO allo-HCT (Table 2). In the 2C→B6D2F1 allo-HCT model, significantly reduced apoptosis was detected at day 3 in GKO 2C T cells compared to WT 2C T cells (Fig. 4B).
Figure 4. Effect of IFN-γ on apoptosis of donor CD8 T cells.
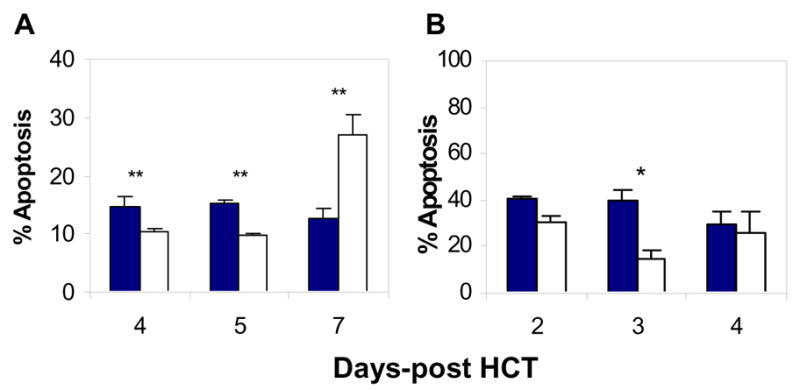
Spleen cells were prepared from the recipients of WT (closed bars) or GKO (open bars) allo-HCT at the indicated time points, and the percentages of apoptotic donor CD8 (or 2C) T cells were determined by FCM analysis. A. Percentages of apoptotic donor CD8 T cells in the spleens of B6→B6D2F1 HCT recipients. B. Percentages of apoptotic donor 2C T cells in the spleens of 2C→B6D2F1 HCT recipients. Data are shown as mean ± SDs (n=3-6 per group). *, p<0.05; **, p<0.01.
To assess donor CD8 T cell division in allo-HCT recipients, lethally-irradiated B6D2F1 mice received T-cell depleted BMCs and CFSE-labeled CD4-depleted spleen cells from WT or GKO B6 donors. Spleen and lymph node cells were prepared from the recipients at days 2 and 4, and analyzed for donor CD8 T cell divisions by flow cytometry. As shown in Figure 5A-B, the numbers of dividing donor CD8 cells, especially those with extensive cell divisions (≥8 divisions), were significantly increased in the mice receiving GKO allo-HCT compared to the recipients of WT allo-HCT.
Figure 5. Effect of IFN-γ on division of donor CD8 T cells.
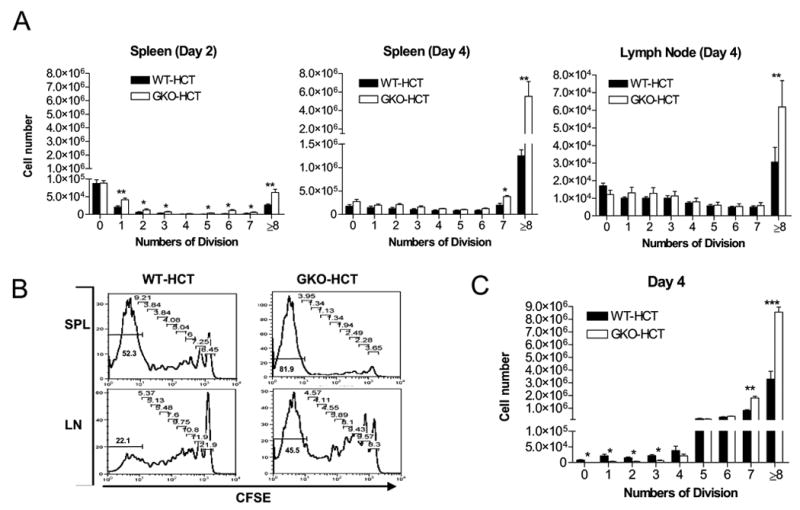
A–B. B6D2F1 mice received BMC and CFSE-labeled CD4-depleted spleen cells from WT (closed bars) or GKO (open bars) B6 donors (n=3 per group). Recipient spleen and lymph node cells were prepared at the indicated time points and analyzed for division of donor CD8 T cells. A. Numbers of donor CD8 T cells with each division in the spleen at days 2 and 4, and in the lymph nodes at day 4 post-HCT. Axillary, brachial and inguinal lymph nodes were harvested at indicated time point, and pooled lymph node cells were analyzed. B. Representative histograms showing CFSE levels in gated donor CD8 T cells at day 4. Numbers indicate the percentages of cells with each number of cell divisions. C. B6D2F1 mice received BMC and CFSE-labeled spleen cells from WT (closed bars) or GKO (open bars) 2C donors (n=3 per group). Data shown are the percentages of donor 2C TCR+ cells with different cell divisions (mean±SDs) in the recipient spleen at day 4 post-HCT. *, p<0.05; **, p<0.01, ***, p<0.001.
Because of the presence of non-GVH-reactive CD8 T cells in the allo-HCT inoculum, it was difficult to conclude if the increased number of extensively divided donor CD8 T cells was due to augmented cell proliferation and/or reduced apoptosis of dividing donor T cells. Thus, we next compared cell division between GKO and WT 2C T cells in the 2C→B6D2F1 allo-HCT model. Similar to the B6→B6D2F1 model, the numbers of host-reactive 2C T cells with 7 or more divisions were significantly greater in the recipients of GKO allo-HCT than in mice receiving WT allo-HCT (Figure 5C). However, the numbers of 2C T cells with less than 4 divisions were lower in mice receiving GKO allo-HCT than in those receiving WT allo-HCT (Figure 5C). Since GKO 2C T cells showed reduced apoptosis in allo-HCT recipients compared to WT 2C T cells (Figure 4B), the reduction in less divided GKO 2C T cells and the increase in GKO 2C T cells with ≥7 divisions is likely a consequence of accelerated proliferation of these cells. Taken together, these results indicate that IFN-γ promotes apoptosis, and also limits the proliferation of donor CD8 T cells in allo-HCT recipients.
T-reg, NKT and memory T cell contents in WT and GKO mice
To determine whether the different ability to induce GVHD between WT and GKO allo-HCT might be attributable to differences in the contents of various T cell subsets in the HCT inocula, we compared the levels of T-reg, NKT and memory T cells in the spleen between WT and GKO B6 mice. In both groups, almost all splenic T-reg (i.e., CD3+Foxp3+) cells express CD4 (Figure 6A). The level of CD4+Foxp3+ T-reg cells (most of these cells also express CD25) was significantly lower in the spleen of GKO mice than in WT mice. However, the levels of CD4− (including CD8+) cells expressing Foxp3 were comparable between WT and GKO mice and both were extremely low. Furthermore, the levels of splenic CD4 and CD8 NKT (i.e., CD4+CD3+NK1.1+ and CD8+CD3+NK1.1+) cells were similar between WT and GKO mice, but more double negative NKT (i.e., CD4−CD8−CD3+NK1.1+) cells were detected in the latter group (Figure 6B).
Figure 6. Comparison of T-reg, NKT and memory T cells in normal WT and GKO B6 mice.
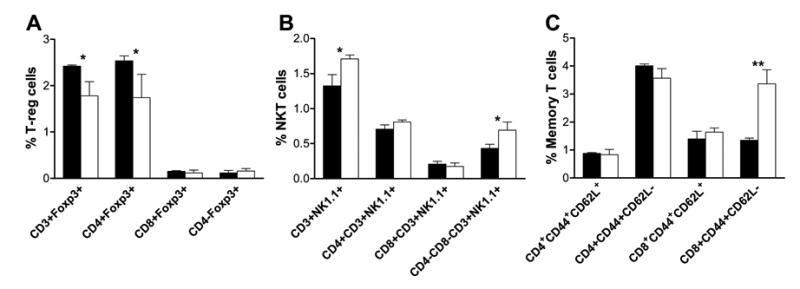
Spleen cells were prepared from naïve WT (▪) and GKO (□) B6 mice (8-week old; n=3 per group) and analyzed for T-reg, NKT and memory T cells by flow cytometry. A. Percentages of total (CD3+Foxp3+), CD4 (CD4+Foxp3+), CD8 (CD8+Foxp3+) and CD4- (CD4-Foxp3+) T-reg cells. B. Percentages of total (CD3+NK1.1+), CD4 (CD4+CD3+NK1.1+), CD8 (CD8+CD3+NK1.1+), and double negative (CD4-CD8-CD3+NK1.1+) NKT cells. C. Percentages of CD4 and CD8 central (CD44+CD62L+) and effector (CD44+CD62L-) memory T cells. Results are presented as Mean ± SDs. * p<0.05; ** p<0.01.
Interestingly, GKO mice showed a significant increase in CD8 T cells with an effector memory phenotype (i.e., CD44+CD62L−) compared to WT mice (Figure 6C), consistent with the increase of alloreactive CD8 memory T cells in allo-HCT recipients (Figure 3). No significant difference was detected between WT and GKO mice in other memory T cell subsets, including CD4 and CD8 central memory (i.e., CD44+CD62L+) T cells, as well as CD4+ effector memory T cells. Since memory T cells are markedly less potent than naïve T cells in inducing GVHD [36–39], the higher incidence of GVHD observed in the recipients of GKO allogeneic cells is unlikely to be due to the inclusion of more CD8 effector memory T cells in the HCT inoculum.
DISCUSSION
This study provides evidence that IFN-γ plays a critical role in the regulation of CD8 T cell alloreactivity in vivo. Allo-HCT recipients of GKO CD8 cells developed severe acute GVHD and succumbed to death within 6 weeks, whereas the majority of the mice receiving similar allo-HCT from WT donors survived long-term (Figures 1–2). The augmented GVHD in recipients of GKO allo-HCT was associated with markedly increased expansion of alloreactive donor CD8 T cells. It has been reported that IFN-γ stimulates death of antigen-specific CD8 T cells in a Listeria infection model [14]. Our results demonstrate that IFN-γ also promotes death of alloreactive CD8 T cells in vivo, and that the increased donor CD8 T cell expansion and augmented GVHD in the recipients of GKO allo-HCT was, at least in part, due to the reduced/delayed death of host antigen-activated donor CD8 T cells.
IFN-γ appears to regulate a critical checkpoint of T cell proliferation. T cell proliferation is governed by the ordered activation of cyclin-dependent kinases (CDKs) [40]. Studies using gene-targeted knockout mice and transgenic mice have demonstrated that the CDK inhibitor, p27Kip1 plays a critical role in controlling T cell proliferation, and the lack of p27Kip1 expression results in a significant increase in T cell numbers [41,42]. A recent study showed that naïve CD8 T cells have high expression of P27Kip1 and low CDK6 and CDK2 kinase activity, whereas G0/G1 memory CD8 T cells have low expression of P27Kip1 and high CDK6 kinase activity, and the latter favors rapid cell division [43]. Furthermore, IFN-γ has been shown to inhibit the proliferation of bronchial epithelial cells by preventing growth factor-induced downregulation of P27Kip1 [44]. However, relatively little is known about the role of IFN-γ in regulating in vivo proliferation of activated CD8 T cells. In the present study, we observed that cell division rates and numbers of donor CD8 cells were markedly increased in the recipients of GKO allo-HCT compared to those in mice that received WT allo-HCT. Using the 2C allo-HCT model that allows specific assessment of the host-reactive CD8 T cells, we found that the lack of IFN-γ results in marked increases in the numbers of host MHC-reactive 2C T cells with 7 or more divisions, which was associated with a significant decrease in the numbers of less divided 2C T cells (Figure 5C). These results indicate that IFN-γ plays an important and previously unidentified role in controlling the proliferation of activated CD8 T cells in vivo, and that the lack of this cytokine accelerates the division of host antigen-reactive donor CD8 T cells in allo-HCT recipients. Since type-1 T cells, which are capable of producing IFN-γ, do not respond to IFN-γ due to the loss of IFN-γR2 expression [45,46], the role of IFN-γ in regulating cell division of host antigen-activated T cells might be mediated via an indirect mechanism. A recent study showed that CD11b+ cells responding to IFN-γ can limit CD8 T cell expansion and promote contraction of this population [47].
Histological examinations revealed that mononuclear cell infiltration and tissue injury in GVHD target tissues was more severe in the recipients of GKO allo-HCT than in those who received WT allo-HCT. It is likely that the augmented donor T cell activation and expression due to reduced apoptosis and accelerated proliferation in the recipients of GKO allo-HCT may result in an overall increase in donor T cell infiltration in GVHD target tissues. The accelerated generation of effector/memory T cells in the recipients of GKO allo-HCT might also contribute to the rapid and severe tissue injury in these mice. Recently, IFN-γ has been shown to inhibit CD8 memory T cell generation in mice after dendritic cell vaccination [17]. In this study, we observed that the ratio of CD8 effector memory (CD8+CD44+CD62L-) T cells in the spleen was significantly greater in GKO mice than in WT mice (Figure 6C). Furthermore, GKO donor CD8 T cells lose CD62L expression more rapidly than WT CD8 T cells upon activation, and that almost all donor CD8 T cells in the spleens of mice receiving GKO allo-HCT acquired an effector memory phenotype (CD62L-CD44+) by day 5 post-HCT (Figure 3). These results indicate that the egress of activated donor CD8 T cells from lymphoid tissues may occur earlier in the recipients of GKO allo-HCT. The data also suggests that the absence of IFN-γ might provide conditions favoring the differentiation and/or expansion of CD8 effector memory T cells in allo-HCT recipients.
T-reg cells have been shown to protect against GVHD [48,49]. Although IFN-γ plays an important role in the generation and function of CD4 T-reg cells (Figure 6A) [16], the lack of this cytokine does not lead to reduction in CD4- T-reg cells in the spleen (Figure 6A). Furthermore, the level of CD4- T-reg cells in the spleen is extremely low in both WT and GKO mice. Since CD4-depleted allo-HCT was given in our studies, these results suggest that the difference in GVHD between the recipients of WT and GKO allo-HCT was unlikely due to the difference in T-reg cell contents in the donor inocula. NKT cells have also been shown to induce allograft tolerance [50] and inhibit GVHD [51,52]. IFN-γ seems to play an important role in NKT-induced allograft tolerance [50], but the GVHD-inhibitory effect of NKT cells has been shown to be dependent on their production of IL-4 [51]. Our results show that GKO mice have more CD4-CD8− NKT cells in the spleen than WT mice (Figure 6B). Further functional analysis is needed to firmly determine the role of NKT cells in IFN-γ-mediated inhibition of GVHD. IFN-γ is produced by multiple cell types, including type 1 T cells, NK/NKT cells, and dendritic cells (DCs) [53–56]. Recent studies demonstrated that a novel DC subset, IFN-producing killer DCs (IKDCs), also produce IFN-γ [57,58]. These cells could serve as the major source of IFN-γ in some tumor models where the tumor cells are poorly recognized by NK cells [58]. However, the role of IKDCs in regulating alloresponses remains unknown. The present study demonstrates that donor-derived IFN-γ protects against GVHD, but has not identified the sources of donor IFN-γ that mediate the protective effect.
In summary, our results demonstrate that IFN-γ is an important regulatory cytokine during CD8 T cell activation in vivo. This cytokine inhibits the activation and expansion of donor CD8 T cells by promoting cell death and suppressing cell division and down-regulates effector/memory CD8 T cell generation in allo-HCT recipients. Importantly, this cytokine mediates graft-vs.-leukemic effects while inhibiting GVHD [30]. Our recent studies indicate that IFN-γ is required for the optimal induction of graft-vs.-host reactions that preferentially attack the host lymphohematopoietic cells (Wang H et al, manuscript in preparation), which has been shown to mediate GVL effects without severe GVHD [59,60]. Further understanding of the molecular mechanisms involved in regulating T cell alloreactivity by IFN-γ will likely shed new light on the development of strategies in clinical GVHD prevention and therapy.
Acknowledgments
We thank Dr. Ronjon Chakraverty (University of Birmingham, UK) for critical review of the manuscript, Dr. Xiaojian Wu for providing technical assistance, Mr. Orlando Moreno for outstanding animal husbandry, and Ms. Luisa Raleza for expert assistance with the manuscript. This work was supported by grants from American Cancer Society (RSG-03-227-01-LIB) and NIH (P01 CA111519 and R01 CA79989). WA was supported by a NIH Training Grant 5T32 HD07529-06. YGY was a recipient of the Orphan Medical Scholar Award from the American Society for Blood and Marrow Transplantation (2002-2004) and Outstanding Overseas Scholar Award from the Natural Science Foundation of China (NSFC #30328012).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Huang S, Hendriks W, Althage A, et al. Immune response in mice that lack the interferon-gamma receptor. Science. 1993;259:1742–1745. doi: 10.1126/science.8456301. [DOI] [PubMed] [Google Scholar]
- 2.Dalton DK, Pitts-Meek S, Keshav S, Figari IS, Bradley A, Stewart TA. Multiple defects of immune cell function in mice with disrupted interferon-gamma genes. Science. 1993;259:1739–1742. doi: 10.1126/science.8456300. [DOI] [PubMed] [Google Scholar]
- 3.Whitmire JK, Tan JT, Whitton JL. Interferon-{gamma} acts directly on CD8+ T cells to increase their abundance during virus infection. J Exp Med. 2005;201:1053–1059. doi: 10.1084/jem.20041463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Refaeli Y, Van Parijs L, Alexander SI, Abbas AK. Interferon {gamma} Is Required for Activation-induced Death of T Lymphocytes. J Exp Med. 2002;196:999–1005. doi: 10.1084/jem.20020666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chu CQ, Wittmer S, Dalton DK. Failure to suppress the expansion of the activated CD4 T cell population in interferon gamma-deficient mice leads to exacerbation of experimental autoimmune encephalomyelitis. J Exp Med. 2000;192:123–128. doi: 10.1084/jem.192.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dalton DK, Haynes L, Chu CQ, Swain SL, Wittmer S. Interferon gamma eliminates responding CD4 T cells during mycobacterial infection by inducing apoptosis of activated CD4 T cells. J Exp Med. 2000;192:117–122. doi: 10.1084/jem.192.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu H, Wipasa J, Yan H, et al. The Mechanism and Significance of Deletion of Parasite-specific CD4+ T Cells in Malaria Infection. J Exp Med. 2002;195:881–892. doi: 10.1084/jem.20011174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seder RA, Ahmed R. Similarities and differences in CD4+ and CD8+ effector and memory T cell generation. Nat Immunol. 2003;4:835–842. doi: 10.1038/ni969. [DOI] [PubMed] [Google Scholar]
- 9.Badovinac VP, Tvinnereim AR, Harty JT. Regulation of antigen-specific CD8(+) T cell homeostasis by perforin and interferon-gamma. Science. 2000;290:1354–1358. doi: 10.1126/science.290.5495.1354. [DOI] [PubMed] [Google Scholar]
- 10.Badovinac VP, Harty JT. Adaptive immunity and enhanced CD8+ T cell response to Listeria monocytogenes in the absence of perforin and IFN-gamma. J Immunol. 2000;164:6444–6452. doi: 10.4049/jimmunol.164.12.6444. [DOI] [PubMed] [Google Scholar]
- 11.Liu Y, Janeway CA. Interferon-gamma plays a critical role in induced cell death of effector T cell: a possible third mechanism of self-tolerance. J Exp Med. 1990;172:1735–1739. doi: 10.1084/jem.172.6.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Novelli F, D'Elios MM, Bernabei P, et al. Expression and role in apoptosis of the alpha- and beta-chains of the IFN-gamma receptor on human Th1 and Th2 clones. J Immunol. 1997;159:206–213. [PubMed] [Google Scholar]
- 13.Oyaizu N, McCloskey TW, Than S, Hu R, Kalyanaraman VS, Pahwa S. Cross-linking of CD4 molecules upregulates Fas antigen expression in lymphocytes by inducing interferon-γ and tumor necrosis factor-γ secretion. Blood. 1994;84:2622–2631. [PubMed] [Google Scholar]
- 14.Badovinac VP, Tvinnereim AR, Harty JT. Regulation of antigen-specific CD8(+) T cell homeostasis by perforin and interferon-gamma [In Process Citation] Science. 2000;290:1354–1358. doi: 10.1126/science.290.5495.1354. [DOI] [PubMed] [Google Scholar]
- 15.Konieczny BT, Dai Z, Elwood ET, et al. IFN-γ is critical for long-term allograft survival induced by blocking the CD28 and CD40 ligand T cell costimulation pathways. J Immunol. 1998;160:2059–2064. [PubMed] [Google Scholar]
- 16.Sawitzki B, Kingsley CI, Oliveira V, Karim M, Herber M, Wood KJ. IFN-{gamma} production by alloantigen-reactive regulatory T cells is important for their regulatory function in vivo. J Exp Med. 2005;201:1925–1935. doi: 10.1084/jem.20050419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Badovinac VP, Messingham KAN, Jabbari A, Haring JS, Harty JT. Accelerated CD8+ T-cell memory and prime-boost response after dendritic-cell vaccination. Nat Med. 2005;11:748–756. doi: 10.1038/nm1257. [DOI] [PubMed] [Google Scholar]
- 18.Niederwieser D, Herold M, Woloszczuk W, et al. Endogenous IFN-gamma during human bone marrow transplantation. Analysis of serum levels of interferon and interferon-dependent secondary messages. Transplantation. 1990;50:620–625. doi: 10.1097/00007890-199010000-00019. [DOI] [PubMed] [Google Scholar]
- 19.Dickinson AM, Sviland L, Hamilton PJ, et al. Cytokine involvement in predicting clinical graft-versus-host disease in allogeneic bone marrow transplant recipients. Bone Marrow Transplant. 1994;13:65–70. [PubMed] [Google Scholar]
- 20.Tanaka J, Imamura M, Kasai M, Sakurada K, Miyazaki T. Cytokine gene expression after allogeneic bone marrow transplantation. LeukLymphoma. 1995;16:413–418. doi: 10.3109/10428199509054427. [DOI] [PubMed] [Google Scholar]
- 21.Das H, Imoto S, Murayama T, et al. Kinetic analysis of cytokine gene expression in patients with GVHD after donor lymphocyte infusion. Bone Marrow Transplant. 2001;27:373–380. doi: 10.1038/sj.bmt.1702799. [DOI] [PubMed] [Google Scholar]
- 22.Guy-Grand D, Vassalli P. Gut injury in mouse graft-vs-host reaction: study of its occurrence and mechanism. J Clin Invest. 1986;77:1584–1595. doi: 10.1172/JCI112474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mowat AM. Antibodies to IFN-gamma prevent immunologically mediated intestinal damage in murine graft-versus-host reaction. Immunology. 1989;68:18–23. [PMC free article] [PubMed] [Google Scholar]
- 24.Klimpel GR, Annable CR, Cleveland MG, Jerrells TR, Patterson JC. Immunosuppression and lymphoid hypoplasia associated with chronic graft versus host disease is dependent upon IFN- gamma production. J Immunol. 1990;144:84–93. [PubMed] [Google Scholar]
- 25.Parfrey NA, El Sheikh A, Monckton EA, Cockfield SM, Halloran PF, Linetsky E. Interferon-gamma gene expression during acute graft-versus-host disease: relationship to MHC induction and tissue injury. J Pathol. 1999;189:99–104. doi: 10.1002/(SICI)1096-9896(199909)189:1<99::AID-PATH404>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 26.Yang YG, Dey B, Sergio JJ, Pearson DA, Sykes M. Donor-derived interferon γ is required for inhibition of acute graft-versus-host disease by interleukin 12. J Clin Invest. 1998;102:2126–2135. doi: 10.1172/JCI4992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dey B, Yang YG, Szot GL, Pearson DA, Sykes M. IL-12 inhibits GVHD through a Fas-mediated mechanism associated with alterations in donor T cell activation and expansion. Blood. 1998;91:3315–3322. [PubMed] [Google Scholar]
- 28.Murphy WJ, Welniak LA, Taub DD, et al. Differential effects of the absence of interferon-γ and IL-4 in acute Graft-Versus-Host Disease after allogeneic bone marrow transplantation in mice. J Clin Invest. 1998;102:1742–1748. doi: 10.1172/JCI3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reddy P, Teshima T, Kukuruga M, et al. Interleukin-18 Regulates Acute Graft-Versus-Host Disease by Enhancing Fas-mediated Donor T Cell Apoptosis. J Exp Med. 2001;194:1433–1440. doi: 10.1084/jem.194.10.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang YG, Qi J, Wang MG, Sykes M. Donor-derived interferon gamma separates graft-versus-leukemia effects and graft-versus-host disease induced by donor CD8 T cells. Blood. 2002;99:4207–4215. doi: 10.1182/blood.v99.11.4207. [DOI] [PubMed] [Google Scholar]
- 31.Sha WC, Nelson CA, Newberry RD, Kranz DM, Russell RD, Loh DY. Selective expression of an antigen receptor on CD8-bearing T lymphocytes in transgenic mice. Nature. 1988;335:271–274. doi: 10.1038/335271a0. [DOI] [PubMed] [Google Scholar]
- 32.Rubio MT, Kim YM, Sachs T, Mapara M, Zhao G, Sykes M. Antitumor effect of donor marrow graft rejection induced by recipient leukocyte infusions in mixed chimeras prepared with nonmyeloablative conditioning: critical role for recipient-derived IFN-{gamma} Blood. 2003;102:2300–2307. doi: 10.1182/blood-2002-12-3949. [DOI] [PubMed] [Google Scholar]
- 33.Ferrara JL, Cooke KR, Pan L, Krenger W. The immunopathophysiology of acute graft-versus-host-disease. Stem Cells. 1996;14:473–489. doi: 10.1002/stem.140473. [DOI] [PubMed] [Google Scholar]
- 34.Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science. 1996;272:60–66. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- 35.Kranz DM, Sherman DH, Sitkowsky MV, Pasternack MS, Eisen HN. Immunoprecipitation of cell surface structures of cloned cytotoxic T lymphocytes by clone-specific antisera. Proc Natl Acad SciUSA. 1984;81:573–577. doi: 10.1073/pnas.81.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anderson BE, McNiff J, Yan J, et al. Memory CD4+ T cells do not induce graft-versus-host disease. J Clin Invest. 2003;112:101–108. doi: 10.1172/JCI17601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Foster AE, Marangolo M, Sartor MM, et al. Human CD62L- memory T cells are less responsive to alloantigen stimulation than CD62L+ naive T cells: potential for adoptive immunotherapy and allodepletion. Blood. 2004;104:2403–2409. doi: 10.1182/blood-2003-12-4431. [DOI] [PubMed] [Google Scholar]
- 38.Zhang Y, Joe G, Zhu J, et al. Dendritic cell-activated CD44hiCD8+ T cells are defective in mediating acute graft-versus-host disease but retain graft-versus-leukemia activity. Blood. 2004;103:3970–3978. doi: 10.1182/blood-2003-09-3135. [DOI] [PubMed] [Google Scholar]
- 39.Chen BJ, Cui X, Sempowski GD, Liu C, Chao NJ. Transfer of allogeneic CD62L- memory T cells without graft-versus-host disease. Blood. 2004;103:1534–1541. doi: 10.1182/blood-2003-08-2987. [DOI] [PubMed] [Google Scholar]
- 40.Sherr CJ. Cancer Cell Cycles. Science. 1996;274:1672–1677. doi: 10.1126/science.274.5293.1672. [DOI] [PubMed] [Google Scholar]
- 41.Kiyokawa H, Kineman RD, Manova-Todorova KO, et al. Enhanced growth of mice lacking the cyclin-dependent kinase inhibitor function of p27(Kip1) Cell. 1996;85:721–732. doi: 10.1016/s0092-8674(00)81238-6. [DOI] [PubMed] [Google Scholar]
- 42.Nakayama K, Ishida N, Shirane M, et al. Mice lacking p27(Kip1) display increased body size, multiple organ hyperplasia, retinal dysplasia, and pituitary tumors. Cell. 1996;85:707–720. doi: 10.1016/s0092-8674(00)81237-4. [DOI] [PubMed] [Google Scholar]
- 43.Veiga-Fernandes H, Rocha B. High expression of active CDK6 in the cytoplasm of CD8 memory cells favors rapid division. Nat Immunol. 2004;5:31–37. doi: 10.1038/ni1015. [DOI] [PubMed] [Google Scholar]
- 44.Takami K, Takuwa N, Okazaki H, et al. Interferon-gamma Inhibits Hepatocyte Growth Factor-Stimulated Cell Proliferation of Human Bronchial Epithelial Cells. Upregulation of p27kip1 Cyclin-Dependent Kinase Inhibitor. Am J Respir Cell Mol Biol. 2002;26:231–238. doi: 10.1165/ajrcmb.26.2.4643. [DOI] [PubMed] [Google Scholar]
- 45.Pernis A, Gupta S, Gollob KJ, et al. Lack of interferon gamma receptor beta chain and the prevention of interferon gamma signaling in TH1 cells. Science. 1995;269:245–247. doi: 10.1126/science.7618088. [DOI] [PubMed] [Google Scholar]
- 46.Bach EA, Szabo SJ, Dighe AS, et al. Ligand-induced autoregulation of IFN-gamma receptor β chain expression in T helper cell subsets. Science. 1995;270:1215–1221. doi: 10.1126/science.270.5239.1215. [DOI] [PubMed] [Google Scholar]
- 47.Sercan O, Hammerling GJ, Arnold B, Schuler T. Cutting Edge: Innate Immune Cells Contribute to the IFN-{gamma}-Dependent Regulation of Antigen-Specific CD8+ T Cell Homeostasis. J Immunol. 2006;176:735–739. doi: 10.4049/jimmunol.176.2.735. [DOI] [PubMed] [Google Scholar]
- 48.Cohen JL, Trenado A, Vasey D, Klatzmann D, Salomon BL. CD4+CD25+ Immunoregulatory T Cells: New Therapeutics for Graft-Versus-Host Disease. J Exp Med. 2002;196:401–406. doi: 10.1084/jem.20020090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ermann J, Hoffmann P, Edinger M, et al. Only the CD62L+ subpopulation of CD4+CD25+ regulatory T cells protects from lethal acute GVHD. Blood. 2005;105:2220–2226. doi: 10.1182/blood-2004-05-2044. [DOI] [PubMed] [Google Scholar]
- 50.Seino Ki, Fukao K, Muramoto K, et al. Requirement for natural killer T (NKT) cells in the induction of allograft tolerance. Proc Natl Acad Sci USA. 2001;98:2577–2581. doi: 10.1073/pnas.041608298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lan F, Zeng D, Higuchi M, Huie P, Higgins JP, Strober S. Predominance of NK1.1+TCR{alpha}{beta}+ or DX5+TCR{alpha}{beta}+ T Cells in Mice Conditioned with Fractionated Lymphoid Irradiation Protects Against Graft-Versus-Host Disease: "Natural Suppressor" Cells. J Immunol. 2001;167:2087–2096. doi: 10.4049/jimmunol.167.4.2087. [DOI] [PubMed] [Google Scholar]
- 52.Haraguchi K, Takahashi T, Matsumoto A, et al. Host-Residual Invariant NK T Cells Attenuate Graft-versus-Host Immunity. J Immunol. 2005;175:1320–1328. doi: 10.4049/jimmunol.175.2.1320. [DOI] [PubMed] [Google Scholar]
- 53.Farrar MA, Schreiber RD. The molecular cell biology of interferon-gamma and its receptor. Ann Rev Immunol. 1993;11:571–611. doi: 10.1146/annurev.iy.11.040193.003035. [DOI] [PubMed] [Google Scholar]
- 54.Peritt D, Robertson S, Gri G, Showe L, Aste-Amezaga M, Trinchieri G. Cutting edge: differentiation of human NK cells into NK1 and NK2 subsets. J Immunol. 1998;161:5821–5824. [PubMed] [Google Scholar]
- 55.Fricke I, Mitchell D, Mittelstadt J, et al. Mycobacteria Induce IFN-{gamma} Production in Human Dendritic Cells via Triggering of TLR2. J Immunol. 2006;176:5173–5182. doi: 10.4049/jimmunol.176.9.5173. [DOI] [PubMed] [Google Scholar]
- 56.Fukao T, Matsuda S, Koyasu S. Synergistic Effects of IL-4 and IL-18 on IL-12-Dependent IFN-{gamma} Production by Dendritic Cells. J Immunol. 2000;164:64–71. doi: 10.4049/jimmunol.164.1.64. [DOI] [PubMed] [Google Scholar]
- 57.Chan CW, Crafton E, Fan HN, et al. Interferon-producing killer dendritic cells provide a link between innate and adaptive immunity. Nat Med. 2006;12:207–213. doi: 10.1038/nm1352. [DOI] [PubMed] [Google Scholar]
- 58.Taieb J, Chaput N, Menard C, et al. A novel dendritic cell subset involved in tumor immunosurveillance. Nat Med. 2006;12:214–219. doi: 10.1038/nm1356. [DOI] [PubMed] [Google Scholar]
- 59.Pelot MR, Pearson DA, Swenson K, et al. Lymphohematopoietic graft-vs-host reactions can be induced without graft-vs-host disease in murine mixed chimeras established with a cyclophosphamide-based non-myeloablative conditioning regimen. Biol Blood Marrow Transplant. 1999;5:133–143. doi: 10.1053/bbmt.1999.v5.pm10392959. [DOI] [PubMed] [Google Scholar]
- 60.Kim YM, Sachs T, Asavaroengchai W, Bronson R, Sykes M. Graft-versus-host disease can be separated from graft-versus-lymphoma effects by control of lymphocyte trafficking with FTY720. J Clin Invest. 2003;111:659. doi: 10.1172/JCI16950. [DOI] [PMC free article] [PubMed] [Google Scholar]


