Abstract
It is well known that herbivores can induce chemical defenses in terrestrial vascular plants, but few examples of inducible production of defense chemicals have been reported for aquatic macrophytes. Furthermore, it is well established that water-borne chemical cues from predators or predator-wounded conspecifics can induce defensive changes of aquatic prey animals, but no such communication between aquatic herbivores and seaweeds has been reported. Here we show that water-borne cues from actively feeding herbivorous gastropods, flat periwinkles (Littorina obtusata), can serve as external signals to induce production of defense chemicals (phlorotannins) in unharmed individuals of seaweeds, knotted wrack (Ascophyllum nodosum), and that the increased levels of defense chemicals deter further feeding by periwinkles. Because seaweeds have poorly developed internal-transport systems and may not be able to elicit systemic-induced chemical defenses through conveyance of internal signals, this mechanism ensures that seaweeds can anticipate future periwinkle attacks without receiving direct damage by herbivores.
The evolution of inducible, as opposed to constitutive, defenses is favored when the risk of attack from predators is unpredictable, when the production of defenses involves fitness costs, and when reliable environmental cues, e.g., direct visual or physical contact or air- and water-borne substances, are available (1, 2). Research on the induction of defensive changes in behavior, life history, and morphology in response to water-borne cues has a long tradition for aquatic animals (2–4). Furthermore, phytoplankton can sense and respond to water-borne chemical cues from herbivorous zooplankton by adjusting their phenotypes (5). The induction of chemical defenses in terrestrial plants subjected to direct grazing is also well documented (1) and can occur in response to airborne chemical cues (6, 7), although this phenomenon is a subject of debate (8, 9). In contrast, there are only three examples of herbivore-induced production of chemical defenses in seaweeds (10–12), all of which have focused on the impact of direct grazing. Here we report on water-borne chemical cues inducing chemical defense in a seaweed species, the knotted wrack (Ascophyllum nodosum).
Knotted wrack is a common brown seaweed species that may form dense monospecific stands in the intertidal zone of sheltered North Atlantic rocky shores. An individual consists of several genetically identical primary shoots that arise from a common holdfast and branch into secondary shoots as the individual grows. Knotted wrack can be inhabited and grazed on by flat periwinkles (Littorina obtusata) that are specialized to live and feed on knotted wrack and a few other brown seaweed species (13). The abundance of flat periwinkles in the study area is highly variable within and among sites (ref. 12; H.P., unpublished data). Partitioning of the variation of the data from a field survey showed that there were highly significant (P < 0.001) differences in periwinkle abundance among sites, but 75% of the total variation was caused by differences among individual plants within sites. Periwinkles inhabited 16% of the monitored plants (H.P., unpublished data). Furthermore, flat periwinkles feed slowly compared with many larger marine herbivores such as fishes and sea urchins, and they are relatively sedentary and probably only move among seaweed individuals growing within a distance of a few meters (13, 14). Given these observations, one can assume that the probability of periwinkle attack for a particular seaweed individual is unpredictable but increases if neighboring individuals are under attack. Because grazing damage made by periwinkles may increase the mortality risk of knotted wrack (12), individuals that are able to assess and respond to water-borne signals that indicate an increase in herbivory risk may have a selective advantage.
Brown seaweeds contain variable levels of secondary polyphenolic metabolites, called phlorotannins (15–17), that may deter feeding by herbivorous gastropods (12, 18). The production of phlorotannins in knotted wrack is negatively correlated with seaweed growth rate (17). Direct grazing by periwinkles induces significant increases of phlorotannin levels in knotted wrack, whereas grazing by isopods (Idotea granulosa) and artificial damage do not (12), indicating that herbivore-specific cues are involved in the elicitation of the response. This observation led us to test the hypothesis that water-borne chemical cues from periwinkles actively feeding on knotted wrack will induce a higher phlorotannin content in unharmed neighboring seaweeds.
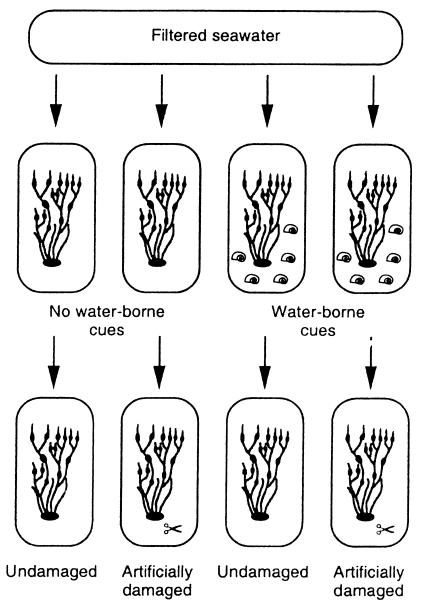
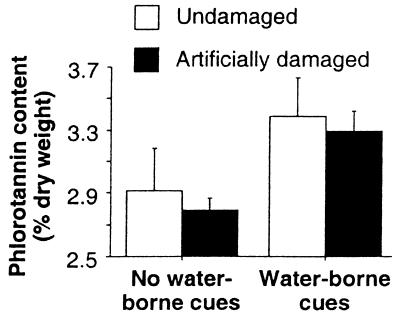
An induction experiment was conducted in a flow-through system where water from aquariums with knotted wrack and adult periwinkles was transferred to aquariums with equally sized artificially wounded or undamaged seaweeds. Knotted wrack and adult (>1 cm) flat periwinkles were collected from their natural habitats in the intertidal zone outside Kristineberg Marine Research Station on the Swedish west coast in July 1999. Then, 40 knotted wrack individuals were placed individually in 2-liter glass aquariums connected to a flow-through system (1 liter⋅min−1) with filtered (100, 10, and 1 μm) seawater. In natural populations, knotted wrack individuals are shaded by neighboring plants, and to expose the plants to a natural daily rhythm of light but not to direct sunlight, the aquariums were placed in the shade outside the laboratory. The time frame of the experiment (20 days) and the density of periwinkles (five individuals per plant) were chosen according to observed feeding activity and maximum abundance of periwinkles in natural knotted wrack populations (unpublished observations). Periwinkles were added to 10 of the aquariums, and water from these and 10 more aquariums containing only seaweed was transferred to aquariums containing either artificially damaged (with a hole-punch) or undamaged seaweeds (Fig. 1). When the experiment was terminated, 10 apical shoots were collected and pooled from each experimental individual in “receiving” aquariums. Phlorotannins were measured by using the Folin–Ciocalteus method (19) for quantification of total phenolics. Phloroglucinol (Chemical Abstract Service no. 6099-90-7) was used as a standard. Data were statistically analyzed by using a two-way analysis of variance with water-borne cues (two levels) and artificial damage (two levels) as fixed orthogonal factors. The mean phlorotannin content in knotted wrack exposed to water from aquariums containing periwinkles actively feeding on seaweeds increased by a significant 15% compared with plants receiving water from aquariums with unharmed seaweed individuals (Fig. 2). Knotted wrack responded to cues irrespective of initial mechanical damage, because induction occurred in both undamaged and artificially damaged plants (Fig. 2). To our knowledge, these are the first published results demonstrating that seaweeds can respond to water-borne cues by increasing levels of secondary metabolites.
Figure 1.
Experimental set-up in the induction experiment. Filtered seawater was transferred through aquariums with knotted wrack only (no water-borne cues) or with knotted wrack and flat periwinkles (water-borne cues) to aquariums containing either undamaged or artificially damaged knotted wrack individuals. Each treatment was replicated five times.
Figure 2.
Phlorotannin content (percentage of dry weight) in undamaged and artificially damaged knotted wrack receiving water from aquariums either with unharmed seaweed individuals (no water-borne cues) or with conspecifics being grazed on by flat periwinkles (water-borne cues). Irrespective of initial damage, water-borne cues induced significantly (two-way analysis of variance, F1,16 = 6.26, P = 0.02) higher levels of chemical defense in knotted wrack. Error bars show SEM (n = 5).
For increases in levels of secondary metabolites to function as effective chemical defenses, increased resistance to further grazing must also be demonstrated (1, 2). Therefore, we tested the hypothesis that induced changes in secondary chemistry (phlorotannins or other compounds) in knotted wrack individuals receiving water-borne cues in the present study will decrease the palatability of the seaweeds to periwinkles. We performed a bioassay whereby flat periwinkles were allowed to choose between artificial diets containing seaweed material from the induction experiment. Two types of artificial diets were produced by pooling all seaweed material collected from experimental individuals that had received water from aquariums with and without water-borne cues. The seaweed material was freeze-dried, ground to a fine powder, and molded into agar discs (1.5% wet-weight agar, 5.6% wet-weight seaweed material). New adult flat periwinkles were placed in 0.2-liter glass aquariums (three periwinkles per aquarium) together with one piece (2 g wet-weight) each of both types of artificial diets. Controls without herbivores were also prepared to measure autogenic weight changes in agar discs during the experiment. Each treatment (periwinkles and controls) was replicated 20 times. The wet-weight of agar discs was determined at the start and at the end of the 6-day experiment by using a standard blotting procedure, and the wet-weight change (WWC) of each agar disk was calculated by subtracting the stop weight from the start weight. To study feeding preference of periwinkles, the difference (D) between weight changes of the two agar discs in each aquarium was calculated as:
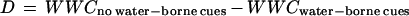 |
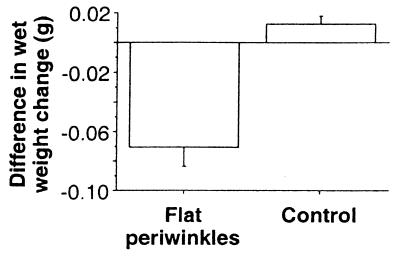
(20) and used as a dependent variable in a one-way analysis of variance with herbivore (two levels) as a fixed factor. Periwinkles were significantly deterred by artificial food containing knotted wrack that had received water-borne cues from aquariums with other periwinkles actively feeding on seaweeds, as indicated by the significantly lower difference in wet-weight changes between agar discs grazed on by periwinkles compared with control agar discs without herbivores (Fig. 3). Because any morphological differences between seaweeds from the induction experiment were eliminated in the artificial diets, this deterrence must be caused by differences in chemistry, although we cannot discern whether deterrence was caused by phlorotannins or some other unknown change in chemical content.
Figure 3.
Difference in wet-weight change (g) between artificial food containing seaweed material from knotted wrack that received water with and without water-borne cues in the induction experiment. Periwinkles were deterred by artificial food containing seaweed material from knotted wrack receiving water-borne cues, as indicated by the significantly (one-way analysis of variance, F1,38 = 35.59, P < 0.0001) lower difference in wet-weight change between the two artificial food types offered to periwinkles compared with wet-weight change between controls without herbivores. Error bars show SEM (n = 20).
The increase in phlorotannin content of knotted wrack subjected to water-borne cues could be caused either by increased production of phlorotannins or by absorption and accumulation of phlorotannins released from wounded conspecifics. Release of phlorotannins from brown seaweeds can be increased in response to stresses such as desiccation (21) or mechanical damage (22). To investigate whether knotted wrack releases phlorotannins in response to herbivore damage, we performed an experiment to measure the total phenolic concentration in filtered seawater from aquariums in which knotted wrack individuals were subjected to herbivory by flat periwinkles. Knotted wrack and flat periwinkles were collected outside Tjärnö Marine Biological Laboratory on the Swedish west coast in October 1999. The experimental set-up was as described in the induction experiment (Fig. 1) except that no seaweeds receiving water-borne cues were used. Empty aquariums with only seawater were used as controls of background levels of total phenolics. After 7 days, the seawater supply was turned off, and phlorotannins were allowed to accumulate for 6 h (22), after which water samples (50 ml) were collected from each aquarium. The water samples were concentrated with ultrafiltration [molecular weight > 1,000; the majority of knotted wrack phlorotannins have a molecular weight of 10,000 (15)], and the total phenolic concentration was measured by using the Folin–Ciocalteus method (19). Data were analyzed statistically by using a one-way analysis of variance with treatment (three levels) as a fixed factor. Herbivory did not increase the release of phlorotannins, because there were no significant differences between water samples from different aquariums (one-way analysis of variance, F2,6 = 0.17, P = 0.85). The phenolic concentration in aquariums containing knotted wrack, knotted wrack and periwinkles, and plain seawater was 0.812 ± 0.070, 0.833 ± 0.195, and 0.925 ± 0.149 mg⋅liter−1, respectively (means ± SEM). Given these results, we conclude that the increase in phlorotannin content in knotted wrack discovered in the induction experiment (Fig. 2) was a result of an increased phlorotannin production and not only an accumulation of phlorotannins released from plants under attack.
The notion of a mechanism that allows plants to “communicate” by means of airborne chemical compounds has been criticized because of the fact that there can be no selective advantage to plants that warn their neighbors of imminent danger (1). However, selection should favor the evolution of inducible production of secondary metabolites that may function as defenses when reliable cues for pathogen detection are present (1, 2, 23), and chemical compounds released by herbivores or wounded plants may serve as such cues (1). Furthermore, induced chemical defenses may be either localized at the site of a wound or systemic throughout the seaweed (24). Induced systemic responses are common in terrestrial vascular plants (1) in response to internal (25, 26) and external (6, 7, 27) signaling cues, and systemically released signals have been found in communication between plants and predators in tritrophic interactions (28, 29). Unlike vascular plants, most seaweed species (except kelps and siphonous green seaweeds) have poorly developed conducting tissues and cannot easily transport chemical compounds long distances within a genetic individual (30). Therefore, most seaweeds cannot use internal signaling efficiently to induce systemic defenses in response to damage, and the selection pressure to evolve mechanisms to respond to external chemical cues is probably greater than for plants with more developed internal transport systems.
We have shown that knotted wrack has evolved a mechanism to sense and respond to the presence of actively feeding flat periwinkles through water-borne cues in the laboratory, although we do not presently know the exact origin of the cue (seaweed or periwinkle) that elicited the induced response. This mechanism may be important for both undamaged seaweed individuals growing nearby an attacked conspecific and for attacked individuals eliciting induced systemic defenses to minimize further herbivory. However, rigorous experimental field investigations are needed to reveal whether this mechanism is present also in knotted wrack individuals in natural populations. We have also shown that flat periwinkles are sensitive to induced changes in the chemistry of their food. Because they are relatively sedentary herbivores, induced systemic chemical defense in knotted wrack is probably effective against this herbivore species, even if it takes days or weeks to be manifested. In conclusion, we propose that external chemical cues may be common elicitors of induced systemic plant defenses in interactions between nonvascular plants and sedentary, slowly feeding herbivores.
Acknowledgments
We are grateful to all staff and students at Kristineberg Marine Research Station and Tjärnö Marine Biological Laboratory for their helpfulness and hospitality. K. Johannesson, P. Nilsson, L. Svärdh, P. Åberg, and three anonymous reviewers provided valuable comments on earlier drafts of the manuscript. The research foundations of Adlerbertska, Ernst Colliander, Lars Hierta, and Carl Stenholm supported this study. H.P. was supported by the Foundation for Strategic Research through the Marine Science and Technology program.
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.250226997.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.250226997
References
- 1.Karban R, Baldwin I T. Induced Responses to Herbivory. Chicago: Univ. of Chicago Press; 1997. [Google Scholar]
- 2.Tollrian R, Harvell C D, editors. The Ecology and Evolution of Inducible Defenses. Princeton: Princeton Univ. Press; 1999. [Google Scholar]
- 3.Chivers D P, Smith R J F. Ecoscience. 1998;5:338–352. [Google Scholar]
- 4.Kats L B, Dill M. Ecoscience. 1998;5:361–394. [Google Scholar]
- 5.Van Donk E, Lürling M, Lampert W. In: The Ecology and Evolution of Inducible Defenses. Tollrian R, Harvell C D, editors. Princeton: Princeton Univ. Press; 1999. pp. 89–103. [Google Scholar]
- 6.Bruin J, Sabelis M W, Dicke M. Trends Ecol Evol. 1995;10:167–170. doi: 10.1016/s0169-5347(00)89033-3. [DOI] [PubMed] [Google Scholar]
- 7.Shonle I, Bergelson J. Ecology. 1995;76:2660–2663. [Google Scholar]
- 8.Fowler S V, Lawton J H. Am Nat. 1985;126:181–195. [Google Scholar]
- 9.Firn R D, Jones C G. Trends Ecol Evol. 1995;10:371. doi: 10.1016/s0169-5347(00)89136-3. [DOI] [PubMed] [Google Scholar]
- 10.Van Alstyne K L. Ecology. 1988;69:655–663. [Google Scholar]
- 11.Cronin G, Hay M E. Ecology. 1996;77:2287–2301. [Google Scholar]
- 12.Pavia H, Toth G. Ecology. 2000;81:3212–3225. [Google Scholar]
- 13.Williams G A. Field Studies. 1990;7:469–482. [Google Scholar]
- 14.Williams G A. Hydrobiologia. 1995;309:143–150. [Google Scholar]
- 15.Ragan M A, Glombitza K-W. Prog Phycol Res. 1986;4:129–241. [Google Scholar]
- 16.Targett N M, Arnold T M. J Phycol. 1998;36:195–205. [Google Scholar]
- 17.Pavia H, Toth G, Åberg P. J Ecol. 1999;87:761–771. [Google Scholar]
- 18.Steinberg P D. J Exp Mar Biol Ecol. 1988;120:221–237. [Google Scholar]
- 19.Van Alstyne K L. J Chem Ecol. 1995;21:45–58. doi: 10.1007/BF02033661. [DOI] [PubMed] [Google Scholar]
- 20.Peterson S H, Renaud P E. Oecologia. 1989;80:82–86. doi: 10.1007/BF00789935. [DOI] [PubMed] [Google Scholar]
- 21.Carlson D J, Carlson M L. Limnol Oceanogr. 1984;29:1077–1087. [Google Scholar]
- 22.Jennings J G, Steinberg P D. Mar Biol (Berlin) 1994;121:349–354. [Google Scholar]
- 23.Karban R, Agrawal A A, Thaler J S, Adler L S. Trends Ecol Evol. 1999;14:443–447. doi: 10.1016/s0169-5347(99)01678-x. [DOI] [PubMed] [Google Scholar]
- 24.Järemo J, Tuomi J, Nilsson P. In: The Ecology and Evolution of Inducible Defenses. Tollrian R, Harvell C D, editors. Princeton: Princeton Univ. Press; 1999. pp. 33–44. [Google Scholar]
- 25.Green T, Ryan C A. Science. 1972;175:776–777. doi: 10.1126/science.175.4023.776. [DOI] [PubMed] [Google Scholar]
- 26.Pearce G, Strydom D, Johnson S, Ryan C A. Science. 1991;253:895–898. doi: 10.1126/science.253.5022.895. [DOI] [PubMed] [Google Scholar]
- 27.Farmer E E, Ryan C A. Proc Natl Acad Sci USA. 1990;87:7713–7716. doi: 10.1073/pnas.87.19.7713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dicke M, Sabelis M W, Takabayashi J, Bruin J, Posthumus M A. J Chem Ecol. 1990;16:3091–3118. doi: 10.1007/BF00979614. [DOI] [PubMed] [Google Scholar]
- 29.Turlings T C J, Loughrin J H, McCall P J, Röse U S R, Lewis W J, Tumlinson J H. Proc Natl Acad Sci USA. 1995;92:4169–4174. doi: 10.1073/pnas.92.10.4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lobban C S, Harrison P J. Seaweed Ecology and Physiology. Cambridge, U.K.: Cambridge Univ. Press; 1994. [Google Scholar]