Abstract
Isoprenoid glycerol dialkyl glycerol tetraethers (GDGTs) and branched glycerol dialkyl diethers are main membrane constituents of cultured hyperthermophilic archaea and eubacteria, respectively, and are found in environments with temperatures >60°C. Recently, we developed a new technique for the analysis of intact core tetraether lipids in cell material and sediments. The application of this technique to recent sediments shows that known and newly identified isoprenoid and branched GDGTs are widespread in low-temperature environments (<20°C) and are structurally far more diverse than previously thought. Their distribution indicates the ubiquitous environmental presence of as yet uncultivated, nonthermophilic organisms that may have independently evolved from hyperthermophilic archaea and eubacteria. The structures of some of the new GDGTs point to the hybridization of both typical archaeal and eubacterial biosynthetic pathways in single organisms.
Hyperthermophilic archaea and eubacteria thrive at temperatures >60°C and are nearly exclusively found in waters near volcanic areas (1). The membranes of the hyperthermophilic archaea currently isolated in cultures are predominantly composed of isoprenoid glycerol dialkyl glycerol tetraethers (GDGTs) (2, 3), whereas those of cultured hyperthermophilic eubacteria are partly composed of branched glycerol dialkyl diethers/glycerol monoalkyl glycerol diethers (4–6). The structural difference from diacyl membrane lipids of nonthermophilic eukaryotes and prokaryotes apparently contributes to the thermal stability of membranes of hyperthermophiles (4). Thus, until recently it was thought that GDGTs were solely present in extreme environments such as hot springs or hydrothermal vents (7). However, recent studies have shown that carbon skeletons present in GDGTs are also present in nonextreme environments, and it was suggested that they derive from GDGTs of marine nonthermophilic Crenarchaeota (8–10). However, these studies did not provide direct evidence for the presence of intact GDGTs or their exact structure.
Recently, we developed a new technique for the direct analysis of intact GDGTs in extracts of archaeal cell material and sediments using high performance liquid chromatography/atmospheric pressure chemical ionization mass spectrometry (HPLC/MS; ref. 11). In contrast to chemical degradation techniques, which are laborious and only reveal partial structure details of GDGTs, HPLC/MS allows rapid analysis of organic matter for intact GDGTs. Here, we report the ubiquitous presence of known and novel GDGTs in low-temperature environments. The findings indicate that low-temperature relatives of hyperthermophilic archaea and eubacteria are widespread in natural environments.
Materials and Methods
Intact GDGTs were identified by HPLC/atmospheric pressure positive ion chemical ionization (APCI) MS using conditions described previously (11). Typically, 1–5 g of freeze-dried sediments were ultrasonically extracted three times with about 5 ml of methanol, three times with about 5 ml of dichloromethane/methanol (1:1, vol/vol), and three times with about 5 ml of dichloromethane. All extracts were combined, and the bulk of the solvent was removed by rotary evaporation under vacuum. The remaining solvent was removed under a stream of nitrogen, and the residue was dissolved by sonication (10 min) in hexane/propanol (99:1, vol/vol). The resulting suspension was centrifuged (1 min, 2,300 × g), and the supernatant was filtered through a 4-mm-diameter PTFE filter (0.45-μm pore size) before injection.
Purified extracts were analyzed by HPLC/MS using an HP (Palo-Alto, CA, USA) 1100 series LC/MS equipped with an autoinjector and Chemstation chromatography manager software. Compounds were separated using an Econosphere NH2 column (4.6 × 250 mm, 5 μm; Alltech Associates), maintained at 30°C. Injection volumes varied from 1 to 5 μl. Tetraethers were eluted isocratically with 99% hexane and 1% propanol for 5 min, followed by a linear gradient to 1.8% propanol in 45 min. Flow rate was 1 ml/min. After each analysis, the column was cleaned by back-flushing hexane/propanol (95:5, vol/vol) at 1 ml/min for 10 min. Solutes were detected in the eluent using APCI/MS. Conditions for APCI/MS were as follows: nebulizer pressure 60 psi, vaporizer temperature 300°C, drying gas (N2) flow 6 liters/min and temperature 250°C, capillary voltage −3 kV, corona 5 μA (≈3.2 kV). Positive ion spectra were generated by scanning m/z 950–1450 in 1.9 s. Mass spectra presented typically represent the peak–apex spectrum and are corrected for background.
For identification of a number of GDGTs, fractions significantly enriched in specific GDGTs were obtained by repeated semipreparative HPLC using a semipreparative Econosphere NH2 column (10 × 250 mm; Alltech Associates) and using the same conditions as described above except that the flow rate was increased to 2 ml/min. These fractions were either studied by high-resolution two-dimensional NMR (as described in detail elsewhere (ref. 12 and J.S.S.D., E.C.H., S.S., and J. A. J. Geenevasen, unpublished results) or by treatment with HI/LiAlH4 or HI/NaSCH3 as described previously (8). Briefly, the fractions were refluxed in a solution of 56 wt % HI in water for 1 h, and the released alkyl iodides were isolated using column chromatography [Al2O3 as stationary phase, hexane/dichloromethane (9:1, vol/vol) as eluents]. Subsequently, the alkyl iodides were treated with either LiAlH4 in 1,4-dioxane for 1 h or NaSCH3 in methanol for 24 h to convert them to hydrocarbons and methylthioethers, respectively. The released compounds were analyzed by gas chromatography and gas chromatography-mass spectrometry (see ref. 8 for details).
Results
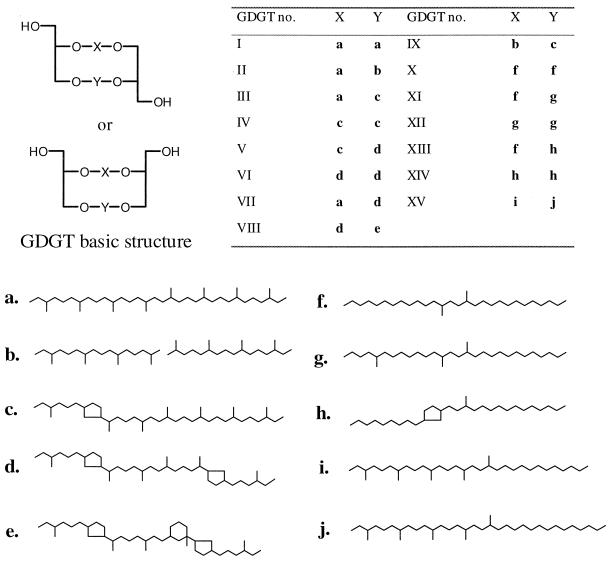
Surface (age <5 Ka) and ancient sediments were analyzed from a range of different continental and aquatic environments (Table 1). GDGTs I–VII were identified in a number of sediments (e.g., Fig. 2). These are GDGTs that have been identified previously as core ether lipids in cultured hyperthermophilic archaea (2, 3). Identifications are based on comparisons of mass spectra and relative retention times with those from a core ether lipid fraction obtained from Sulfolobus solfaticarus with known composition (11). GDGTs are easily recognizable by their characteristic APCI mass spectra, which contain, besides the [M + H]+ ions, fragment ions of [M + H]+ − 18 (loss of hydroxyl group as H2O) and [M + H]+ − 74 (loss of glycerol group) (11). In addition to known GDGTs, a suite of other compounds having characteristic GDGT APCI mass spectra were detected (GDGTs VIII–XV; Figs. 1–3). Their relative abundances were frequently higher than those of known GDGTs (Table 1). GDGT VIII was structurally identified by isolation and high-field two-dimensional NMR techniques. Briefly, signals in the 13C NMR spectrum of GDGT VIII were very comparable to those reported for GDGT VI (3). However, the presence of a quarternary carbon atom was unequivocally established by the Distortionless Enhancement by Polarization Transfer technique. Two-dimensional NMR techniques revealed that this carbon atom is part of a cyclohexane moiety attached to one of the cyclopentane rings, which leaves two possible structures of which one is shown in Fig. 1. Mass spectral data of carbon skeleton e (J.S.S.D., E.C.H., S.S., and J. A. J. Geenevasen, unpublished results) is in good agreement with this structural assignment. GDGTs X–XII were also structurally identified by isolation and high-field two-dimensional NMR techniques as reported elsewhere (12).
Table 1.
Relative distribution of GDGTs as a percentage of total tetraether membrane lipids in different archaea and recent and ancient sediments (structures refer to Fig. 1)
| Archaea | Type | Environmental conditions | I (%) | II (%) | III–VII (%) | VIII (%) | IX (%) | X–XII (%) | XIII–XIV (%) | XV (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| S. solfaticarus | Thermophilic archaeon | T > 50°C, pH < 5 | 0.1 | ND | 99 | ND | ND | ND | ND | ND |
| Metallosphaera sedula | Thermophilic archaeon | T = 57°C, pH = 2 | 7 | 0.2 | 93 | ND | ND | ND | ND | ND |
| Methanosarcina barkerii* | Methanogenic archaeon | T < 40°C, neutral pH | 100 | ND | ND | ND | ND | ND | ND | ND |
| Holocene sediments | Location | Environment | ||||||||
| Black Sea | Black Sea | Open marine | 45 | 0.3 | 8 | 42 | ND | 5 | ND | ND |
| Arabian Sea | Indian Ocean | Open marine | 30 | ND | 24 | 36 | ND | 5 | 0.9 | 5 |
| Peru Margin | Off shore Peru | Open marine | 36 | ND | 14 | 46 | ND | 3 | ND | ND |
| Skagerrak | North Sea | Open marine | 44 | ND | 5 | 29 | ND | 13 | 9 | 2 |
| Cariaco Basin | Off shore Venezuela | Open marine | 30 | 0.1 | 30 | 37 | ND | 2 | ND | 0.6 |
| Aegean Sea | Mediterranean Sea | Open marine | 39 | ND | 18 | 41 | ND | 2 | ND | ND |
| Saanich Inlet | Canada | Coastal marine | 56 | 0.6 | 7 | 34 | ND | 2 | 0.3 | ND |
| Kyllaren Fjord | Norway | Coastal marine | 23 | ND | ND | 7 | ND | 65 | 5 | ND |
| Wadden Sea | The Netherlands | Coastal marine | 19 | ND | 2 | 12 | ND | 57 | 11 | ND |
| Mok Bay | The Netherlands | Coastal marine | 29 | ND | 5 | 21 | ND | 34 | 11 | ND |
| Olimpi Mud Vulcano | Mediterranean Sea | Cold seep | 23 | 2 | 70 | 3 | 0.6 | 0.9 | 0.3 | 0.6 |
| Carbonate Crust | Mediterranean Sea | Cold seep | 15 | 0.3 | 67 | 16 | ND | 0.6 | 0.1 | 0.5 |
| Lake Ciso | Catalonia, Spain | Monomictic lake | 10 | ND | 4 | 5 | ND | 50 | 28 | 3 |
| Ace Lake | Antarctica | Meromictic lake | 100 | ND | ND | ND | ND | ND | ND | ND |
| Lake Paloma | Chile | Artificial lake | 40 | ND | 2 | 3 | ND | 43 | 12 | ND |
| Bargerveen | The Netherlands | Peat bog | 12 | 0.2 | 2 | ND | ND | 82 | 4 | ND |
| Carbury | Ireland | Peat bog | 14 | 0.1 | 3 | ND | ND | 78 | 6 | ND |
| Ancient sediments | Location (age, My) | Environment | ||||||||
| Walvis Bay | Off shore Africa (0.05) | Open marine | 48 | 0.4 | 10 | 37 | 0.2 | 1 | 0.3 | 2 |
| Funza Lake | Columbia (0.8) | Lacustrine | 42 | 0.5 | 20 | ND | ND | 31 | 6 | 1 |
| Ptolomas Basin | Greece (2) | Lacustrine | 27 | ND | 17 | ND | ND | 43 | 12 | ND |
| Vena del Gesso | Italy (6) | Hypersaline lagoon | 35 | 0.5 | 16 | 30 | ND | 11 | ND | 8 |
ND, not detected.
Koga et al. (2).
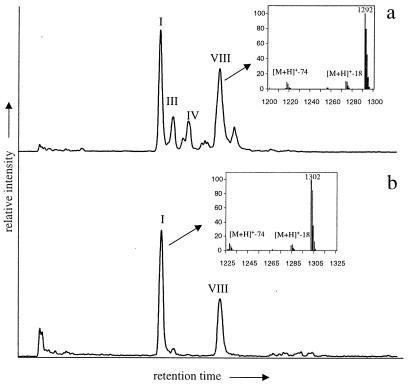
Figure 2.
HPLC/MS total ion current traces of tetraether membrane lipids in Arabian Sea (Indian Ocean) (a) and Skagerrak (North Sea) (b) revealing the abundance of isoprenoid GDGTs I and VIII in marine sediments. Roman numbers refer to structures in Fig. 1. Insets show the APCI mass spectra of GDGTs I and VIII.
Figure 1.
Structures of known and novel tetraether membrane lipids identified in different sediments (see Table 1). The basic structure of a GDGT is composed of two glycerol moieties linked by two carbon chains, here denoted X and Y, whereby X and Y may be interchanged. The table gives the structures of X and Y for the different GDGT isomers. For the basic structure of a GDGT, both the parallel and anti-parallel configurations are drawn, as this has not been established. However, chemical degradation experiments on GDGT I from archaeal cell material indicate a racemic mixture (26).
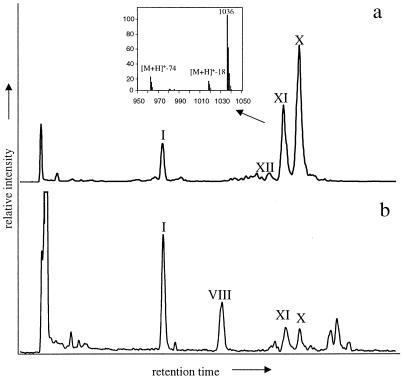
Figure 3.
HPLC/MS chromatograms of tetraether membrane lipids in Carbury peat bog (Ireland) (a) and Mok Bay (NE Netherlands) (b) revealing the abundance of non-isoprenoid GDGTs X and XI in terrestrial and coastal environments. Roman numbers refer to structures in Fig. 1. The inset shows the APCI mass spectrum of GDGT XI.
GDGT IX was tentatively identified on the basis of its relative retention time and additional fragments in its APCI-mass spectrum indicating the loss of a phytanyl moiety (for details, see ref. 11). GDGTs XIII–XV were tentatively identified by HI degradation of preparative HPLC fractions enriched in these tetraether lipids. This degradation technique cleaves ether bonds and thus releases the carbon skeletons of the GDGTs as alkyliodides (e.g., refs. 8 and 9). Subsequently, the released alkyliodides were either treated by LiAlH4 to reduce them to hydrocarbons or treated by NaSCH3 to transform them into methylthioether derivatives. In the case of GDGTs XIII and XIV, two and one C30-branched alkane(s), respectively, were released upon treatment with HI/LiAlH4. One was identified as 13,16-dimethyloctadocosane, using an authentic standard, whereas the other was tentatively identified, based on mass spectral data and retention times (Table 2), as 1-(3′-methylpentadecyl)-4-nonylcyclopentane (carbon skeleton h, Fig. 1). Further confirmation of the structures of XIII and XIV was obtained by HI/NaSCH3 degradation, which released two C30-branched α,ω-methylthioether alkanes, indicating that the alkanes were indeed ether bound at their terminal carbon atoms. The mass spectrum of the α,ω-methylthioether derivative of carbon skeleton h indicated the presence and the position of the cyclopentane ring (Table 2; Fig. 4a) by characteristic fragmentations next to the cyclopentane ring (9). In the case of GDGT XV, C35- and C37-branched alkanes were released after HI/LiAlH4 degradation, as were C35- and C37-branched α,ω-methylthioether alkanes after HI/NaSCH3 degradation. Mass spectral data of the hydrocarbons and the methylthioether derivatives (Fig. 4b), combined with comparisons of the measured retention indices with those of the calculated retention indices of the alkanes (Table 2), suggested that the C35 alkane was 3,7,11,15,18-pentamethyltriacontane (carbon skeleton i, Fig. 1), whereas the C37 alkane was identified as 3,7,11,15,18-pentamethyldotriacontane (carbon skeleton j, Fig. 1).
Table 2.
Protonated molecular ions in APCI mass spectra of tentatively identified GDGT XIII–XV and pseudo Kovats indices and diagnostic ions in electron mass spectra of the carbon skeletons released by HI/LiAlH4 degradation of these GDGTs (see Fig. 1)
| GDGT* | [M+H]+† | Carbon skeleton* | RImeasured | RIcalculated‡ | Diagnostic ions mass spectrum hydrocarbon |
|---|---|---|---|---|---|
| XIII | 1020 | f§ | 2912 | 2914 | 196/197, 253 |
| h | 2945 | —¶ | 193/194/195/196, 210, 251, 420 | ||
| XIV | 1018 | h | 2945 | — | 193/194/195/196, 210, 251, 420 |
| h | 2945 | — | 193/194/195/196, 210, 251, 420 | ||
| XV | 1190 | i | 3187 | 3196 | 127, 197, 211, 253, 323, 393, 462 |
| j | 3400 | 3396 | 127, 197, 211, 239, 281, 351, 421, 491 |
See Fig. 1.
As determined by APCI/MS mass spectra.
See ref. 25 for details.
Identified by authentic standard.
No retention indices can be calculated due to lack of data on retention shifts by cyclopentyl moieties. However, the later elution time of carbon skeleton h compared to carbon skeleton f is in agreement with the later elution time of carbon skeleton c compared to carbon skeleton a.
Figure 4.
Electron impact mass spectra of the α,ω-methylthioether derivatives of carbon skeleton h (a) and carbon skeleton i (b). Fragment ions of M+-63 are characteristic of α,ω-methylthioether alkanes (27).
Discussion
Distribution and Origin of Known GDGTs.
GDGT I (calderarchaeol) is found in all samples studied, and its relative abundance varies between 10 and 100% of total GDGTs (Table 1, Figs. 2 and 3). This component is a minor constituent in nonthermophilic methanogenic archaea (2). Although a number of our samples were taken at sites where methane production is likely to be low or absent, we cannot completely exclude the presence of methanogenic archaea in our samples. Thus, the presence of GDGT I may be due partly to methanogenic archaea living in anoxic niches in the aquatic and terrestrial environments examined here. However, it is surprising that GDGTs II–VII are present in low-temperature (<20°C) environments, as they are only known from cultured hyperthermophilic archaea (1, 3). The presence of GDGTs II–VII in low-temperature environments clearly indicates the presence of nonthermophilic archaea whose membranes are composed (partly) of these cyclic tetraether lipids as suggested previously (8–10). Stable carbon isotope analysis revealed in some instances (e.g., Olimpi Mud Vulcano) that GDGTs II–VII are isotopically depleted in 13C (δ13C <−50%), indicating that these unknown archaea may be involved in the anaerobic oxidation of methane (13–15). However, in other instances they were not isotopically depleted (−19% <δ13C <−28%) (8), suggesting that they are also biosynthesized by nonthermophilic archaea with different physiological characteristics.
Novel GDGT VIII in the Marine Environment.
GDGT VIII occurs predominantly in open and coastal marine environments and is often an abundant component (7–46% of total GDGTs; e.g., Fig. 2 and Table 1). Its structure is composed of a dicyclic biphytane (carbon skeleton d; Fig. 1) and a tricyclic biphytane (carbon skeleton e; Fig. 1). Only the former carbon skeleton is known from GDGTs of cultured hyperthermophilic archaea (3). The latter carbon skeleton is structurally rather different from that of tricyclic biphytanes from cultured archaea because it possesses an additional cyclohexyl ring rather than a cyclopentyl ring (J.S.S.D., E.C.H., S.S., and J. A. J. Geenevasen, unpublished results). This carbon skeleton, whose structure is now proven to be different from previously suggested (8–10), has been found in water column and recent sediment samples (8, 9), several water column samples known to contain nonthermophilic Crenarchaeota (10), and in Cenarchaeum symbiosum (10). Thus, it was proposed to exclusively occur in nonthermophilic Crenarchaeota (8, 9), which are widespread in today's oceans (16, 17). Our results now unequivocally establish that this tricyclic biphytane carbon skeleton is derived from a single GDGT isomer composed of a tricyclic and a dicyclic biphytane carbon skeleton. Genetic evidence for related nonthermophilic Crenarchaeota has been reported for lacustrine (18) and terrestrial environments (19), but GDGT VIII was only found in a few lacustrine settings and not in any peats (Table 1). This suggests that GDGT VIII is predominantly biosynthesized by marine nonthermophilic Crenarchaeota, although more nonmarine settings need to be surveyed to substantiate this hypothesis. These findings are consistent with recent molecular biological analyses, which revealed that planktonic marine nonthermophilic Crenarchaeota are dominated by relatively few cosmopolitan phylotypes that are not directly related to nonthermophilic Crenarchaeota from terrestrial environments (20). The structure of GDGT VIII is more asymmetrical than those of cyclic GDGTs of known hyperthermophilic archaea. This will affect the structure and fluidity of the membrane of the marine nonthermophilic Crenarchaeota significantly and may be an adaptation of their hyperthermophilic ancestors to low-temperature environments.
Novel GDGTs X–XIV of Terrestrial Origin.
GDGTs X–XII are newly identified tetraether lipids that are unlike any known GDGTs because their carbon skeletons are not composed of isoprenoids but of branched skeletons, i.e., 13,16-dimethyloctadocosane (carbon skeleton f; Figs. 1 and 3) in the case of GDGT X. In fact, GDGT X can be formed by biosynthetic condensation of two diethers containing two iso-pentadecyl moieties, lipids previously reported (21, 22). This would be similar to GDGT I being formed from two archaeols, i.e., diethers containing two ether-bound phytanyl moieties (23). Diethers containing two iso-pentadecyl moieties and other structurally comparable diethers have been reported in a number of hyperthermophilic eubacteria (5, 6, 22). In addition, structurally similar glycerol monoalkyl glycerol diether lipids containing 14,15-dimethyloctadocosane carbon skeletons have been found in the hyperthermophilic eubacteria Aquifex pyrophilis (5) and Thermotoga marina (6). Combined with the fact that cultured archaea exclusively biosynthesize isoprenoid ether lipids, it suggests that GDGTs X–XII are derived from nonthermophilic eubacteria, which may have evolved from hyperthermophilic ancestors. The possession of a unique archaeal feature, GDGT production, in a eubacterium would fit into the recently proposed occurrence of lateral gene transfer between (hyperthermophilic) archaea and eubacteria (24). The tentative identification of GDGTs XIII and XIV in the same settings as GDGTs X–XII provides additional evidence for the hybridization of archaeal and eubacterial biosynthetic pathways in single organisms. GDGTs XIII and XIV are structurally very similar to GDGT X except that one or two, respectively, of the methyl groups have reacted intramolecularly to form a cyclopentane ring. The occurrence of cyclopentane rings in membrane lipids has, until now, also been recognized as a unique feature in cultured hyperthermophilic archaea (3, 6, 23). However, the structure of GDGTs XII and XIV shows that this cyclization may also occur with non-isoprenoid tetraether lipids biosynthesized by low-temperature organisms, most likely eubacteria.
Similar to GDGT VIII, the carbon skeletons of GDGT X–XIV are different (e.g., additional methylation of the carbon skeleton) from those of known glycerol monoalkyl glycerol diether lipids, likely affecting the structure and fluidity of their membranes compared with those of hyperthermophilic prokaryotes. Again, this may be an evolutionary adaptation of hyperthermophilic relatives to low-temperature environments. GDGTs X–XIV are predominantly found in terrestrial samples and in lacustrine and some coastal marine sediments that are known to receive a significant terrestrial input because they are close (<500 m) to the coast (e.g., Fig. 3 and Table 1); they can comprise up to 82% of total GDGTs. This suggests that they are biosynthesized by organisms thriving predominantly in terrestrial environments and possibly lake settings as well.
Distribution and Origin of GDGT XV.
The tentatively identified GDGT XV occurs in minor amounts in marine and lacustrine settings (up to 8% of total GDGTs; Table 1). It includes two different alkyl moieties that are unique compared with those of previously discussed GDGTs. One is composed of phytane and iso-pentadecane, which have been coupled to form α,ω-diether-bound 3,7,11,15,18-pentamethyltriacontane (carbon skeleton i; Fig. 1). The other carbon skeleton is composed of phytane and iso-heptadecane, which have been coupled to form α,ω-diether-bound 3,7,11,15,18-pentamethyldotriacontane (carbon skeleton j; Fig. 1). In this way, its structure resembles partly those of archaea (phytanyl moiety) and of eubacteria (iso-alkyl moiety). This makes it difficult to speculate to which domain the organism belongs that biosynthesizes this specific membrane lipid. Further work is needed to rigorously identify GDGT XV, but its presence demonstrates the high structural diversity in naturally occurring tetraether lipids and potential utility as chemotaxonomic markers.
Implications.
Our results show that nonthermophilic organisms with membranes composed of tetraether lipids are widespread in nature. The GDGTs they produce are sometimes present in very high abundances (e.g., up to 3 mg/g total organic carbon for GDGT VIII and up to 0.5 mg/g total organic carbon for GDGT X) compared with other common eukaryotic and eubacterial lipids. In some cases, for instance in surface sediments of the Indian Ocean (8), they are even the predominant lipids. Selective preservation of GDGTs compared with other lipids is an unlikely explanation for these high abundances. HPLC analyses of Vena del Gesso samples heated at different temperatures by hydrous pyrolysis show that GDGTs degrade thermally at rates similar to those of other lipids, as suggested previously (9). Also, comparison of GDGT concentrations in Indian Ocean sediments deposited under suboxic and oxic conditions (J.S.S.D., W. I. C. Rijpstra, and G. J. Reichart, unpublished results) indicates that the oxidative degradation rates of GDGTs are similar to those of other lipids. Hence, the organisms producing the GDGTs must be a significant component of present prokaryotic communities.
Another clear impact of our data is that the structures of tetraether lipids are far more diverse than previously thought and may reflect an evolutionary adaptation of hyperthermophilic ancestors to low-temperature environments. The structures of some GDGTs can only be explained by the coexistence of typical archaeal and eubacterial biosynthetic pathways in single organisms. Molecular biological analysis and cultivation efforts should now focus on detecting and isolating these ubiquitous organisms, thereby providing more detailed insights into their ecological significance and phylogenetic position.
Acknowledgments
Prof. J. M. Hayes (Woods Hole Oceanographic Institute) and three anonymous referees are thanked for providing useful comments on an earlier draft. P. Schaeffer and P. Albrecht (University of Strasbourg) are thanked for providing the authentic standard of 13,16-dimethyloctadocosane. Thanks to A. Boom, H.-J. Bosch, R. Smittenberg, B. van Dongen, M. Baas, I. Rijpstra, E. Schefuss, T. Eglinton, G. Fuchs, M. van der Meer, A. Gambacorta, W. Hartgers, D. Adams, B. van Geel, J. Volkman, the Medinaut Shipboard Scientific party, J. Werne, and W. Helder for supplying a number of the studied sediments. This is Netherlands Institute for Sea Research contribution no. 3520.
Abbreviations
- GDGT
isoprenoid glycerol dialkyl glycerol tetraether
- APCI
atmospheric pressure positive ion chemical ionization
Footnotes
See commentary on page 14033.
References
- 1.Stetter K O. In: Extremophiles: Microbial Life in Extreme Environments. Horikoshi K, Grant W D, editors. New York: Wiley–Liss; 1998. pp. 1–17. [Google Scholar]
- 2.Koga Y, Nishihara M, Morii H, Akagawa-Matsushita M. Microbiol Rev. 1993;57:164–182. doi: 10.1128/mr.57.1.164-182.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DeRosa M, Gambacorta A. Prog Lipid Res. 1988;27:153–175. doi: 10.1016/0163-7827(88)90011-2. [DOI] [PubMed] [Google Scholar]
- 4.van den Vossenberg J C M, Driessen A J M, Konings W N. Extremophiles. 1998;2:163–170. doi: 10.1007/s007920050056. [DOI] [PubMed] [Google Scholar]
- 5.Huber R, Wilharm T, Huber D, Trincone A, Burggraf S, König H, Rachel R, Rockinger I, Fricke H, Stetter K O. Syst Appl Microbiol. 1992;15:340–351. [Google Scholar]
- 6.DeRosa M, Gambacorta A, Huber R, Lanzotti V, Nicolaus B, Stetter K O, Trincone A. In: Microbiology of Extreme Environments and Its Potential for Biotechnology. Da Costa M S, Duarte J C, Williams R A D, editors. London: Elsevier; 1989. pp. 167–173. [Google Scholar]
- 7.Ward D M, Brassell S C, Eglinton G. Nature (London) 1985;318:656–659. [Google Scholar]
- 8.Hoefs M J L, Schouten S, King L, Wakeham S G, De Leeuw J W, Sinninghe Damsté J S. Appl Environ Microbiol. 1997;63:3090–3095. doi: 10.1128/aem.63.8.3090-3095.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schouten S, Hoefs M J L, Koopmans M P, Bosch H-J, Sinninghe Damsté J S. Org Geochem. 1998;29:1305–1319. [Google Scholar]
- 10.DeLong E F, King L L, Massana R, Cittone H, Murray A, Schleper C, Wakeham S G. Appl Environ Microbiol. 1998;64:1133–1138. doi: 10.1128/aem.64.3.1133-1138.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hopmans E C, Schouten S, Pancost R D, van der Meer M J T, Sinninghe Damsté J S. Rapid Comm Mass Spectrom. 2000;14:585–589. doi: 10.1002/(SICI)1097-0231(20000415)14:7<585::AID-RCM913>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 12.Sinninghe Damsté J S, Hopmans E C, Pancost R D, Schouten S, Geenevasen J A J. J. Chem. Soc. Chem. Commun. 2000. 1683–1684. [Google Scholar]
- 13.Pancost R, Sinninghe Damsté J S, De Lint S, Van Der Maarel M J E C, Gottschal J C Medinaut Shipboard Scientific Party. Appl Environ Microbiol. 2000;66:1126–1132. doi: 10.1128/aem.66.3.1126-1132.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hinrichs K U, Hayes J M, Sylva S P, Brewer P G, DeLong E F. Nature (London) 1999;398:802–805. doi: 10.1038/19751. [DOI] [PubMed] [Google Scholar]
- 15.Thiel V, Peckmann J, Seifert R, Wehrung P, Reitner J, Michaelis W. Geochim Cosmochim Acta. 1999;63:3959–3968. [Google Scholar]
- 16.DeLong E F, Wu K Y, Prezelin B B, Jovine R V M. Nature (London) 1994;371:695–697. doi: 10.1038/371695a0. [DOI] [PubMed] [Google Scholar]
- 17.Fuhrman J A, McCallum K, Davis A A. Nature (London) 1992;356:148–149. doi: 10.1038/356148a0. [DOI] [PubMed] [Google Scholar]
- 18.MacGregor B J, Moser D P, Alm D W, Nealson K H, Stahl D A. Appl Environ Microbiol. 1997;63:1178–1181. doi: 10.1128/aem.63.3.1178-1181.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hershberger K L, Barns S M, Reysenbach A L, Dawson S C, Pace N R. Nature (London) 1996;384:420. doi: 10.1038/384420a0. [DOI] [PubMed] [Google Scholar]
- 20.Massana R, DeLong E F, Pedros-Alio C. Appl Environ Microbiol. 2000;66:1777–1787. doi: 10.1128/aem.66.5.1777-1787.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chappe B, Albrecht P, Michaelis W. Science. 1982;217:65–66. doi: 10.1126/science.217.4554.65. [DOI] [PubMed] [Google Scholar]
- 22.Langworthy T A, Holzer G, Zeikus J G, Tornabene T G. Syst Appl Microbiol. 1983;4:1–17. doi: 10.1016/S0723-2020(83)80029-0. [DOI] [PubMed] [Google Scholar]
- 23.DeRosa M, Gambacorta A, Gliozzi A. Microbiol Rev. 1986;50:70–80. doi: 10.1128/mr.50.1.70-80.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nelson K E, Clayton R A, Gill S R, Gwinn M L, Dodson R J, Haft D H, Hickey E K, Peterson J D, Nelson W C, Ketchum K A, et al. Nature (London) 1999;399:323–329. doi: 10.1038/20601. [DOI] [PubMed] [Google Scholar]
- 25.Kissin Y V, Feulmer G P, Payne W B. J Chromatogr Sci. 1986;24:164–169. [Google Scholar]
- 26.Gräther O, Arigoni D. J. Chem. Soc., Chem. Commun. 1995. 405–407. [Google Scholar]
- 27.Kohnen M E L, Sinninghe Damsté J S, Kock-van Dalen A C, de Leeuw J W. Geochim Cosmochim Acta. 1991;55:1375–1394. [Google Scholar]