Abstract
Ecologists hold two views about the role of herbivory in ecosystem dynamics. First, from a food web perspective in population/community ecology, consumption by herbivores reduces plant abundance. Second, from a nutrient cycling perspective in ecosystem ecology, herbivory sometimes slows down cycling, which decreases plant abundance, but at other times speeds up cycling, which possibly increases plant abundance. The nutrient cycling perspective on herbivory has been experimentally addressed more thoroughly in aquatic systems than in terrestrial systems. We experimentally examined how grasshoppers influence nutrient cycling and, thereby, plant abundance and plant species composition over a period of 5 years. We examined how grasshoppers influence nutrient (nitrogen) cycling (i) by their excrement, (ii) by changing the abundance of and the decomposition rate of plant litter, and (iii) by both. Grasshoppers may speed up nitrogen cycling by changing the abundance and decomposition rate of plant litter, which increases total plant abundance (up to 32.9 g/m2 or 18%), especially, the abundance of plants that are better competitors when nitrogen is more available. However, whether grasshoppers enhance plant abundance depends on how much they consume. Consequently, ecosystems and food web perspectives are not mutually exclusive. Finally, under some conditions, grasshoppers may decrease nutrient cycling and plant abundance.
If other factors are not limiting plant production (e.g., water availability, temperature, and sunlight), plant abundance should decrease when nutrient cycling is slowed down and increase when nutrient cycling is speeded up. It has long been hypothesized that herbivory may affect the speed of nutrient cycling (1). Herbivores change nutrient cycling by deposition of excrement, by changing the quantity and quality (nutrient content and decomposition rate) of plant litter, and by sequestering nutrients in their bodies. However, because consumption reduces plant abundance, herbivory can increase plant abundance only if the enhancement of nutrient cycling exceeds the depressing effect of consumption.
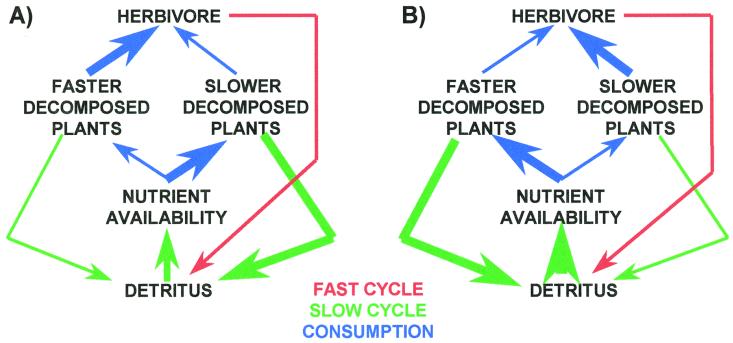
Herbivory's effects on nutrient cycling are summarized in Fig. 1. Nutrient release from excrement and dead herbivores has been termed the fast cycle (2), because this detritus rapidly decomposes and releases nutrients for plant uptake. Release of nutrients from plant litter has been termed the slow cycle (2), because this detritus slowly decomposes and releases nutrients for plant uptake. Herbivory affects the slow cycle by changing the quantity of plant litter and its quality, if herbivores preferentially feed on plants that differ in how rapidly their litter decomposes (3, 4). Preferential feeding on plants that produce slower decomposing litter reduces their relative abundance, speeding up the slow cycle, whereas preferential feeding on plants that produce faster decomposing litter slows down the slow cycle.
Figure 1.
Conditions for herbivores to modify nutrient cycling and NPP are presented as nutrient cycling slows down and NPP decreases (A) and nutrient cycling speeds up and NPP increases (B). Green lines, slow cycle; red lines, fast cycle; blue lines, consumption. Line thickness reflects the relative magnitude of consumption and nutrient cycling.
Shifts in proportion of nutrients released by fast versus slow cycles may change nutrient availability to plants, which may, in turn, modify plant production and species composition. If preferential feeding on slower decomposing plants overshadows deleterious effects of consumption on plants, it may accelerate nutrient cycling and increase plant production (3). However, preferential feeding on fast decomposing plants may decelerate nutrient cycling and decrease plant production (3). Nutrient cycling changes are one of several factors that affect plant species composition. For example, if slow decomposing plants are better competitors when nutrients are less available, and fast decomposing plants are better competitors when nutrients are more available, herbivore-induced changes in nutrient cycling may affect plant competition and, thereby, vegetation communities (3). Furthermore, changes in nutrient cycling, with resulting changes in plant species composition, may further accentuate changes in nutrient cycling, plant species composition, and plant production, thereby creating a self-enhancing or positive feedback.
Herbivory's role in nutrient cycling has been investigated experimentally in aquatic systems where both plants and herbivores are small-bodied and short-lived. In those environments, herbivory tends to accelerate nutrient cycling and increase plant production (5). In terrestrial systems, the role of mammalian herbivores in nutrient cycling has been studied most often, because mammals, with their large body sizes, consume large quantities of plants. Mammals may either decelerate nutrient cycling and diminish plant abundance (4–6) or accelerate cycling and increase plant abundance (2, 7, 8). These findings tend to be observational because of the difficulty in performing experiments on mammals with their large bodies and extensive home ranges. However, insect herbivores, because of their abundance and rapid turnover (short life span), may also strongly influence terrestrial nutrient cycling and may generally accelerate it (9, 10). Furthermore, because of their small size, short life span, and small home ranges, insect herbivores and their role in nutrient cycling can be studied experimentally.
For 5 years (1994–1999), we experimentally examined the role that grasshoppers play in nutrient cycling, plant production and plant species composition in an ecosystem where large mammalian herbivores are abundant, the Palouse prairie at the National Bison Range in Montana.
Methods
Study System.
Precipitation averages 350 mm/year, primarily falling as spring rain (May–June). A 4-ha (1 ha = 104 m2) flat site with homogeneous vegetation (plant biomass and species composition) at an elevation of ≈750 m was chosen. Three monocot species (Elymus smithii, Poa pratensis, and Poa compressa) composed more than 90% of plant biomass, with a variety of herbaceous dicots being the remainder. Net aboveground primary production (NPP) varied annually from 108 to 237 g (dry weight)/m2. NPP is limited in part by nitrogen (N) availability, because plant growth increased with addition of N fertilizer (11).
The grasshopper (Melanoplus sanguinipes) annually was 50–70% of all grasshoppers. Grasshopper hatchlings annually varied from 11 to 91 hatchlings per m2, and peak adult densities were from 4 to 36 adults per m2. Peak grasshopper biomass annually varied from 2 to 8 g/m2. During the 5-year study, grasshopper density increased and then declined. High grasshopper densities are typical for Palouse prairie (12, 13). At this site, mammalian herbivores, ranging in size from Bison bison (636 kg) to Microtus pennsylvanicus (3.5 × 10−2 kg), were abundant (≈2.5 g/m2), densities similar to those reported by early explorers of the Great Plains (5–9 g/m2) (14, 15). Nevertheless, grasshoppers annually consume 1.25–2.25 times more plant biomass than mammals at our study site, even if we ignore cut but unconsumed vegetation, which can be considerable.
Experiment.
In May 1994, we established replicated mesocosms of the site's ecosystem: 24 areas of 1 m2 (1 m × 1 m) and 3 areas of 9 m2 (3 m × 3 m) that were delineated by burying plastic edging 15 cm in the soil. For the first 4 years, we covered 18 of the 1-m2 areas with an insect screen so that grasshopper densities could be manipulated to assess how grasshopper density influences nutrient cycling, annual aboveground plant production (aboveground NPP − herbivore consumption), and plant species composition. We left 9 areas uncaged (6 areas of 1 m2 and 3 areas of 9 m2) to serve as controls. For the fifth year (May 1998–April 1999), we did not cover any areas with screen, allowing field grasshoppers to inhabit previously manipulated and control areas to assess whether observed density effects persisted even though density manipulations had ended. Finally, we examined aboveground plant production and nutrient cycling without grasshoppers in 10 cages of 0.36 m2 that were randomly placed over vegetation from May 1994 to October 1995 and again from May 1996 to October 1997 (5 cages with no grasshoppers and 5 cages with each year's field density; ref. 16).
We manipulated each 1-m2 caged mesocosm in one of three ways (six cages per way) from May 1994 to May 1998: (i) Grasshopper density was manipulated (50% or 125% of each year's field density: three replicates), with each mesocosm having its litter removed and replaced with litter produced in a 1-m2 unenclosed area (field grasshopper density), to examine fast cycle effects (variable consumption, but litter produced by constant consumption levels). (ii) Grasshopper density was kept constant (each year's field density), with each mesocosm having its litter removed and replaced with litter produced in mesocosms with different grasshopper densities (50% or 125% of each year's field density–litter produced in cages from way i above: three replicates) to examine slow cycle effects (constant consumption, but litter produced by different consumption levels). (iii) Grasshopper density was manipulated (50% or 125% of each year's field density: three replicates) with litter annually produced in situ clipped and placed back in the mesocosm (see below) to examine combined slow and fast cycles.
We defined litter as the aboveground portions of plants in October that become dormant over the winter and grow from stem bases in the following spring. We manipulated litter by clipping the aboveground portions at a height of 1.5 cm, removing them, weighing them, and then spreading them on the appropriate area. To keep clipped litter in each area from October to May when cages were removed, we staked netting (2.54-cm mesh) over areas. We manipulated litter in the six control mesocosms of 1 m2 as above, but we placed it back on each respective area. We did not manipulate litter in the three uncaged mesocosms of 9 m2.
We measured field densities of grasshoppers weekly in the 9-m2 control areas by counting grasshoppers in 0.1-m2 rings (eight rings per control area) at times of about 1100 and 1600 (17). We stocked the 1-m2 caged mesocosms with M. sanguinipes nymphs (≤3rd instar) in mid-June to 50%, 100%, or 125% of current field density. We counted grasshoppers in each caged mesocosm weekly in three 0.05-m2 rings at about 1100 and 1600. We added or removed M. sanguinipes individuals weekly from cages to maintain experimental densities relative to the field. We added individuals of the current most common developmental stage and removed individuals by killing and leaving them in each cage, which had no significant impact on N availability (16).
We made the following measurements each year in each of the 27 mesocosms, and we made measurements 1 and 7 in the 10 0.36-m2 mesocosms (16).
Measurement 1.
An index of N availability (NH4, NO2, and NO3) to plants was provided by ion-exchange resin (Rexyn, Fisher Scientific) bags (18, 19). A bag was buried (15-cm depth) in May and removed in October to reflect N availability during the plant-growing season, and another bag was buried in October and removed in May for N availability at initiation of spring plant growth. N absorbed in resin bags was correlated with N mineralization (NH4, NO2, and NO3) measured by soil cores and in situ incubation tubes (ref. 20; r2 = 0.47, df = 10, P < 0.01). Samples were kept frozen until analyzed.
Measurement 2.
Soil percent water content [(1 − dry weight in g/wet weight in g) × 100%] was measured with soil cores (3 cm in diameter × 15 cm deep) taken in May and October. This measure does not reflect absolute water availability to plants but does reflect whether soil moisture was greater in mesocosms receiving one type of treatment compared with mesocosms receiving other treatments.
Measurement 3.
Plant N content for the most common plant species (E. smithii, P. pratensis, and P. compressa) was measured in early June (time of peak biomass) from a 5-g dry sample (<2% of plant biomass).
Measurement 4.
Litter N content was measured in October (time of onset of dormancy and litter manipulation) from a 5-g dry sample (<2% of litter biomass).
Measurement 5.
Decomposition rates (percent change in dry matter and total N per period) were measured for common sources (field) of the two most abundant grasses (P. pratensis and E. smithii). Four nylon mesh (1-mm mesh) bags of each plant species (10 g collected in May) were placed on soil in May and collected in October and May over the next 2 years.
Measurement 6.
Plant species composition and proportion of bare ground were measured in July by point-sampling (21) 100 points along four transects.
Measurement 7.
Living-plant aboveground (green) biomass was measured by radiometer every 2 weeks from May to October. Radiometers measure the ratio of far-red/infrared reflected radiation, which is used to estimate living-plant biomass (22, 23) by regressions, which are based on clipped living-plant biomass and radiometer readings for 10 areas of 0.10 m2 in early May and 4 areas of 0.1 m2 every 2 weeks until October. Annual aboveground NPP, less herbivore consumption, equals May plant biomass plus all positive differences between consecutive biweekly measures from May to October. Radiometer measures (0.1 m2) were made at three permanent locations in each 1-m2 and 0.36-m2 area and at four permanent locations in each 9-m2 area.
Measurement 8.
Grasshopper feeding on each of the most common grasses (P. pratensis, P. compressa, and E. smithii) was measured as the percentage of 25 blades exhibiting damage.
Plant matter was dried at 60°C for 48 h, and soil was dried at 100°C for 48 h. Plant and litter N measurements were made by extracting N by using micro-Kjeldahl methods (28). Inorganic N in the soil and resin bags was extracted with 2 M KCl (24, 25) after samples were thawed. The extractant N [g/g (dry weight) of soil, plant, or litter] was assessed colorimetrically with an autoanalyzer (24, 26–28).
We expressed measurements from manipulated mesocosms as percent change relative to control areas since the start of the experiment (May 1994):
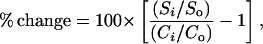 |
where Si is the measure in year i from mesocosms, So is the measure from those mesocosms in 1994 (start of experiment), Ci is the average measure in year i for 1-m2 control areas, and Co is the average measure in 1994 for 1-m2 control areas. Percent change was normalized by logit transformation for statistical tests [analysis of variance (ANOVA), analysis of covariance (ANCOVA), and t tests].
We compared measurements from 1-m2 control mesocosms with those from 9-m2 areas to determine whether litter manipulation or mesocosm area created differences. We compared measurements from 1-m2 control mesocosms with those from 0.36-m2 mesocosms containing each year's field density to examine for cage effects.
Results
Control mesocosms (1 m2), where litter was clipped, did not differ from 9 m2 control areas for N availability (ANOVA: F = 0.03; df = 1, 28; P < 0.86), annual aboveground NPP less herbivory (ANOVA: F = 0.21; df = 1, 39; P < 0.64), and plant species composition (χ2 = 0.14, df = 1, P < 0.85). This indicated that mesocosm size and litter manipulation methods did not account for differences between mesocosms where grasshopper density and litter were manipulated. Control mesocosms (1 m2) did not differ from 0.36-m2 caged areas that contained each year's grasshopper density (1994, 1996) for N availability (ANOVA: F = 0.25; df = 1, 15; P < 0.63) and annual aboveground NPP less herbivory (ANOVA: F = 0.59; df = 1, 18; P < 0.45). This indicated that there were no cage effects on ecosystem processes. Therefore, we conclude that mesocosms reflect processes in the larger ecosystem.
Next year's aboveground NPP less consumption increases at a decreasing rate because the previous year's grasshopper density increases from 0% to 125% of field density (Fig. 2A). N availability increases in a similar manner (r2 = 0.68, df = 4, P < 0.04) and changes in N correlate with changes in aboveground NPP less consumption (Fig. 2B). Because N additions increase NPP at this site (11), grasshopper herbivory appears to increase aboveground NPP after consumption by increasing available N for plant growth. Over the 5 years of the study, herbivory increased aboveground NPP by 19.9–32.9 g (dry weight) per m2 per year.
Figure 2.
Impacts in 1995 (red) and 1996 (green) of grasshopper density (0%, 50%, and 125% of year's field density) on percent change in aboveground NPP less herbivory (A) and its relationship to percent change in N availability (B).
We examined how grasshoppers increase NPP by using the 1-m2 mesocosm experiments as follows:
Experiment 1: Fast Cycle Experiment (Varied Grasshopper Density/ Control Litter).
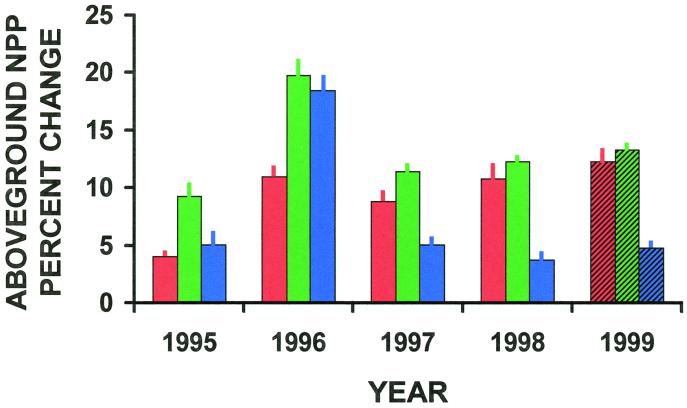
Aboveground NPP less consumption was 4–12% greater when grasshoppers were at 125% versus 50% of field density (Fig. 3; ANOVA: F = 6.8; df = 1, 26; P < 0.01). This is expected if the fast cycle's magnitude is increased when more grass is produced at higher densities, which makes more N available to plants.
Figure 3.
Percent increase (mean ± SEM) in aboveground NPP with increased (125%) compared with decreased (50%) grasshopper density for fast (red), slow (green), and combined fast and slow cycle (blue) effects. Cross-hatching denotes the last year when mesocosms were opened to field densities of grasshoppers.
Experiment 2: Slow Cycle Experiment (Control Grasshopper Density/ Varied Litter).
Aboveground NPP less consumption was 9–20% greater with litter from mesocosms with 125% versus 50% field density (Fig. 3; ANOVA: F = 21.71; df = 1, 26; P < 0.0001). This is expected if grasshoppers speed up the slow cycle, releasing more N by decomposition of litter.
Experiment 3: Combined Fast and Slow Cycle Experiment (Varied Grasshopper Density/in Situ Litter).
Aboveground NPP less consumption was 4–18% greater when grasshoppers were at 125% versus 50% of field density (Fig. 3; ANOVA: F = 4.39; df = 1, 26; P < 0.04). This is expected given that both fast and slow cycles increase NPP. However, when combined effects were correlated with associated fast (experiment 1) and slow cycle (experiment 2) effects, the fast cycle was found to diminish the slow cycle, and the slow cycle was twice as important as the fast cycle (r2 = 0.99, df = 3, P < 0.003). Antagonism between fast and slow cycles is expected, because greater consumption (increased fast cycle effects) reduces litter abundance, which diminishes the magnitude of the slow cycle. Our result that NPP enhancement is greatest when grasshopper density is neither too great nor too small (Fig. 4) supports this “tradeoff” between fast and slow cycle effects.
Figure 4.
A regression relating changes in aboveground NPP with grasshopper density is presented.
As expected, N availability to plants is greater when grasshoppers were at 125% versus 50% of field density (Fig. 5: t = 3.10, df = 6, P < 0.02), and we suggest that this increases NPP because N additions increase NPP at this site (11). Grasshoppers influence the more important slow cycle by changing the amount of litter and its N content. As consumption increases (125% versus 50% field density), litter abundance decreases (Fig. 5: t = 2.9, df = 6, P < 0.04), which reduces the amount of N contained in the slow cycle. However, as consumption increases, litter N content increases (Fig. 5: t = 4.69, df = 6, P < 0.009), which increases the amount of N contained in the slow cycle and its rate of cycling. Slow cycle speed increases because decomposition is more rapid as litter N content increases (r2 = 0.91, df = 4, P < 0.04). For example, E. smithii decomposed 11% slower than P. pratensis (t = 5.10, df = 2, P < 0.03), and E. smithii contained 28% less N than P. pratensis (t = 13.76, df = 8, P < 0.0001). Increased litter N content, in part, is due to increases in relative abundance of grasses with greater N content (P. pratensis and P. compressa versus E. smithii; Fig. 5: t = 48.18, df = 6, P < 0.0005), which is partially due to preferential feeding on E. smithii (χ2 = 9.45, df = 1, P < 0.002).
Figure 5.
Average percent changes (±SEM) in ecosystem characteristics with decreased (red) and increased (green) grasshopper densities.
Increased grasshopper density can counter the slow cycle in two ways. (i) Less litter might increase soil evaporation so that plants become water-limited, but soil moisture was not diminished in our experiments (Fig. 5: t = 0.55, df = 14, P < 0.70). (ii) Less litter and higher litter and soil N might make the soil microbes that are responsible for decomposition carbon-limited rather than N-limited, and thereby, decrease decomposition rates (29, 30). However, decomposition rates for common litter sources did not change with grasshopper density in our experiments (Fig. 5: t = 0.59, df = 4, P < 0.62). Therefore, countermechanisms to herbivory-enhancing slow-cycle effects and aboveground NPP were not observed.
All of the above fast, slow, and combined cycle effects were maintained in the last year (e.g., NPP in 1999; Fig. 3) when mesocosms were not caged, so that all areas were open to field grasshopper densities (for fast cycle, ANOVA: F = 0.2; df = 1, 26; P < 0.66; for slow cycle, ANOVA: F = 0.57; df = 1, 26; P < 0.46; for combined cycles, ANOVA: F = 3.15; df = 1, 26; P < 0.09). This suggests long-lasting influences of grasshoppers on nutrient cycling and NPP.
Discussion
Grasshopper herbivory appears to affect the Palouse prairie in the manner depicted in Fig. 1B, because N availability for plants and plant abundance increased with grasshopper density. Plant abundance probably increased because of greater N availability, because N additions are known to increase NPP at this site (11). In this scenario (Fig. 1B), N availability is primarily enhanced when the herbivore speeds up the slow cycle, which requires the herbivore to feed preferentially on plants of lower N content that decompose more slowly, as observed for E. smithii and P. pratensis.
The above ecosystem changes (Fig. 1B) may be self-enhancing (positive feedback) and no longer dependent on herbivory, if plants with higher N content and decomposition rates are competitively superior to other plants once N is more available. P. pratensis is competitively superior to E. smithii at higher N availability (31, 32), and Poa increased relative to E. smithii (Fig. 5). Poa's increase is not solely caused by preferential feeding by grasshoppers on E. smithii, because Poa increased when grasshopper consumption was held constant and litter was manipulated (slow cycle experiment 1; t = 5.42, df = 4, P < 0.005). Self-enhancement was further supported in 1999 when mesocosms were not caged and grasshoppers had equal access to all areas, because increased plant abundance and N availability were maintained in areas previously experiencing higher grasshopper densities (Fig. 2).
The observed increase in plant abundance is not due to herbivores benefiting consumed plants in a mutualistic fashion (33, 34), because consumed plants do not grow or survive as well as unconsumed plants (16). Rather, consumed and unconsumed plants grow better when herbivory makes more nitrogen available, an ecosystem function. Thus, herbivory can increase plant abundance if neither too little nor too much is consumed (Fig. 3). If too little is consumed, herbivory will have too little influence on nutrient cycling to increase plant production. If too much is consumed, herbivory will depress plant growth and survival more than can be compensated for by greater growth as nutrient cycling increases.
In conclusion, our results indicate that ecosystem processes (nutrient cycling), functions (NPP) and structure (plant composition) are interrelated and strongly influenced by biotic interactions (35–37). We cannot assess how typical our observations are for terrestrial systems. Other grasslands have lower grasshopper densities (38, 39) and nutrient cycling that depends more on fire (40). Mammalian herbivory is not equivalent to grasshopper herbivory, because mammals, especially ungulates, uproot and trample plants and compact soil, which can decrease nutrient cycling and NPP (41). However, generalizing about herbivory's ecosystem effects depends on whether herbivores preferentially feed on slower decomposing plants. Grasshoppers within 4 km of our study site fed preferentially on P. pratensis over E. smithii. P. pratensis decomposed faster and had higher N content, providing conditions for herbivory to diminish nutrient cycling and NPP (Fig. 1A). Consequently, ecosystem processes may vary because of biotic influences at smaller spatial scales than typically considered.
Acknowledgments
D. Branson and J. Chase aided in establishing the long-term experiments. We thank The Utah State Agricultural Experiment Station and The National Science Foundation (DEB-9317984, DEB-9707654) for financial support.
Abbreviation
- NPP
net primary production
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.250483797.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.250483797
References
- 1.Hutchinson G E, Deevey E S. Biol Prog. 1949;1:325–359. [Google Scholar]
- 2.McNaughton S J, Ruess R W, Seagle S W. BioScience. 1988;38:794–800. [Google Scholar]
- 3.Pastor J, Naiman R J. Am Nat. 1992;139:690–705. [Google Scholar]
- 4.Pastor J, Naiman R J, Dewey B, McInnes P. BioScience. 1988;38:770–777. [Google Scholar]
- 5.DeAngelis D L. Dynamics of Nutrient Cycling and Food Webs. London: Chapman & Hall; 1992. [Google Scholar]
- 6.Pastor J, Dewey B, Naiman R J, McInnes P F, Cohen Y. Ecology. 1993;74:467–480. [Google Scholar]
- 7.Frank D A, McNaughton S J. Oecologia. 1993;96:157–161. doi: 10.1007/BF00317727. [DOI] [PubMed] [Google Scholar]
- 8.Holland E A, Parton W J, Detling J K, Coppock D L. Am Nat. 1992;140:685–706. doi: 10.1086/285435. [DOI] [PubMed] [Google Scholar]
- 9.Seastedt T R, Crossley D A. BioScience. 1984;34:157–161. [Google Scholar]
- 10.Lovett G M, Ruesink A E. Oecologia. 1996;104:133–138. doi: 10.1007/BF00328577. [DOI] [PubMed] [Google Scholar]
- 11.Schmitz O J. Oecologia. 1993;93:327–335. doi: 10.1007/BF00317874. [DOI] [PubMed] [Google Scholar]
- 12.Shelford V E. The Ecology of North America. Urbana: Univ. Illinois Press; 1963. [Google Scholar]
- 13.Sheldon J K, Rogers L E. Oecologia. 1978;32:85–92. doi: 10.1007/BF00344692. [DOI] [PubMed] [Google Scholar]
- 14.Watts L R, Stewart G, Connaughton C, Palmer L J, Talbot M W. US Senate Doc. 1936;199:501–522. [Google Scholar]
- 15.French N R, Steinhorst R K, Swift D M. In: Perspectives in Grassland Ecology. French N, editor. New York: Springer; 1979. pp. 59–87. [Google Scholar]
- 16.Belovsky G E. In: Grasshoppers and Grassland Health. Lockwood J A, Latchininsky A V, Sergeev M G, editors. Dordrecht, The Netherlands: Kluwer; 2000. pp. 7–29. [Google Scholar]
- 17.Onsager J A, Henry J E. Acridida. 1977;6:231–237. [Google Scholar]
- 18.Binkley D, Matson P. Soil Sci Soc Am J. 1983;47:1050–1052. [Google Scholar]
- 19.Binkley D. Soil Sci Soc Am J. 1984;48:1181–1184. [Google Scholar]
- 20.Binkley D, Hart S C. Adv Soil Sci. 1989;10:57–112. [Google Scholar]
- 21.Daubenmire R F. Plants and Environment. New York: Wiley; 1947. [Google Scholar]
- 22.Pearson R L, Miller L D, Tucker C J. Applied Optics. 1976;15:416–418. doi: 10.1364/AO.15.000416. [DOI] [PubMed] [Google Scholar]
- 23.Milton E J. Int J Remote Sensing. 1987;8:1807–1827. [Google Scholar]
- 24.Page A L, Miller R H, Keeney D R. Methods of Soil Analysis. Madison, WI: Am. Soc. Agron. Soil Sci. Soc. Am.; 1982. , Part 2. [Google Scholar]
- 25.Adamsen F J, Bigelow D S, Scott G R. Com Soil Sci Plant Anal. 1985;16:883–898. [Google Scholar]
- 26.Adamski J M. Anal Chem. 1976;48:1194–1197. doi: 10.1021/ac50002a033. [DOI] [PubMed] [Google Scholar]
- 27.Nelson D W, Sommers L E. J Assoc Off Agric Chem. 1980;63:770–778. [Google Scholar]
- 28.Association of Official Agricultural Chemists. Official Methods of Analysis. Washington, DC: Assoc. Off. Agric. Chem.; 1984. [Google Scholar]
- 29.Seastadt T R. In: The Changing Prairie: North American Grasslands. Joern A, Keeler K H, editors. New York: Oxford Univ. Press; 1995. pp. 157–174. [Google Scholar]
- 30.Zheng D W, Ågren G I, Bengtsson J. Oikos. 1999;86:430–442. [Google Scholar]
- 31.Smika D E, Hass H J, Power J F. Agron J. 1965;57:483–486. [Google Scholar]
- 32.Tilman D. Plant Strategies and the Dynamics and Structure of Plant Communities. Princeton: Princeton Univ. Press; 1988. [Google Scholar]
- 33.Dyer M, Bokhari U G. Ecology. 1976;57:762–772. [Google Scholar]
- 34.Owen D F, Wiegert R G. Oikos. 1982;38:258–259. [Google Scholar]
- 35.Tilman D. In: Nature's Services: Societal Dependence on Natural Ecosystems. Daly G C, editor. Washington, DC: Island; 1997. pp. 685–700. [Google Scholar]
- 36.Chapin F S, Walker B H, Hobbs R J, Hooper D U, Lawton J H, Sala O E, Tilman D. Science. 1997;277:500–504. [Google Scholar]
- 37.Hooper D U, Vitousek P M. Science. 1997;277:1302–1305. [Google Scholar]
- 38.Evans E W. Ecology. 1989;70:435–444. [Google Scholar]
- 39.Mitchell J E, Pfadt R E. Environ Entomol. 1974;3:358–360. [Google Scholar]
- 40.Bragg T B. In: The Changing Prairie: North American Grasslands. Joern A, Keeler K H, editors. New York: Oxford Univ. Press; 1995. pp. 49–81. [Google Scholar]
- 41.Wallace L L, Dyer M I. In: The Changing Prairie: North American Grasslands. Joern A, Keeler K H, editors. New York: Oxford Univ. Press; 1995. pp. 177–198. [Google Scholar]