Abstract
OBJECTIVES
The purpose of this paper is to describe the accumulation of aging-associated health disorders by a cumulative measure known as a frailty index (FI) and to evaluate its ability to differentiate long- and short-life phenotypes as well as the FIs connection to aging-associated processes in the elderly.
DESIGN
Retrospective cross-sectional and longitudinal studies.
SETTING
The National Long Term Care Survey (NLTCS) data that assessed health and functioning of the U.S. elderly (65 and older) individuals in 1982, 1984, 1989, 1994, and 1999 were analyzed. The NLTCS sample in each survey represents a mixture of longitudinal and cross-sectional components.
PARTICIPANTS
About five thousand individuals in each survey.
MEASUREMENTS
A cumulative index of health/well-being deficiencies (disabilities, signs, diseases) was calculated as a count of deficits observed in an individual divided by the total number of all considered deficits.
RESULTS
It is shown that males and females died before age 75 years and those who lived long and died at age 85 and older exhibit remarkably similar FI frequency patterns despite the ten-year age difference between age profiles in these samples. Long life is consistently characterized in longitudinal analyses by lower levels of the FIs. FI dynamics are found to be strongly sex-sensitive.
CONCLUSIONS
The FI appears to be a sensitive age-independent indicator of sex-specific physiological decline in aging individuals and sex-specific discriminator of survival chances. The FI is a promising characteristic suitable for improving sex-sensitive forecasts of risks of adverse health outcomes in the elderly.
Keywords: Frailty, longevity, aging, sex differences
INTRODUCTION
Experimental studies indicate that for many diseases incidence rates increase among adult and old individuals and then decline among the oldest old [1,2]. The forces shaping such age patterns leave the potential for a certain portion of survivors to escape major chronic diseases [2–4]. The total mortality rate, however, continues to increase to those ages. The recently developed concept of frailty appears to be helpful in addressing such a paradox, since frailty is consistently associated with pure health and death [5–8] and is a state of an organism with non-specific vulnerability to stressors [9]. Frailty appears to be a result of decreasing physiological reserves and deregulation of multiple physiologic systems. However, despite agreement on the physiological origin of frailty, its operational definition remains controversial [10].
One approach to operationalize frailty is through multiple physical indicators, e.g., declined grip strength, reduction of lean bodyweight, slowing mobility, poor endurance [5,11]. These factors (apart from a multitude of physiological indicators, see, e.g., [12]) were also considered as predictors of mortality both as individual indicators [13,14] and composite indices [7,9]. Another approach for assessing frailty is to track health and well-being changes in aging individuals [15]. Unlike more specific approaches [5,11], this approach defines frailty more broadly [16], assuming that more frail individuals will experience more health problems than those who are less frail [17]. An advantage of the FI concept is that it allows one to use conventional data collected in typical population-based surveys. The frailty state can be described by a cumulative index of health/well-being deficiencies that is assessable for each individual as the fraction of deficits among a list of items that measure health/well-being (called a frailty index, FI). According to this systemic approach, the frailty state appears to be characterized not by the substance of specific traits used to define the FI but by their aggregate (or systemic) ability to describe the decline in physiological performance in an organism – and, thus, to characterize its overall function [17].
The validity of such a conceptualization of frailty has been examined in a number of studies [6,16–21]. The concept of a cumulative index (FI) is also useful in studies of aging, health, and survival in which FI appears to be a promising alternative to chronological age to characterize aging-associated processes in elderly individuals and to better predict chances of adverse events for them [20–23]. Indeed, the FI is typically constructed using a set of so-called “mild-effect” variables, i.e., variables whose individual effect on various outcomes is often non-significant due to insufficient sample size. In many studies, such variables are simply ignored. The FI gathers them into a single measure that can provide a coherent effect, increasing the statistical power of estimates [22]. Consequently, more data can be used for the analyses, which has the potential to better assess aging-associated processes. Extant studies of FI properties were largely limited to cross-sectional settings. This paper focuses on connections of the FI with aging-associated processes in longitudinal settings. We apply the Rockwood-Mitnitski approach to construct the FI and perform longitudinal analyses in a sample of U.S. elderly individuals using National Long Term Care Survey (NLTCS) data. The problem to be addressed is the ability of the FI to differentiate long- and short-life phenotypes.
METHODS
Settings and Samples
The data are the 1982, 1984, 1989, 1994, and 1999 NLTCS and the linked Medicare vital statistics (from 1982 to August, 2003). The NLTCS uses a sample of elderly individuals (65+) that is drawn from national Medicare enrollees. The survey instruments used in all NLTCS waves asked the same questions in a similar way. The same data collection agency, the U.S. Census Bureau, was used in all NLTCS waves, so all procedures are consistent across surveys. This minimizes bias in longitudinal estimates. The likelihood of bias is also reduced by the high (95%) response rates in five NLTCS waves.
To complete the NLTCS, a two-stage interviewing process was used. A screening interview assessing chronic disability (90+ days) was given to all participants. A detailed interview was given to i) those who reported at least one chronic impairment in (instrumental) activities of daily living (I)ADL, ii) institutionalized individuals, and iii) those who received a detailed interview in a previous survey. For each new survey, a cohort sample of about 5,000 persons was added to the surviving sample to replace deaths occurring since the previous survey and to ensure that the new sample was representative of the U.S. elderly population. Such a procedure ensures a valid longitudinal and cross-sectional design for the survey (see [24]).
The Frailty Index Definition
Following arguments in [17], the FI is defined as an unweighted count of the number of deficits divided by the total number of all potential deficits considered for a person. Basically, we considered the following set of 32 deficits – which is similar to that assessed from the Canadian Study of Health and Aging (Mitnitski et al., 2001), i.e., difficulty with eating, dressing, walking around, getting in/out bed, taking a bath, using the toilet, using the telephone, going out, shopping, cooking, light housework, taking medicine, managing money, arthritis, Parkinson’s disease, glaucoma, diabetes, stomach problems, history of heart attack, hypertension, history of stroke, flu, broken hip, broken bones, trouble with bladder/bowels, dementia, self-rated health, as well as problems with vision, hearing, ear, teeth, and feet. Since the NLTCS has a high overall response rate, the percentage of missing values for most of these traits is fewer than 1%. To assess the robustness of the FI, certain deficits from this list were randomly replaced by other deficits (e.g., headaches, trouble sleeping, difficulty with doing laundry, indicators of physical performance, mental and emotional problems, cognitive ability). In all cases, the results were qualitatively similar, which is in concordance with extant studies indicating relative independence of the FI concept on the nature of particular deficits [16,17]. Consequently, throughout this study the original definition of the FI on the basis of the listed 32 deficits was retained.
Selection of Long- and Short-life Phenotypes
To characterize connections between FI and long- and short-life phenotypes, three groups of individuals with distinct survival profiles were selected: 1) died early (short-livers; SL); defined as aged between 65 and 74 years at the date of death (note that since NLTCS focuses on 65+ Medicare enrollees, the age profile for short-livers defined at the date of the respective interview is also 65–74 years); 2) died but lived long (LLD); defined as aged 85+ years at the date of death (i.e., their age profile is 65+), and 3) lived long and stayed alive at the end of the observation period (LLA); defined as individuals aged 85+ years at August 6, 2003 (age profile is 65+). Although basic reasoning was applied, the choice of the age cutoffs distinguishing these categories is flexible. Specifically, different age cutoffs adjusted to available sample sizes were tested to ensure that the results are stable against such uncertainty.
Additionally, a sub-sample of individuals from the LLD group who was aged 85+ years at the date of interview (LLD85+) was selected. The reason was to adjust for individuals aged less than 85 years at the interview who, by definition, remained alive until 85 years and, thus, might have been in better health early in life. After such an adjustment, the LLD85+ sub-sample becomes similar to the short-livers’ sample from the viewpoint of survival chances.
Analysis
All analyses were performed using SPSS software (release 12.0, Chicago, Illinois, USA). First, cross-sectional analysis focused on the FI frequency distributions and their statistical characteristics was performed. The frequency distributions provide valuable information on systemic processes in aging individuals which reflect biological changes at the organism and cellular levels [25,26]. For instance, the shape parameters (skewness and kurtosis) can distinguish between healthy/unhealthy phenotypes [25,27]. To assess the FI’s prognostic power to differentiate long- and short-life phenotypes, two types of longitudinal analyses were performed. First, cohorts of early NLTCS were followed longitudinally. Second, two samples of participants of early (E-sample) surveys (i.e., individuals from LLA and LLD groups who participated in three consecutive NLTCS – 1984, 1989, and 1994 – and short-livers who participated in the 1984 and 1989 NLTCS) and later (L-sample) surveys (1989, 1994, 1999 for LLA/LLD and 1989, 1994 for SL) were selected to adjust for the effect of selection of robust individuals.
Ethics
Our research complies with the ethical rules of the Declaration of Helsinki. We used only secondary data for the analyses, which were approved by the Institutional Review Board at Duke University.
RESULTS
Surprisingly, despite essential differences in age profiles between short-livers (SL) and individuals who died at age 85+ (LLD), the analyses reveal similar FI frequency distributions for them (Figure 1). Moreover, this similarity becomes even more pronounced when considering the LLD85+ sub-sample. Quantitatively, Figure 1 shows that the mean FIs for died (SL/LLD/LLD85+) males and females are similar, while they are considerably larger than those for living individuals (LLA). The standard deviation is also larger for died than for alive groups, although the relative difference between these values for each sex is not as large as in the case of mean FI. The distributions for died individuals have similar shapes characterized by considerably smaller skewness than for alive individuals. Kurtosis shows a qualitatively different sharpness of these distributions, i.e., for alive individuals it is positive – indicating a “peaked” distribution, while for died individuals kurtosis is negative – indicating a “flat” distribution. Moreover, kurtosis for the short-livers and the LLD85+ group is practically identical for males and females. Figure 1 also shows FI cutoff values that distinguish quartiles in each group. Again, all cut-points are considerably larger for died groups than for the alive group. For instance, 75% of surviving males have a FI less than or equal to 20%. For died individuals, only 25% of males have a smaller FI. The remarkable fact is that males from the short-lived and LLD85+ samples have practically the same cut points. It can be also seen that living males have significantly smaller mean FIs (as well as quartiles), and considerably larger skewness and kurtosis, than the living females.
Figure 1.
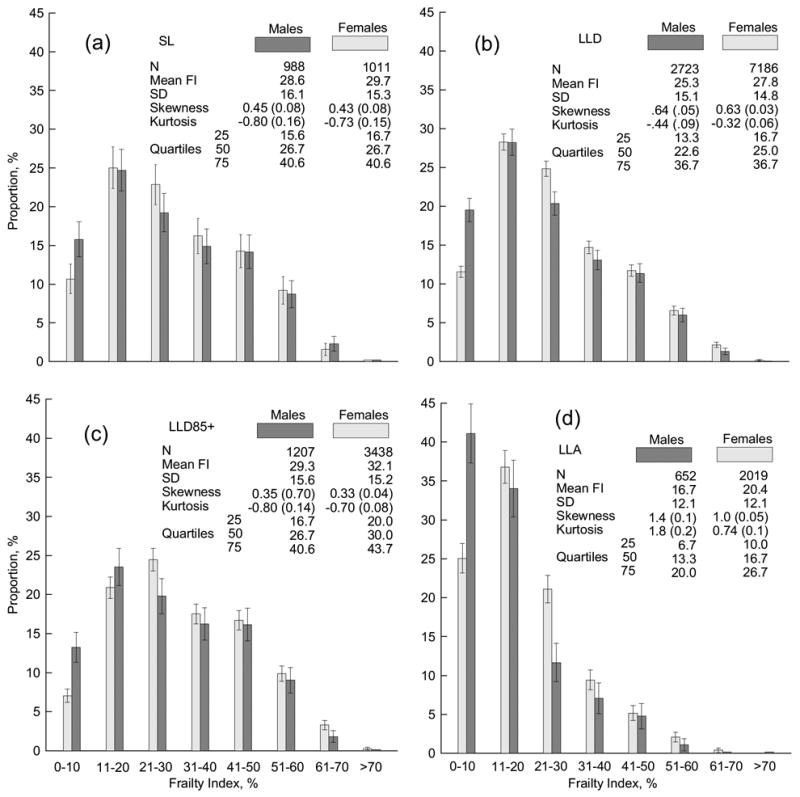
The sex-specific frailty index (measured in percentages) frequency distributions for the (a) short-livers (SL), (b) individuals who died at age 85+ (LLD), (c) sub-sample of the LLD group with individuals aged 85+ years at the date of interview (LLD85+), and (d) long-living individuals aged 85+ years at the date of interview (LLA). Bars show 95% confidence intervals. Insets show statistical characteristics of the respective groups. SD means standard deviation. Numbers in parentheses show standard errors.
The longitudinal analysis was performed for the two earliest cohorts of the 1982 and 1984 NLTCS, since they were followed for the longest time periods. The analysis shows no qualitative differences between them. As an example, Figure 2 shows the sex-specific FI dynamics for SL, LLD, and LLA groups of the 1984 cohort. It can be seen that the FIs for short-livers are larger than those for individuals who died at age 85+, and the FIs for the latter group are larger than the FI for living individuals. For died males, the FI remains nearly constant, while the FI for died females increases with time. For all groups, the FI for females is larger than for males.
Figure 2.
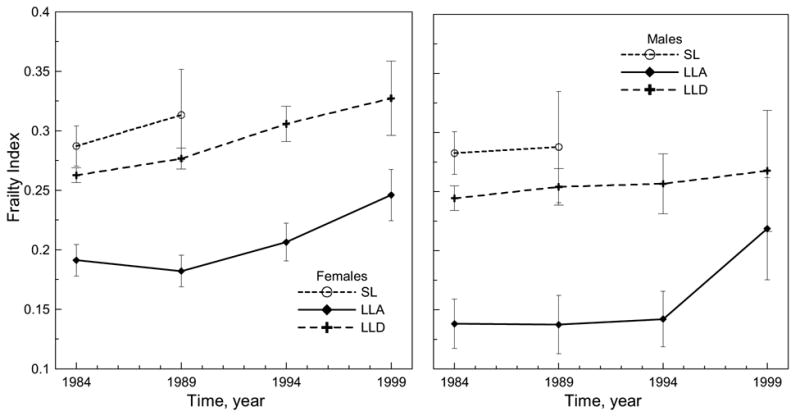
Longitudinal changes of the mean frailty index (theoretical range is between 0 and 1) for the deceased (SL and LLD) and living (LLA) females and males from the 1984 cohort. Bars show 95% confidence intervals.
Figures 3a,b show sex-specific dynamics for short- and long-livers for the E- and L-samples (note that the LLA group is shown for comparison, since drop-outs not related to death for this sample are not essential). Apparently different dynamics between sexes and/or the E- and L-samples are seen. The E-sample of living males has a flat FI pattern. For the L-sample, the FI is the same in the 1989 and 1994 NLTCS, but it significantly increases in the 1999 NLTCS. An intriguing observation is that the same longitudinal pattern is characteristic of the living females of the E-sample. In the L-sample, the living females accumulate deficits, however, with each survey.
Figure 3.
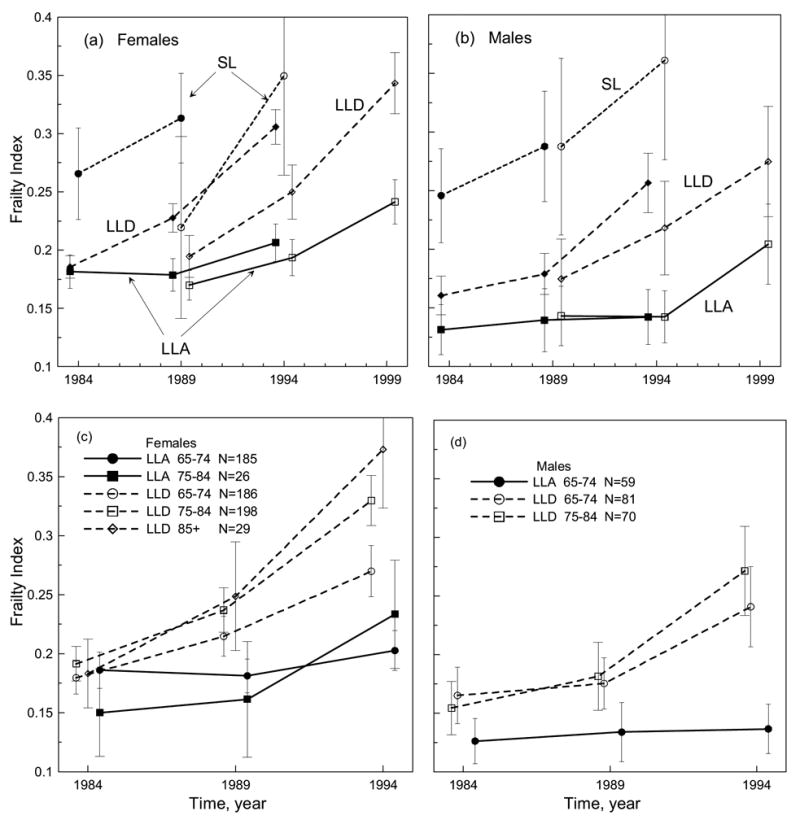
(a-b) Time behavior of the frailty index for the males’ and females’ subgroups of the deceased (SL: short-dashed line; LLD: dashed line) and living (LLA: solid line) individuals who participated in early surveys (1984–1994 for LLA and LLD and 1984–1989 for SL; denoted by filled symbols) and later surveys (1989–1999 for LLA and LLD and 1989–1994 for SL; denoted by open symbols). Bars show 95% confidence intervals. (c-d) The same as in (ab) but only for the early surveys and 10-year age groups (shown in the figures along with sample sizes) as defined on April 1, 1984.
The LLD males have a larger FI than the long-living males. The FI dynamics for them are also different, showing the tendency to increase over time. The LLD females of the E-sample start with the same FI as the long-living females. However, their patterns diverge: for the long-living females the pattern is flatter. Similar divergent dynamics are also seen for the L-sample. Short-lived males and females have larger FIs and, generally, larger rates of FI increase than those of other groups.
Finally, Figures 3c,d show the age-specific FI dynamics for long-livers of the E-sample. We distinguish three groups, aged 65–74, 75–84, and 85+ years on April 1, 1984. Nearly all living males in the E-sample (except one) are aged 65–74 years in 1984. Therefore, the FI dynamics resemble those in Figure 3b for the respective group. There are two (65–74 and 75–84) age groups of died males which do accumulate deficits in a similar fashion despite essentially different ages; no significant differences between the respective FIs are seen. The tendency for a more accelerated FI increase over time for older males is, however, noticeable.
The younger group of the living females (65–74) has non-significantly larger FIs early in life (in 1984 and 1989) than the older group (75–84). Both these groups show upward trend in FI over time which is larger for older group than for younger group. For died females, no qualitative differences in the longitudinal patterns are observed; all show acceleration with time. The rates of deficit accumulation, however, increase with age (i.e., from younger to older groups).
DISCUSSION
This study focuses on samples of U.S. elderly (65+) males and females having qualitatively distinct age and survival profiles. Specifically, we select individuals who died early (before age 75 years), who died at age 85+, and long-living individuals aged 85+ years who remained alive at the end of the follow up period in the NLTCS.
The analysis reveals that short-lived individuals and individuals who died at age 85+ exhibit nearly identical FI frequency patterns despite the essential difference in their age profiles, i.e., the FI frequency distributions for deceased individuals appear to be practically age-insensitive. This finding demonstrates the FIs ability to capture the association between risks of death and individuals’ aging-related decline in physiological performance that occurs regardless of chronological age. Qualitative differences between FI frequency patterns for deceased and living individuals suggest that the FI is a sensitive characteristic, discriminating individuals with respect to their survival chances in heterogeneous populations. Consequently, the FI has a potential to better predict adverse health events in the elderly individuals than chronological age. These findings are consistent with the FI’s utility to discriminate healthy/unhealthy phenotypes [25,27]. They are also consistent with the results of the cross-sectional analyses which show (practically age-independent) associations of the FI with mortality, time to death, and relative risks of death [21], providing the reasoning for the observed similarities. It is also worth noting that although the FI theoretically ranges from 0 to 100%, the FI frequency distributions suggest that there is limit which is about 70% irrespective of survival profile. Interestingly, the same limit is observed in other settings that might be associated with limits of the organism’s stress resistance [20,28].
Long life is consistently characterized in longitudinal analyses by lower levels of the FIs than short life (Figure 2). However, the difference between deceased and living individuals is even more pronounced, supporting our conclusion on the FIs utility for selection of more homogenous groups in studies of aging, health, and longevity.
Since individuals with larger FIs are more likely to die (Figure 1), longitudinal patterns of the FI for short-livers and individuals who died at age 85+ represent interplay of at least two processes. One is selection of robust individuals (i.e., with small FIs) who have larger chances to survive to old ages. This process results in lowering the mean FI over time. The other process is associated with aging-related changes in individuals loaded by environmental exposures. This results in an increase of the mean FI over time. In the NLTCS (Figure 2), these processes can largely be balanced for males, resulting in flat patterns for decedents. For females, the selection effects are not as prominent as for males. Consequently, superposition of both processes results basically in the accelerated FI patterns for these groups. This finding indicates lesser female vulnerability to the accumulated deficits. This conclusion is supported by the fact of a lower mortality rate for females than for males despite the fact that females can have larger FIs than males [6,21].
Further insight into the association between the FI and aging-related processes is obtained by following longitudinally the long-living individuals, as well as those individuals from the deceased groups who were interviewed in several surveys (i.e., adjusting for selection effects; Figure 3). This analysis suggests that to live long individuals should have low FI levels. The FI dynamics are, however, found to be strongly sex-sensitive. Specifically, males from the long-living group do not accumulate deficits over time (note that the increase of the FI in the 1999 NLTCS – see Figure 3b – is attributed to limited length of the follow-up). The long-living females, however, can accumulate deficits. Therefore, to survive for a long time period, males should remain in a good health (i.e., have a small FI) and not accumulate deficits. Females can have a larger FI and can accumulate deficits to larger FI levels and survive at least the same time period as males. Similar conclusions can be drawn from analysis of the respective patterns for individuals who died at age 85+: high level of the accumulated deficits indicates that such individuals have large chances to die soon. Again, males accumulate deficits at a larger rate than females. Such sex differences can be attributed to two factors acting either separately or together. The first is that females may have lesser vulnerability to the accumulated deficits (e.g., due to higher stress resistance). The second is that females have better chances for adaptation than males, since they accumulate deficits at a smaller rate and during larger time periods. The latter factor highlights possible evolutional differences between the sexes.
Age-specific patterns in Figures 3c,d confirm our conclusion from Figure 1 on the weak age-dependence of the process of deficit accumulation. This finding indicates that FI is a promising characteristic of aging-associated processes in the elderly. Consequently, the FI can be a sensitive age-independent indicator of physiological decline in aging individuals and survival chances suitable for modeling risks of adverse health outcomes in the elderly [22,23].
Acknowledgments
Financial Disclosure: The research reported in this paper was supported by the NIH/NIA grants 5UO1-AG-007198-18, 1R01 AG028259-01, 1R01-AG-027019-01 and 5P01-AG-008761-16 from the National Institute on Aging (NIA). Each co-author asserts no proprietary interest in the results and no financial conflict of interest.
Author contributions: All authors had full access to the data. Drs. Kulminski, Ukraintseva, and Yashin take responsibility for the data integrity and the accuracy of the data analyses, study design, interpretation of the data, and drafting the manuscript. Drs. Akushevich and Arbeev contributed to the final version. Dr. Kulminski was responsible for statistical analyses.
Sponsor’s Role: Sponsor institution (NIA) did not played a direct role in the study design, conducts, management, data analysis, review, or authorization for submission.
Footnotes
Funding sources and related paper presentations
The research reported in this paper was supported by the NIH/NIA grants 5UO1-AG-007198-18, 1R01 AG028259-01, 1R01-AG-027019-01 and 5P01-AG-008761-16 from the National Institute on Aging (NIA).
A. Kulminski, S. Ukraintseva, I. Akushevich, K. Arbeev, and A. Yashin. Cumulative index of health disorders and longevity. Forthcoming oral presentation at 2007 Population Association of America annual meeting, New York, March 2007.
References
- 1.Ukraintseva SV, Yashin AI. How individual age-associated changes may influence human morbidity and mortality patterns. Mech Ageing Dev. 2001;122:1447–1460. doi: 10.1016/s0047-6374(01)00277-9. [DOI] [PubMed] [Google Scholar]
- 2.Ukraintseva SV, Yashin AI. Individual aging and cancer risk: How are they related? Demographic Research. 2003;9:163–196. [Google Scholar]
- 3.Anisimov VN, Ukraintseva SV, Yashin AI. Cancer in rodents: does it tell us about cancer in humans? Nat Rev Cancer. 2005;5:807–819. doi: 10.1038/nrc1715. [DOI] [PubMed] [Google Scholar]
- 4.Evert J, Lawler E, Bogan H, et al. Morbidity profiles of centenarians: survivors, delayers, and escapers. J Gerontol A Biol Sci Med Sci. 2003;58:232–237. doi: 10.1093/gerona/58.3.m232. [DOI] [PubMed] [Google Scholar]
- 5.Ferrucci L, Guralnik JM, Cavazzini C, et al. The frailty syndrome: a critical issue in geriatric oncology. Crit Rev Oncol Hematol. 2003;46:127–137. doi: 10.1016/s1040-8428(02)00177-4. [DOI] [PubMed] [Google Scholar]
- 6.Mitnitski A, Song X, Skoog I, et al. Relative fitness and frailty of elderly men and women in developed countries and their relationship with mortality. J Am Geriatr Soc. 2005;53:2184–2189. doi: 10.1111/j.1532-5415.2005.00506.x. [DOI] [PubMed] [Google Scholar]
- 7.Puts MT, Lips P, Deeg DJ. Sex differences in the risk of frailty for mortality independent of disability and chronic diseases. J Am Geriatr Soc. 2005;53:40–47. doi: 10.1111/j.1532-5415.2005.53008.x. [DOI] [PubMed] [Google Scholar]
- 8.Newman AB, Gottdiener JS, McBurnie MA, et al. Associations of subclinical cardiovascular disease with frailty. J Gerontol A Biol Sci Med Sci. 2001;56:M158–166. doi: 10.1093/gerona/56.3.m158. [DOI] [PubMed] [Google Scholar]
- 9.Fried LP, Ferrucci L, Darer J, et al. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci. 2004;59:255–263. doi: 10.1093/gerona/59.3.m255. [DOI] [PubMed] [Google Scholar]
- 10.Bortz WM., 2nd A conceptual framework of frailty: a review. J Gerontol A Biol Sci Med Sci. 2002;57:M283–288. doi: 10.1093/gerona/57.5.m283. [DOI] [PubMed] [Google Scholar]
- 11.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 12.Goldhill DR, Sumner A. APACHE II, data accuracy and outcome prediction. Anaesthesia. 1998;53:937–943. doi: 10.1046/j.1365-2044.1998.00534.x. [DOI] [PubMed] [Google Scholar]
- 13.Willcox BJ, He Q, Chen R, et al. Midlife risk factors and healthy survival in men. JAMA. 2006;296:2343–2350. doi: 10.1001/jama.296.19.2343. [DOI] [PubMed] [Google Scholar]
- 14.Rantanen T, Harris T, Leveille SG, et al. Muscle strength and body mass index as long-term predictors of mortality in initially healthy men. J Gerontol A Biol Sci Med Sci. 2000;55:M168–173. doi: 10.1093/gerona/55.3.m168. [DOI] [PubMed] [Google Scholar]
- 15.Mitnitski A, Graham J, Mogilner A, et al. Frailty, fitness and late-life mortality in relation to chronological and biological age. BMC Geriatr. 2002;2:1. doi: 10.1186/1471-2318-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fisher AL. Just what defines frailty? J Am Geriatr Soc. 2005;53:2229–2230. doi: 10.1111/j.1532-5415.2005.00510.x. [DOI] [PubMed] [Google Scholar]
- 17.Rockwood K, Mitnitski A, Song X, et al. Long-term risks of death and institutionalization of elderly people in relation to deficit accumulation at age 70. J Am Geriatr Soc. 2006;54:975–979. doi: 10.1111/j.1532-5415.2006.00738.x. [DOI] [PubMed] [Google Scholar]
- 18.Goggins WB, Woo J, Sham A, et al. Frailty index as a measure of biological age in a Chinese population. J Gerontol A Biol Sci Med Sci. 2005;60:1046–1051. doi: 10.1093/gerona/60.8.1046. [DOI] [PubMed] [Google Scholar]
- 19.Woo J, Goggins W, Sham A, et al. Public health significance of the frailty index. Disabil Rehabil. 2006;28:515–521. doi: 10.1080/09638280500215867. [DOI] [PubMed] [Google Scholar]
- 20.Kulminski A, Yashin A, Ukraintseva S, et al. Accumulation of health disorders as a systemic measure of aging: Findings from the NLTCS data. Mech Ageing Dev. 2006;127:840–848. doi: 10.1016/j.mad.2006.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kulminski A, Yashin A, Arbeev K, et al. Cumulative Index of Health Disorders as an Indicator of Aging-Associated Processes in the Elderly: Results From Analyses of the National Long Term Care Survey. Mech Ageing Dev. 2007:000–000. doi: 10.1016/j.mad.2006.12.004. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yashin AI, Arbeev KG, Kulminski A, et al. Cumulative index of elderly disorders and its dynamic contribution to mortality and longevity. Rejuvenation Research. 2007:000–000. doi: 10.1089/rej.2006.0500. in press. [DOI] [PubMed] [Google Scholar]
- 23.Yashin AI, Arbeev KG, Kulminski A, et al. Health Decline, Aging And Mortality: How Are They Related? Biogerontology. 2007:000–000. doi: 10.1007/s10522-006-9073-3. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manton KG, Gu X, Lamb VL. Change in chronic disability from 1982 to 2004/2005 as measured by long-term changes in function and health in the U.S. elderly population. Proc Natl Acad Sci U S A. 2006;103:18374–18379. doi: 10.1073/pnas.0608483103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rockwood K, Mogilner A, Mitnitski A. Changes with age in the distribution of a frailty index. Mech Ageing Dev. 2004;125:517–519. doi: 10.1016/j.mad.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 26.Kirkwood TB. Molecular gerontology. J Inherit Metab Dis. 2002;25:189–196. doi: 10.1023/a:1015625811569. [DOI] [PubMed] [Google Scholar]
- 27.Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. ScientificWorldJournal. 2001;1:323–336. doi: 10.1100/tsw.2001.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rockwood K, Mitnitski A. Limits to deficit accumulation in elderly people. Mech Ageing Dev. 2006;127:494–496. doi: 10.1016/j.mad.2006.01.002. [DOI] [PubMed] [Google Scholar]


