Abstract
We report an alternative, hydroxylating pathway for the metabolism of vitamin D2 in a cytochrome P450 side chain cleavage (P450scc; CYP11A1) reconstituted system. NMR analyses identified solely 20-hydroxyvitamin D2 and 17,20-dihydroxyvitamin D2 derivatives. 20-Hydroxyvitamin D2 was produced at a rate of 0.34 mol·min−1·mol−1 P450scc, and 17,20-dihydroxyvitamin D2 was produced at a rate of 0.13 mol·min−1·mol−1. In adrenal mitochondria, vitamin D2 was metabolized to six monohydroxy products. Nevertheless, aminoglutethimide (a P450scc inhibitor) inhibited this adrenal metabolite formation. Initial testing of metabolites for biological activity showed that, similar to vitamin D2, 20-hydroxyvitamin D2 and 17,20-dihydroxyvitamin D2 inhibited DNA synthesis in human epidermal HaCaT keratinocytes, although to a greater degree. 17,20-Dihydroxyvitamin D2 stimulated transcriptional activity of the involucrin promoter, again to a significantly greater extent than vitamin D2, while the effect of 20-hydroxyvitamin D2 was statistically insignificant. Thus, P450scc can metabolize vitamin D2 to generate novel products, with intrinsic biological activity (at least in keratinocytes).
Keywords: cytochrome P450scc, keratinocytes, skin, vitamin D2
Vitamin D2 (ergocalciferol) is a product of UVB-mediated transformation of ergosterol, a 5,7-diene phytosterol, which is synthesized by fungi and phytoplankton but not in the animal kingdom [1]. The physicochemical reactions that generate vitamin D2 are similar to those involved in the generation of vitamin D3 from 7-dehydrocholesterol: UVB energy converts ergosterol into previtamin D2, while thermal energy (at 37 °C) converts previtamin D2 into vitamin D2 [1]. Vitamin D2 differs from vitamin D3 in exhibiting a lesser hypercalcemic effect [2,3], making it a potential precursor for effective drugs in therapy for cancer [1,3–5], or for proliferative cutaneous diseases [1,6]. Such use is based on the non-metabolic actions of vitamin D apart from its effect on calcium. These include modulation of immune and neuroendocrine activities, cellular proliferation, differentiation and apoptosis in cells of different lineages, and protection of DNA against oxidative damage and action as a cell membrane antioxidant [1,3,6,7].
Structurally, vitamin D2 differs from vitamin D3 in that its side chain has a C24 methyl group and a C22–C23 double bound. These features are responsible for the differences in oxidative processes occurring on the side chain relative to those observed for vitamin D3 [8,9]. However, the main steps of metabolic conversion of vitamins D3 and D2 in vivo are mediated by the same enzymes, with similar products that include 24- and 25-hydroxy derivatives [1,5, 10,11]. These are further hydroxylated at position 1 to generate 1α,24-dihydroxyvitamin D2 and the metabolite with the highest biological activity, 1α,25-dihydroxyvitamin D2 [12]. Additional hydroxylations produce 1α,24(S),26-trihydroxyvitamin D2 and 1α,24(R),25-trihydroxyvitamin D2, and further hydroxylation at position 26 or 28 results in tetrahydroxyvitamin D2 [12]. 24-Hydroxyvitamin D2 and 25-hydroxyvitamin D2 are inactivated through the transformations to 24(S),26-dihydroxyvitamin D2 and 24(R),25-dihydroxyvitamin D2, respectively [12]. Additional derivatives that have been identified are generated through other modifications of the side chain or of the A-ring [12].
Cytochrome P450scc (CYP11A1) catalyzes the first step in steroid synthesis, the cleavage of the side chain of cholesterol to produce pregnenolone [13–15]. This reaction proceeds via the enzyme-bound reaction intermediates 22R-hydroxycholesterol and 20α,22R-dihydroxycholesterol [13–15]. Recently, it has been demonstrated that in addition to cholesterol, P450scc can also use 7-dehydrocholesterol, vitamin D3 and ergosterol as substrates [16–19]. P450scc cleaves the side chain of 7-dehydrocholesterol, producing 7-dehydropregnenolone [18]. With ergosterol and vitamin D3, P450scc hydroxylates the substrate but cleavage of the side chain is not observed [17,19]. P450scc converts vitamin D3 to 20-hydroxyvitamin D3 and 20,22-dihydroxyvitamin D3 [16,17] and metabolizes ergosterol to 17α,24-dihydroxyergosterol [19]. Thus, a new family of metabolites can be generated by the action of P450scc, with the nature of the modifications differing between substrates of animal (7-dehydrocholesterol and vitamin D3) and plant (ergosterol) origin. To further characterize these novel metabolic pathways, we have investigated the action of mammalian cytochrome P450scc on vitamin D2, utilizing both purified enzyme in a reconstituted system and adrenal mitochondria, with products being identified by MS and NMR.
Results and Discussion
Metabolism of vitamin D2 by purified P450scc in a reconstituted system
Vesicle-reconstituted P450scc metabolized vitamin D2 to two novel products as shown by TLC; these were not seen in control incubations where the electron source was omitted (Fig. 1). As expected, there was production of a little pregnenolone from cholesterol that copurified with bovine P450scc, confirming the side chain-cleaving activity of the enzyme. Following their elution from TLC plates, both vitamin D2 metabolites displayed UV absorbance corresponding to an intact vitamin D chromophore (λmax at 265 nm and λmin at 228 nm). For metabolite 1, the molecular ion had m/z = 412 with fragment ions m/z = 394 (412—H2O), m/z = 379 (394—CH3), m/z = 376 (412—2H2O) and m/z = 361 (379—H2O). The molecular ion of metabolite 2 had m/z = 428, with fragment ions at m/z = 410 (428—H2O), m/z = 392 (428—2H2O), m/z = 395 (410—CH3) and m/z = 377 (428—2H2O–CH3). Since vitamin D2 has m/z = 396, metabolite 1 was identified as hydroxyvitamin D2, and metabolite 2 as dihydroxyvitamin D2 (Fig. 1C).
Fig. 1.
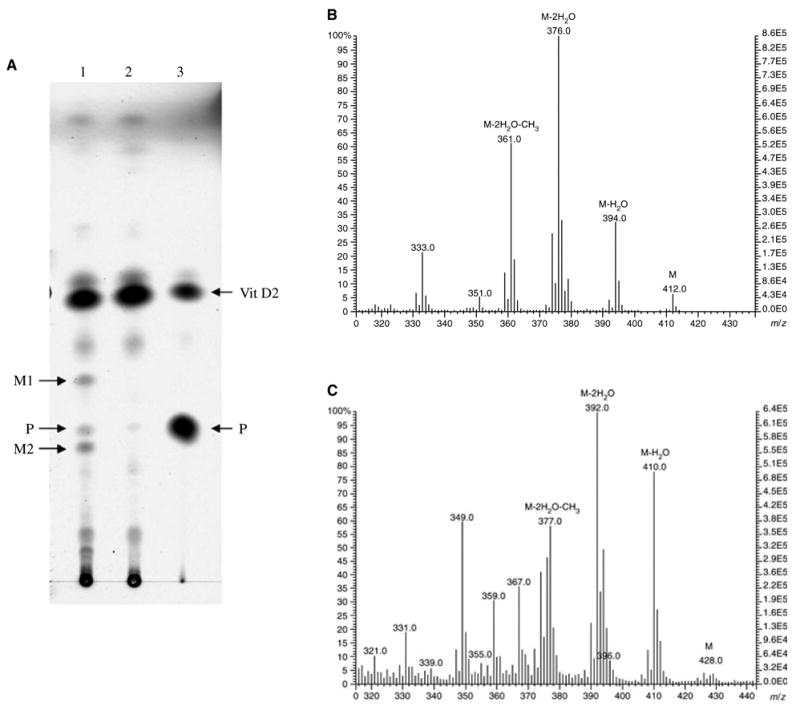
Metabolism of vitamin D2 by purified bovine cytochrome P450 side chain cleavage (P450scc). Incubations were carried out in a reconstituted system comprising purified P450scc (3 μM), adrenodoxin reductase, adrenodoxin and phospholipid vesicles containing vitamin D2 at a molar ratio to phospholipid of 0.2. (A) Reaction products were analyzed by TLC and visualized by charring. Lane 1, Experimental incubation with NADPH; lane 2, control incubation without NADPH; lane 3, pregnenolone (P) and vitamin D2 standards. Metabolite 1 (M1) and metabolite 2 (M2) are marked by arrows. (B) Electron impact MS of metabolite 1. (C) Electron impact MS of metabolite 2.
Identification of the structure of vitamin D2 metabolites
Incubation of P450scc (2.0 μM) with vitamin D2 in phospholipid vesicles (40 mL) for 1 h produced 70 μg of TLC-purified hydroxyvitamin D2 (4% yield) and 60 μg of TLC-purified dihydroxyvitamin D2 (3.3% yield). Products from two 40 mL incubations were pooled and used for structural analysis by NMR.
Identification of metabolite 1 was accomplished by analysis of proton 1D, COSY and proton–carbon correlation spectroscopy (HSQC) spectra of this compound and of parent vitamin D2 (Fig. 2). The high-order pattern in proton NMR of vitamin D2 at 5.19 p.p.m. (22-CH) and 5.20 p.p.m. (23-CH) became separated to 5.54 p.p.m. (22-CH) and 5.42 p.p.m. (23-CH) in metabolite 1 (Fig. 2, projections on COSY spectra). The scalar coupling between 22-CH and 20-CH did not exist in this metabolite (Fig. 2B). At the same time, the doublet of the 21-methyl in vitamin D2 (proton at 1.01 p.p.m. and carbon at 21.2 p.p.m.; Fig. 2C) became a singlet in metabolite 1 with a down-field shift (proton at 1.30 p.p.m. and carbon at 29.5 p.p.m.; Fig. 2D), also indicating the removal of scalar coupling from 20-CH. Other regions of the spectra are similar between vitamin D2 and metabolite 1. All these changes can be readily explained by the presence of a 20-OH group in metabolite 1. The impurities present in metabolite 1 have strong NMR signals in the low chemical shift region but not in the high chemical shift region, and they probably derive from the TLC plate used in the purification process.
Fig. 2.
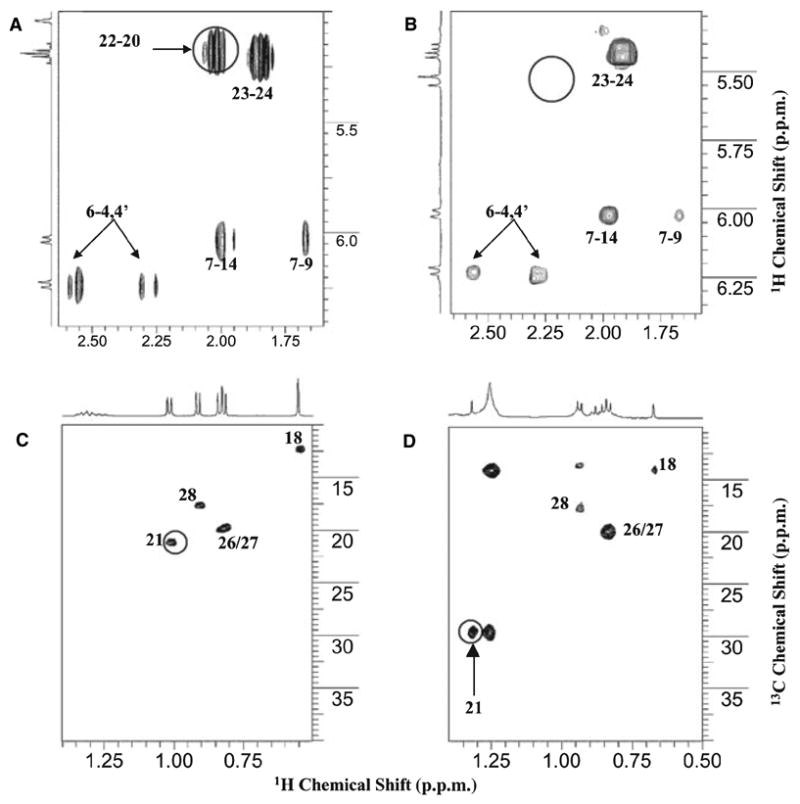
NMR spectra of vitamin D2 metabolite 1 identified as 20-hydroxyvitamin D2. (A) Proton–proton COSY of vitamin D2 standard. (B) COSY of vitamin D2 metabolite 1. (C) Proton–carbon correlation spectroscopy (HSQC) of vitamin D2 standard. (D) HSQC of vitamin D2 metabolite 1. The separation of 22/23 proton signals in metabolite 1 and the lack of scalar coupling between 20-CH and 22-CH at 5.54 p.p.m. (circle in (B)) clearly indicates hydroxylation at 20-C. The doublet-to-singlet transition of proton NMR with concurrent downfield shift of the 21-methyl signal (1.01 p.p.m. and 21.2 p.p.m. to 1.3 p.p.m. and 29.5 p.p.m.) confirms hydroxylation at the 20 position. Impurities in the methyl regions are probably from the TLC purification process.
The HSQC spectrum of the methyl region in metabolite 2 was cleaner and similar to that of metabolite 1, indicating the presence of 20-OH and no other hydroxyl group on the side chain (Fig. 3D). The A-ring and double bond linker were also intact in this metabolite, indicating that the second hydroxylation is either at the B-ring or at the C-ring (Fig. 3). The well-isolated proton NMR signals of 9-CH2 (1.68 p.p.m. and 2.82 p.p.m.) have very similar position and coupling patterns in vitamin D2 and metabolite 2, indicating that the B-ring stays intact. Therefore, the second hydroxylation must occur in the C-ring. The 14-CH in this metabolite has a large downfield shift in its proton NMR (1.99 p.p.m. in vitamin D2 and 2.68 p.p.m. in metabolite 2; Fig. 3A and Fig. 3B), while the proton NMR of the 17-CH in the vitamin D2 standard at 1.32 p.p.m. disappeared. The shift of the 14-CH is caused by the formation of 17-OH in this metabolite. Hence, this dihydroxylated metabolite is most likely to be 17,20-dihydroxyvitamin D2.
Fig. 3.
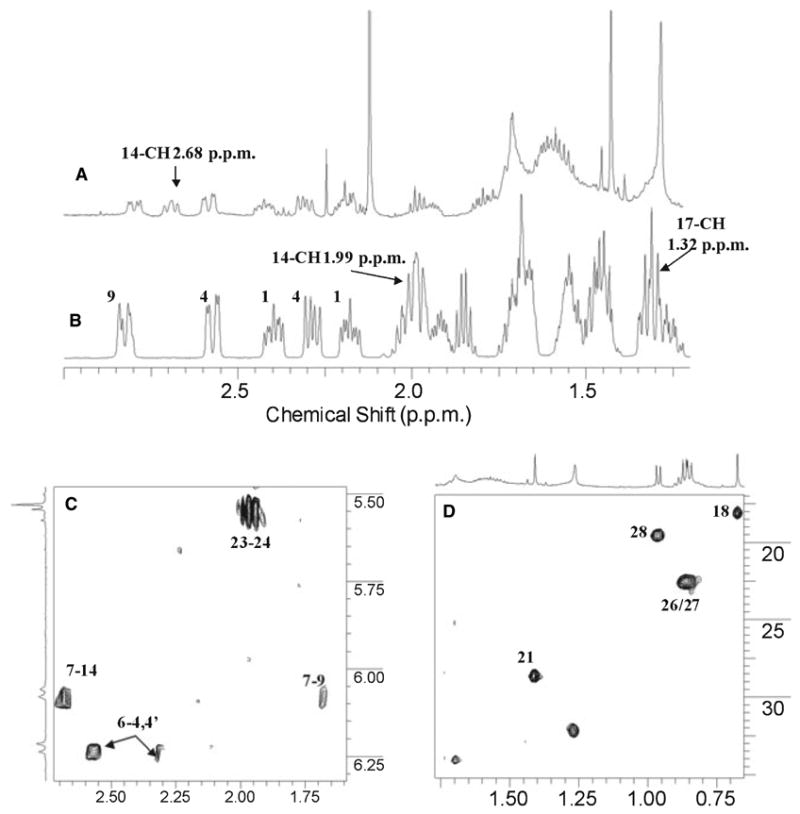
NMR spectra of vitamin D2 metabolite 2 identified as 17,20-dihydroxyvitamin D2. (A) Proton spectra of metabolite 2. (B) Proton spectra of vitamin D2. (C) COSY of metabolite 2. (D) Proton–carbon correlation spectroscopy (HSQC) of methyl regions of metabolite 2. Numbers in (B) indicate proton positions in the vitamin D2 standard. In metabolite 2, the 20-hydroxyl is clearly present and there are no other changes in the side chain as indicated by COSY and HSQC. The large downfield shift of 14-CH from 1.99 p.p.m. to 2.68 p.p.m. with disappearance of the 17-CH signal at 1.32 p.p.m. indicates that hydroxylation has occurred at the 17-C position.
We have therefore shown that P450scc hydroxylates vitamin D2, and generates hydroxyvitamin D2 and dihydroxyvitamin D2 as main products in approximately equivalent amounts. NMR analysis further showed that these products correspond to 20-hydroxyvitamin D2 and 17,20-dihydroxyvitamin D2, and also revealed that the initial hydroxylation occurs at position 20, and is followed by a second hydroxylation at C17 (Fig. 4). The explanation for hydroxylation in these positions lies in the structure of vitamin D2, which has a C22–C23 double bond that both prevents hydroxylation at C22 and apparently limits hydroxylation of the side chain to C20. Hydroxylation of the C-ring at position 17 indicates a shift in substrate orientation in the active site, as compared to cholesterol, vitamin D3, or 24α-methylcholesterol (campesterol), where P450scc is free to hydroxylate at C20 and C22 [16,17,20]. Interestingly, ergosterol (provitamin D2) is hydroxylated at C17 similar to vitamin D2, but the second hydroxylation is at C24 rather than C20 [19]. The detected accumulation of 20-hydroxyvitamin D2 (Fig. 1A) suggests that it can be released from the active site of P450scc, with only a portion remaining bound or rebinding for subsequent hydroxylation at C17. This is again in contrast to the P450scc-mediated metabolism of ergosterol, where the accumulation of monohydroxy product is only minor, and also in contrast to the conversion of cholesterol into pregnenolone, where hydroxycholesterol intermediates are not normally released [20,21].
Fig. 4.
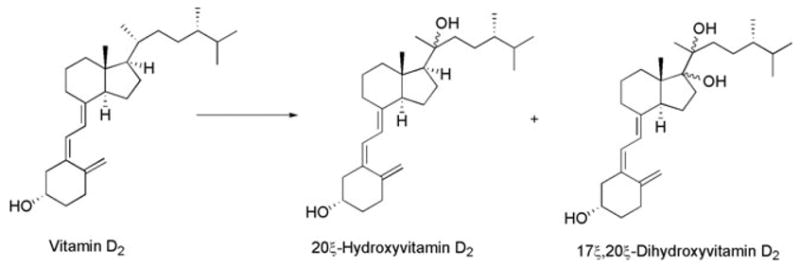
Proposed sequence for the cytochrome P450 side chain cleavage (P450scc)-catalyzed transformation of vitamin D2 with chemical structures of the reaction products.
The rate of vitamin D2 metabolism by purified P450scc
To obtain an estimate of the initial rate of vitamin D2 metabolism by P450scc, vitamin D2 at a molar ratio to phospholipid of 0.4 was incubated with P450scc for 5 min at 35 °C. The 20-hydroxyvitamin D2 and 17,20-dihydroxyvitamin D2 products were extracted, purified by TLC and quantitated from their absorbance at 264 nm. 20-Hydroxyvitamin D2 was produced at a rate of 0.34 mol·min−1·mol−1 P450scc, and 17,20-dihydroxy vitamin D2 was produced at a rate of 0.13 mol·min−1·mol−1 P450scc. Under similar conditions, this preparation of P450scc converted cholesterol to pregnenolone at a rate of 14.4 mol·min−1·mol−1 P450scc. The rate of hydroxylation of vitamin D2 by P450scc is slightly lower than the rate of hydroxylation of its precursor, ergosterol [19].
Vitamin D2 metabolism by adrenal mitochondria
To evaluate the biological relevance of the above findings, we incubated purified adrenal mitochondria, which contain a high concentration of P450scc, with vitamin D2. Tests were performed in the presence (experimental) or absence (control) of NADPH and isocitrate. When the reaction products were subjected to LC/MS or LC with UV spectral analysis, we detected six new products by monitoring at 265 nm of HPLC-separated fractions (Fig. 5). These products had UV absorbance spectra characteristic of the vitamin D triene, with λmax at 265 nm and λmin at 228 nm, and displayed a molecular ion [M + 1]+ at m/z = 413 and a major fragment ion at m/z = 395 (413—H2O), indicating that they represent isomers of hydroxyvitamin D2 (not shown). The molecular ion [M + 1]+ for vitamin D2 (not shown) had the expected m/z = 397.
Fig. 5.
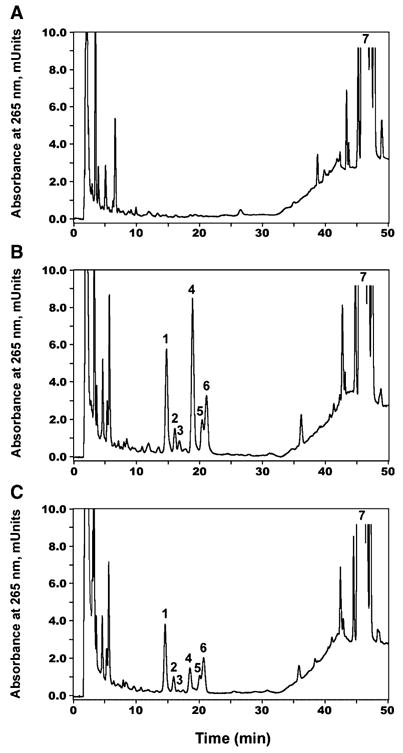
RP-HPLC separation of products of vitamin D2 metabolism by adrenal mitochondria. (A) Incubation of mitochondria in the absence of NADPH and isocitrate. (B) Experimental incubation with NADPH and isocitrate. (C) Experimental incubation with 200 μM aminoglutethimide. The HPLC elution profile was monitored by absorbance at 265 nm. 1–6, novel vitamin D2 metabolites; 7, vitamin D2.
To further study the possible involvement of P450scc in the formation of the vitamin D2 metabolites, aminoglutethimide, a specific inhibitor of cytochrome P450scc in rat adrenal mitochondria [22,23], was added to the reaction mixture. The formation of the unknown metabolites 1, 2, 3, 5 and 6 decreased in a parallel fashion (Fig. 5C). More profound inhibition was observed in the case of metabolite 4, which suggests that it represents 20-hydroxyvitamin D2. This provides further evidence that vitamin D2 hydroxylation in adrenal mitochondria is catalyzed by P450scc, especially for production of metabolite 4 (putative 20-hydroxyvitamin D2).
Initial tests for biological activity of vitamin D2 metabolites
Cultured human epidermal HaCaT keratinocytes were incubated with HPLC-purified 20-hydroxyvitamin D2 or 17,20-dihydroxyvitamin D2 added to the culture media at a concentration of 10−10 M. This caused inhibition of DNA synthesis, significantly greater than that seen with vitamin D2 itself (Fig. 6A). A similar inhibitory effect of both hydroxy metabolites was also seen in an additional independent experiment (not shown). We also tested for an effect of hydroxyvitamin D2 products on keratinocyte differentiation, with vitamin D2 and 5 mM Ca2+ as positive controls. This was done using the firefly luciferase reporter gene plasmid IVL-Luc, containing the involucrin gene promoter region (−668 bp to + 34 bp) (Fig. 6B). Since involucin expression is characteristically proportional to keratinocyte differentiation [24–28], these assays are typically used in models testing for keratinocyte differentiation [24]. All of the tested compounds stimulated transcriptional activity of the involucrin promoter; the most significant effects were shown by Ca2+ and 17,20-dihydroxyvitamin D2, which simulated luciferase activity 25-fold and 12-fold, respectively (Fig. 6B). The stimulatory effect of 17,20-dihydroxy-vitamin D2 was significantly higher than that of vitamin D2 (P < 0.05), while the effect of 20-hydroxyvitamin D2 on involucrin promoter activity was statistically insignificant (P > 0.05). Thus, the data above indicate that vitamin D2 can be converted to product(s) of higher biological activity by P450scc.
Fig. 6.
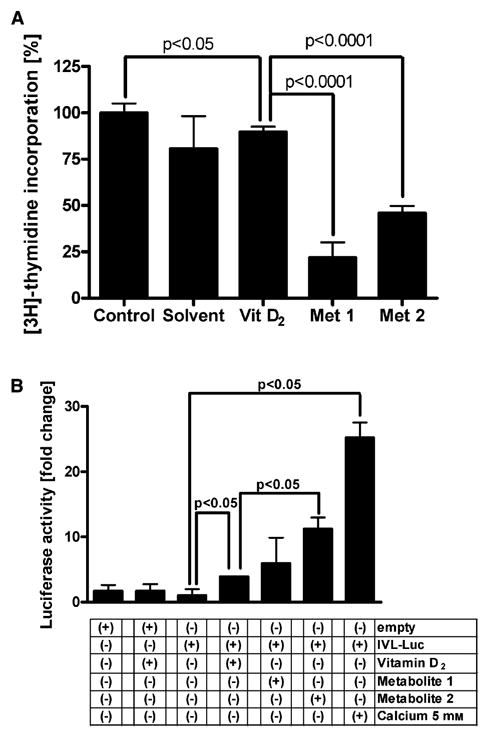
Metabolites of vitamin D2 inhibit DNA synthesis and stimulate differentiation in human HaCaT keratinocytes. (A) HaCaT keratinocytes were synchronized and incubated for 24 h in Ham’s F10 medium containing serum and vitamin D2 or its metabolites, and [3H]-thymidine. (B) HaCaT keratinocytes were transfected with a construct containing the involucrin promoter (IVL-Luc) or with empty (promoter-free) construct, synchronized and incubated for 24 h in Ham’s F10 medium containing serum and vitamin D2 or its metabolites. Data are shown as mean ± SEM (n = 3–8).
Conclusions
The novel hydroxylating activity of mammalian P450scc towards vitamin D2 to generate 20-monohydroxyvtamin D2 and 17,20-dihydroxyvitamin D2 raises questions of a new role for this enzyme and on the biological activity of its products. It was previously shown that P450scc cleaves the side chain of 7-dehydrocholesterol to produce 7-dehydropregnenolone [16,18], that it hydroxylates vitamin D3 to 20S-hydroxyvitamin D3 and 20,22-dihydroxyvitamin D3 [17], and that it hydroxylates ergosterol to 24-monohydroxyergosterol and 17α,24-dihydroxyergosterol [19]. Thus, it is becoming apparent that metabolism by mammalian P450scc opens novel pathways, where processing is determined by both substrate structure (5,7-dienes vs. secosteroids) and origin (animal kingdom vs. fungi or phytoplankton). The human disease, Smith Lemli Opitz Syndrome, illustrates the significance of the former pathway; defective 7-delta reductase leads to excessive accumulation of 7-dehydropregnenolone and its hydroxy derivatives, with characteristic pathologic features [29,30]. Conversely, in the case of vitamin D3, P450scc action may prevent its sequential transformation to bioactive calcitriol, although the activity of the alternative products remains to be tested [17]. The transformation of ergosterol [19] results in distinct products that are also biologically active. Both 20-monohydroxyvitamin D2 and 17,20-dihydroxyvitamin D2 inhibit proliferation of human keratinocytes to a greater degree than vitamin D2 itself, while only 17,20-dihydroxyvitamin D2 is able to stimulate activity of the involucrin promoter (higher than vitamin D2). Thus, both products show biological activity, differentially expressed depending on the phenotypic feature measured. Within the context of the recently described pleiotropic activity of vitamin D [1,4,5], the new hydroxy derivatives of vitamin D2 could have a place in the management of epithelial hyperproliferative disorders or skin diseases. This may be particularly important when considering the limitation in clinical use imposed by the potentially toxic hypercalcemic action [1,4,5]. Since vitamin D2 is absorbed by the alimentary tract, it could be metabolized in any organ expressing high levels of P450scc, such as adrenal glands (see above), gonads [31] or placenta [32], raising the possibility of additional systemic effects. Organs expressing low levels of P450scc, which include brain [33], gastrointestinal tract [34], kidney [35], and skin [18], could alternatively produce and use the same metabolites in local paracrine, autocrine or intracrine roles.
Our previous work [17–19] and current findings have clearly uncovered a new biological significance for an ancient enzyme, cytochrome P450scc. We have shown that P450scc opens new metabolic pathways, thus generating novel steroidal and secosteroidal derivatives. Of these, some have already been shown to possess biological activity (vitamin D2 and ergosterol hydroxy derivatives), while for others the biological activity remains to be defined.
Experimental procedures
Enzymatic assays
Metabolism of vitamin D2 by purified cytochrome P450scc
The detailed methodology has been described before [17]. Briefly, bovine cytochrome P450scc and adrenododoxin reductase were isolated from adrenals [36,37]. Adrenodoxin was expressed in Esccherichia coli and purified as previously described [38]. The reaction mixture comprised 510 μM phospholipid vesicles (dioleoyl PC plus 15 mol% cardiolipin) with a vitamin D2/phospholipid molar ratio of 0.2, 50 μM NADPH, 2 mM glucose 6-phosphate, 2 U·mL−1 glucose-6-phosphate dehydrogenase, 0.3 μM adrenodoxin reductase, 6.5 μM adrenodoxin, 3.0 μM cytochrome P450scc and buffer pH 7.4. After incubation at 35 °C for 3 h, the mixture was extracted with methylene chloride and dried under nitrogen. Products were analyzed and purified by preparative TLC on silica gel G with three developments in hexane/ethyl acetate (3 : 1, v/v). For NMR and MS analyses, they were eluted from the silica gel with chloroform/methanol (1 : 1, v/v), dried separately under nitrogen, and shipped on dry ice.
Metabolism of vitamin D2 by adrenal mitochondria
Adrenals were obtained from male Wistar rats aged 3 months, killed under anesthesia. The animals were housed at the vivarium of the Department of Biotestings of Bio-organic Chemistry Institute, Minsk, Belarus. The experiments were approved by the Belarus University Animal Care and Use Committee. The reactions were run as described previously [17,18]. Briefly, mitochondria prepared from the adrenals were preincubated (10 min at 37 °C) with 100 μM vitamin D2 (dissolved in 45% 2-hydroxypropyl-cyclodextrin) in buffer comprising 0.25 M sucrose, 50 mM Hepes pH 7.4, 20 mM KCl, 5 mM MgSO4, and 0.2 mM EDTA. The reactions were started by adding NADPH (0.5 mM) and isocitrate (5 mM) to the samples, and after 90 min mixtures were extracted with methylene chloride and the organic layers combined and dried. The residues were dissolved in methanol and subjected to LC/MS analysis as detailed below.
NMR
Samples of the purified hydroxy metabolites of vitamin D2 (the masses of the compounds were confirmed by MS) were dissolved in 40 μL of ‘100% D’ CDCl3 (Cambridge Isotope Laboratories, Inc., Andover, MA), and NMR spectra were acquired using a Varian Inova-500 M NMR spectrometer equipped with a 4 mm inverse gHX Nanoprobe (Varian NMR, Inc., Palo Alto, CA). The total volume in the NMR rotor was 40 μL, and all spectra were acquired at a temperature of 294 K with a spinning rate of 2500 Hz. Proton 1D NMR, COSY and HSQC spectra were acquired and processed with standard parameters. Possible positions of the hydroxyl groups in the metabolite were analyzed by comparing the acquired spectra with those of parent vitamin D2.
MS
Products of vitamin D2 metabolism by purified P450scc were eluted from TLC plates and dissolved in ethanol, and electron impact (EI) mass spectra were recorded with a Micromass VG Autospec Mass Spectrometer (Waters, Milford, MA) operating at 70 eV with scanning from 800 to 50 at 1 s·per decade.
The products of mitochondrial transformation (see above) were dissolved in methanol and analyzed on an HPLC mass spectrometer (LCMS-QP8000α, Shimadzu, Kyoto, Japan) equipped with a Restec Allure C18 column (150 × 4.6 mm; 5 μm particle size, and 60 Å pore size), UV/VIS photodiode array detector (SPD-M10Avp) and quadrupole mass spectrometer. The LC/MS WORKSTATION CLASS 8000 software was used for system control and data acquisition (Shimadzu). Elution was carried out at a flow rate of 0.75 mL·min−1 at 40 °C. The mobile phases consisted of 85% methanol and 0.1% acetic acid from 0 to 25 min, followed by a linear gradient to 100% methanol and 0.1% acetic acid from 25 to 35 min, and 100% methanol and 0.1% acetic acid from 35 to 50 min. The mass spectrometer was operated in atmospheric pressure chemical ionization (APCI) positive ion mode and nitrogen was used as the nebulizing gas. The MS parameters were as follows: nebulizer gas flow rate 2.5 L·min−1; probe high voltage 4.5 kV; probe temperature 250 °C; curved desolvation line heater temperature 230 °C. Analyses were carried out in the scan mode from m/z 370 to 430 or in SIM mode.
Cell culture experiments
HaCaT keratinocytes were grown in DMEM with 5% fetal bovine serum and 1% antibiotic solution as described previously [39]. Vitamin D2 metabolites, produced by purified P450scc and isolated by TLC, were further purified by RP-HPLC through a Restec Allure C18 column (150 × 4.6 mm; 5 μm particle size; 60 Å pore size) following the procedure described for LC/MS (see above). For testing biological activity, vitamin D2 and its metabolites were dissolved in cyclodextrin, as described previously [19].
DNA synthesis
Cells were seeded at 5000 per well into 96-well plates in growth medium. After 6 h, medium was discarded and serum-free Ham’s F10 medium was added. After 12 h, this medium was changed to 5% fetal bovine serum Ham’s F10 medium containing compounds to be tested at 10−10 and 10−12 M. After 12 h, medium was discarded and replaced with 5% fetal bovine serum Ham’s medium containing test compounds and [3H]thymidine (1 μCi·mL−1), and incubated for an additional 12 h. After treatment, media were discarded, cells were detached with trypsin and harvested on a glass fiber filter, and radioactivity proportional to methyl-[3H]thymidine incorporated into DNA was counted with a Packard direct beta counter (Packard, Meriden, CA).
Reporter gene assay
The effect of vitamin D2 metabolites on the transcriptional activity of the involucrin promoter was assessed with a reporter gene assay. Cells were seeded at 20 000 per well in 24-well plates in growth medium. After 6 h, cells were transfected using transfection reagents (sc-29528 and sc-36868) from Santa Cruz Biotechnology Inc., Santa Cruz, CA in serum-free F10 medium with firefly luciferase reporter gene plasmid IVL-Luc containing the involucrin gene promoter region (−668 bp to + 34 bp; added at 1 μg per well) and with phRL-TK (expresses Renilla luciferase and serves as normalization control; Promega, Madison, WI; added at 1 μg per well). IVL-Luc and p-Luc (control without promoter, empty vector) plasmids were constructed as described previously [18]. Twelve hours after transfection, the medium was changed to 5% fetal bovine serum Ham’s F10 medium containing vitamin D2 and its hydroxy derivatives. Compounds were added again after 12 h. After another 12 h (entire incubation with compounds lasted 24 h), cells were lysed with passive lysis buffer and luciferase, and Renilla luciferase signals were recorded after sequential addition of Luciferase Assay Reagent II and Stop-Glo Reagent (Promega, Madison, WI) using a TD-20/20 luminometer (Turner Designs, Sunnyvale, CA). After subtraction of background, the specific signal was divided by the Renilla signal. Resulting values were divided by the mean value for controls (cells transfected with IVL-Luc construct and incubated without compounds).
Statistical analysis
Data are presented as mean ± SEM (n = 3–8) and analyzed with Student’s t-test. Each experiment was performed independently two times.
Acknowledgments
The work was supported in part by NIH grant AR052190 to AS.
Abbreviations
- APCI
atmospheric pressure chemical ionization
- EI
electron impact
- P450scc
cytochrome P450 side chain cleavage
- HSQC
proton–carbon correlation spectroscopy
References
- 1.Holick MF. Vitamin D: a millenium perspective. J Cell Biochem. 2003;88:296–307. doi: 10.1002/jcb.10338. [DOI] [PubMed] [Google Scholar]
- 2.Armas LA, Hollis BW, Heaney RP. Vitamin D2 is much less effective than vitamin D3 in humans. J Clin Endocrinol Metab. 2004;89:5387–5391. doi: 10.1210/jc.2004-0360. [DOI] [PubMed] [Google Scholar]
- 3.Brown AJ, Ritter CS, Holliday LS, Knutson JC, Strugnell SA. Tissue distribution and activity studies of 1,24-dihydroxyvitamin D2, a metabolite of vitamin D2 with low calcemic activity in vivo. Biochem Pharmacol. 2004;68:1289–1296. doi: 10.1016/j.bcp.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 4.Albert DM, Kumar A, Strugnell SA, Darjatmoko SR, Lokken JM, Lindstrom MJ, Damico CM, Patel S. Effectiveness of 1alpha-hydroxyvitamin D2 in inhibiting tumor growth in a murine transgenic pigmented ocular tumor model. Arch Ophthalmol. 2004;122:1365–1369. doi: 10.1001/archopht.122.9.1365. [DOI] [PubMed] [Google Scholar]
- 5.Holick MF. Evolution and function of vitamin D. Recent Results Cancer Res. 2003;164:3–28. doi: 10.1007/978-3-642-55580-0_1. [DOI] [PubMed] [Google Scholar]
- 6.Mitani H, Naru E, Yamashita M, Arakane K, Suzuki T, Imanari T. Ergocalciferol promotes in vivo differentiation of keratinocytes and reduces photodamage caused by ultraviolet irradiation in hairless mice. Photodermatol Photoimmunol Photomed. 2004;20:215–223. doi: 10.1111/j.1600-0781.2004.00116.x. [DOI] [PubMed] [Google Scholar]
- 7.Wiseman H. Vitamin D is a membrane antioxidant. Ability to inhibit iron-dependent lipid peroxidation in liposomes compared to cholesterol, ergosterol and tamoxifen and relevance to anticancer action. FEBS Lett. 1993;326:285–288. doi: 10.1016/0014-5793(93)81809-e. [DOI] [PubMed] [Google Scholar]
- 8.Reddy GS, Tserng KY. Isolation and identification of 1,24,25-trihydroxyvitamin D2, 1,24,25,28-tetrahydroxyvitamin D2, and 1,24,25,26-tetrahydroxyvitamin D2: new metabolites of 1,25-dihydroxyvitamin D2 produced in rat kidney. Biochemistry. 1986;25:5328–5336. doi: 10.1021/bi00366a051. [DOI] [PubMed] [Google Scholar]
- 9.Shankar VN, Propp AE, Schroeder N, Surber BW, Makin HL, Jones G. In vitro metabolism of 19-nor-1alpha, 25-(OH)2D2 in cultured cell lines: inducible synthesis of lipid- and water-soluble metabolites. Arch Biochem Biophys. 2001;387:297–306. doi: 10.1006/abbi.2000.2239. [DOI] [PubMed] [Google Scholar]
- 10.Prosser DE, Jones G. Enzymes involved in the activation and inactivation of vitamin D. Trends Biochem Sci. 2004;29:664–673. doi: 10.1016/j.tibs.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 11.Sakaki T, Kagawa N, Yamamoto K, Inouye K. Metabolism of vitamin D3 by cytochromes P450. Front Biosci. 2005;10:119–134. doi: 10.2741/1514. [DOI] [PubMed] [Google Scholar]
- 12.Rao DS, Dayal R, Siu-Caldera ML, Horst RL, Uskokovic MR, Tserng KY, Reddy GS. Isolation and identification of 4,25-dihydroxyvitamin D2: a novel A-ring hydroxylated metabolite of vitamin D2. J Steroid Biochem Mol Biol. 1999;71:63–70. doi: 10.1016/s0960-0760(99)00125-9. [DOI] [PubMed] [Google Scholar]
- 13.Burstein S, Gut M. Intermediates in the conversion of cholesterol to pregnenolone: kinetics and mechanism. Steroids. 1976;28:115–131. doi: 10.1016/0039-128x(76)90131-8. [DOI] [PubMed] [Google Scholar]
- 14.Hume R, Kelly RW, Taylor PL, Boyd GS. The catalytic cycle of cytochrome P-450scc and intermediates in the conversion of cholesterol to pregnenolone. Eur J Biochem. 1984;140:583–591. doi: 10.1111/j.1432-1033.1984.tb08142.x. [DOI] [PubMed] [Google Scholar]
- 15.Tuckey RC. Progesterone synthesis by the human placenta. Placenta. 2005;26:273–281. doi: 10.1016/j.placenta.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 16.Guryev O, Carvalho RA, Usanov S, Gilep A, Estabrook RW. A pathway for the metabolism of vitamin D3: unique hydroxylated metabolites formed during catalysis with cytochrome P450scc (CYP11A1) Proc Natl Acad Sci USA. 2003;100:14754–14759. doi: 10.1073/pnas.2336107100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Slominski A, Semak I, Zjawiony J, Wortsman J, Li W, Szczesniewski A, Tuckey RC. The cytochrome P450scc system opens an alternate pathway of vitamin D3 metabolism. FEBS J. 2005;272:4080–4090. doi: 10.1111/j.1742-4658.2005.04819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Slominski A, Zjawiony J, Wortsman J, Semak I, Stewart J, Pisarchik A, Sweatman T, Marcos J, Dunbar C, Tuckey RC. A novel pathway for sequential transformation of 7-dehydrocholesterol and expression of the P450scc system in mammalian skin. Eur J Biochem. 2004;271:4178–4188. doi: 10.1111/j.1432-1033.2004.04356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Slominski A, Semak I, Zjawiony J, Wortsman J, Gandy MN, Li J, Zbytek B, Li W, Tuckey RC. Enzymatic metabolism of ergosterol by cytochrome P450scc to biologically active 17alpha,24-dihydroxyergosterol. Chem Biol. 2005;12:931–939. doi: 10.1016/j.chembiol.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 20.Tuckey RC, Cameron KJ. Human placental cholesterol side-chain cleavage: enzymatic synthesis of (22R)-20 alpha,22-dihydroxycholesterol. Steroids. 1993;58:230–233. doi: 10.1016/0039-128x(93)90024-h. [DOI] [PubMed] [Google Scholar]
- 21.Tuckey RC. Cholesterol side-chain cleavage by mitochondria from the human placenta. Studies using hydroxycholesterols as substrates. J Steroid Biochem Mol Biol. 1992;42:883–890. doi: 10.1016/0960-0760(92)90097-3. [DOI] [PubMed] [Google Scholar]
- 22.Toaff ME, Schleyer H, Strauss JF., 3rd Metabolism of 25-hydroxycholesterol by rat luteal mitochondria and dispersed cells. Endocrinology. 1982;111:1785–1790. doi: 10.1210/endo-111-6-1785. [DOI] [PubMed] [Google Scholar]
- 23.Gower DB. Modifiers of steroid-hormone metabolism: a review of their chemistry, biochemistry and clinical applications. J Steroid Biochem. 1974;5:501–523. doi: 10.1016/0022-4731(74)90051-x. [DOI] [PubMed] [Google Scholar]
- 24.Efimova T, Eckert RL. Regulation of human involucrin promoter activity by novel protein kinase C isoforms. J Biol Chem. 2000;275:1601–1607. doi: 10.1074/jbc.275.3.1601. [DOI] [PubMed] [Google Scholar]
- 25.Bikle DD, Ng D, Oda Y, Hanley K, Feingold K, Xie Z. The vitamin D response element of the involucrin gene mediates its regulation by 1,25-dihydroxyvitamin D3. J Invest Dermatol. 2002;119:1109–1113. doi: 10.1046/j.1523-1747.2002.19508.x. [DOI] [PubMed] [Google Scholar]
- 26.Bikle DD, Oda Y, Xie Z. Calcium and 1,25(OH)2D: interacting drivers of epidermal differentiation. J Steroid Biochem Mol Biol. 2004;89–90:355–360. doi: 10.1016/j.jsbmb.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 27.Hanley K, Wood L, Ng DC, He SS, Lau P, Moser A, Elias PM, Bikle DD, Williams ML, Feingold KR. Cholesterol sulfate stimulates involucrin transcription in keratinocytes by increasing Fra-1, Fra-2, and Jun D. J Lipid Res. 2001;42:390–398. [PubMed] [Google Scholar]
- 28.Reichrath J, Perez A, Muller SM, Chen TC, Kerber A, Bahmer FA, Holick MF. Topical calcitriol (1,25-dihydroxyvitamin D3) treatment of psoriasis: an immunohistological evaluation. Acta Derm Venereol. 1997;77:268–272. doi: 10.2340/0001555577268272. [DOI] [PubMed] [Google Scholar]
- 29.Shackleton CH, Roitman E, Kelley R. Neonatal urinary steroids in Smith–Lemli–Opitz syndrome associated with 7-dehydrocholesterol reductase deficiency. Steroids. 1999;64:481–490. doi: 10.1016/s0039-128x(99)00022-7. [DOI] [PubMed] [Google Scholar]
- 30.Tint GS, Irons M, Elias ER, Batta AK, Frieden R, Chen TS, Salen G. Defective cholesterol bio-synthesis associated with the Smith–Lemli–Opitz syndrome. N Engl J Med. 1994;330:107–113. doi: 10.1056/NEJM199401133300205. [DOI] [PubMed] [Google Scholar]
- 31.Hanukoglu I, Hanukoglu Z. Stoichiometry of mitochondrial cytochromes P-450, adrenodoxin and adrenodoxin reductase in adrenal cortex and corpus luteum. Implications for membrane organization and gene regulation. Eur J Biochem. 1986;157:27–31. doi: 10.1111/j.1432-1033.1986.tb09633.x. [DOI] [PubMed] [Google Scholar]
- 32.Tuckey RC, Kostadinovic Z, Cameron KJ. Cytochrome P-450scc activity and substrate supply in human placental trophoblasts. Mol Cell Endocrinol. 1994;105:103–109. doi: 10.1016/0303-7207(94)90041-8. [DOI] [PubMed] [Google Scholar]
- 33.Baulieu EE, Robel P. Neurosteroids: a new brain function? J Steroid Biochem Mol Biol. 1990;37:395–403. doi: 10.1016/0960-0760(90)90490-c. [DOI] [PubMed] [Google Scholar]
- 34.Cima I, Corazza N, Dick B, Fuhrer A, Herren S, Jakob S, Ayuni E, Mueller C, Brunner T. Intestinal epithelial cells synthesize glucocorticoids and regulate T cell activation. J Exp Med. 2004;200:1635–1646. doi: 10.1084/jem.20031958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dalla Valle L, Toffolo V, Vianello S, Belvedere P, Colombo L. Expression of cytochrome P450scc mRNA and protein in the rat kidney from birth to adulthood. J Steroid Biochem Mol Biol. 2004;88:79–89. doi: 10.1016/j.jsbmb.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 36.Tuckey RC, Stevenson PM. Properties of ferredoxin reductase and ferredoxin from the bovine corpus luteum. Int J Biochem. 1984;16:489–495. doi: 10.1016/0020-711x(84)90165-4. [DOI] [PubMed] [Google Scholar]
- 37.Tuckey RC, Stevenson PM. Properties of bovine luteal cytochrome P-450scc incorporated into artificial phospholipid vesicles. Int J Biochem. 1984;16:497–503. doi: 10.1016/0020-711x(84)90166-6. [DOI] [PubMed] [Google Scholar]
- 38.Woods ST, Sadleir J, Downs T, Triantopoulos T, Headlam MJ, Tuckey RC. Expression of catalytically active human cytochrome P450scc in Escherichia coli and mutagenesis of isoleucine-462. Arch Biochem Biophys. 1998;353:109–115. doi: 10.1006/abbi.1998.0621. [DOI] [PubMed] [Google Scholar]
- 39.Slominski A, Pisarchik A, Zbytek B, Tobin DJ, Kauser S, Wortsman J. Functional activity of serotoninergic and melatoninergic systems expressed in the skin. J Cell Physiol. 2003;196:144–153. doi: 10.1002/jcp.10287. [DOI] [PubMed] [Google Scholar]


