Abstract
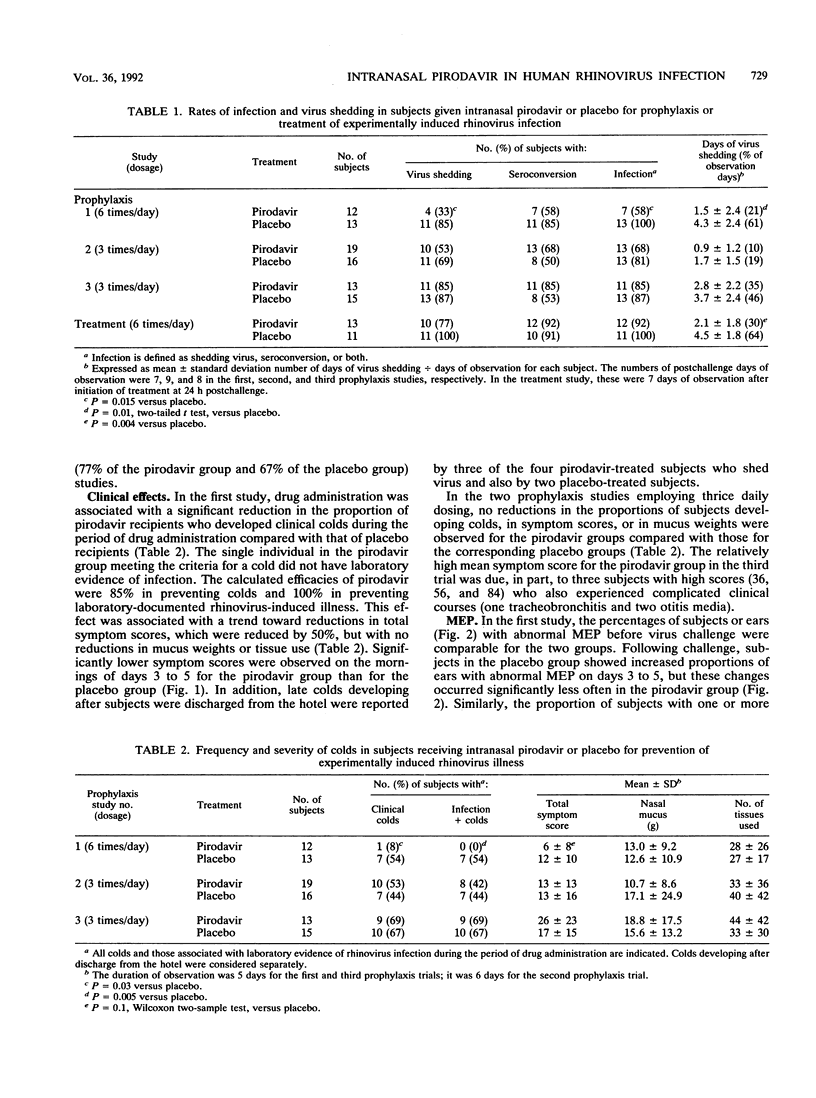
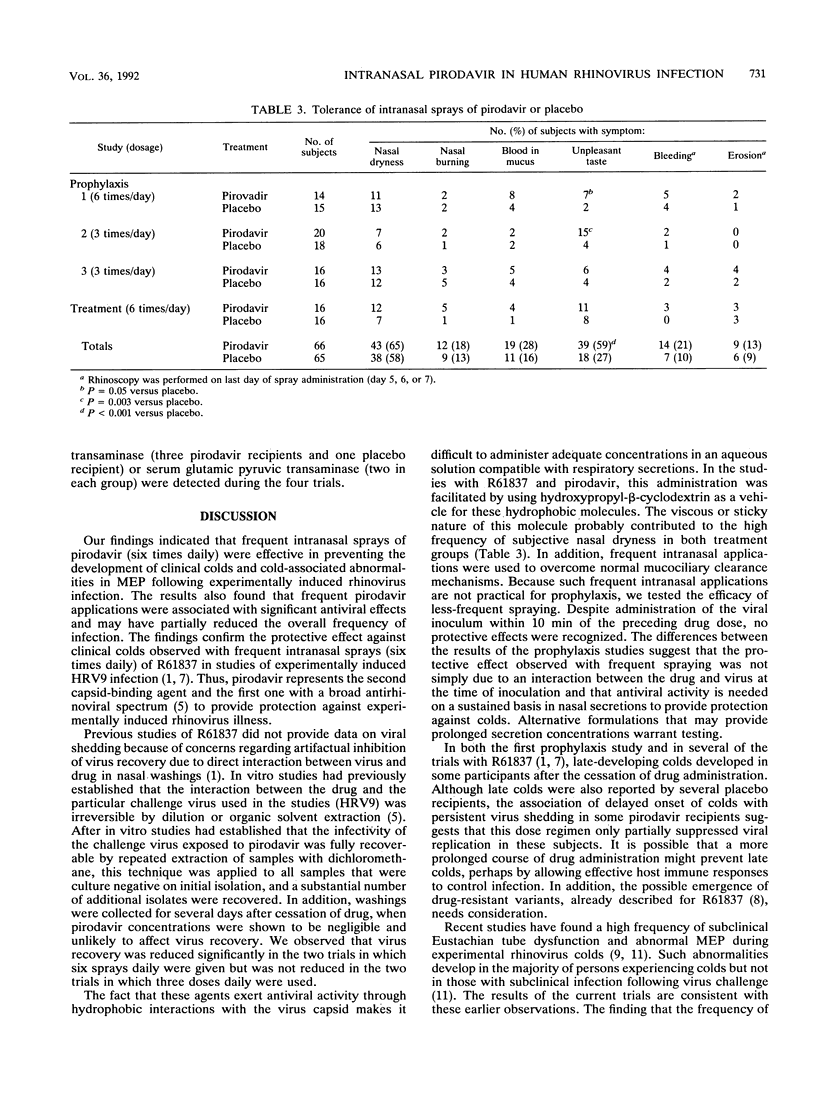
Pirodavir (R77975) is a capsid-binding, antipicornaviral agent with in vitro activity against most rhinovirus (RV) serotypes. We conducted four double-blind, controlled trials to assess the efficacy of intranasal pirodavir in experimentally induced RV infection of susceptible volunteers. Intranasal pirodavir (2 mg per dose) or the hydroxypropyl-beta-cyclodextrin vehicle as a placebo was given by metered pump spray. In three prophylaxis trials, subjects were inoculated with RV within 10 min of the second and third doses. When sprays were given six times per day for a total of 25 doses, infection, detected by either virus shedding or seroconversion, developed in 100% of the 13 placebo-treated subjects and 58% of the 12 pirodavir-treated subjects (P = 0.015). Clinical colds developed in 54% of placebo-treated subjects and 8% of pirodavir-treated subjects during drug administration (efficacy = 85%, P = 0.03), although late-developing colds developed in several subjects in both groups. Significant reductions in morning symptom scores and in the frequency of abnormal middle-ear pressures were also found in the pirodavir group. In contrast, in two prophylaxis studies using three doses daily, no significant antiviral or clinical benefits were observed. When frequent sprays were initiated at 24 h after RV challenge, significant reductions in virus shedding but no clinical benefits were found. Intranasal pirodavir was generally well tolerated but was associated with an excess rate of transient unpleasant taste. The findings indicated that frequent intranasal sprays of pirodavir were effective in preventing experimentally induced RV illness.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Nakib W., Higgins P. G., Barrow I., Tyrrell D. A., Lenox-Smith I., Ishitsuka H. Intranasal chalcone, Ro 09-0410, as prophylaxis against rhinovirus infection in human volunteers. J Antimicrob Chemother. 1987 Dec;20(6):887–892. doi: 10.1093/jac/20.6.887. [DOI] [PubMed] [Google Scholar]
- Al-Nakib W., Willman J., Higgins P. G., Tyrrell D. A., Shepherd W. M., Freestone D. S. Failure of intranasally administered 4', 6-dichloroflavan to protect against rhinovirus infection in man. Arch Virol. 1987;92(3-4):255–260. doi: 10.1007/BF01317482. [DOI] [PubMed] [Google Scholar]
- Andries K., Dewindt B., De Brabander M., Stokbroekx R., Janssen P. A. In vitro activity of R 61837, a new antirhinovirus compound. Arch Virol. 1988;101(3-4):155–167. doi: 10.1007/BF01310997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andries K., Dewindt B., Snoeks J., Wouters L., Moereels H., Lewi P. J., Janssen P. A. Two groups of rhinoviruses revealed by a panel of antiviral compounds present sequence divergence and differential pathogenicity. J Virol. 1990 Mar;64(3):1117–1123. doi: 10.1128/jvi.64.3.1117-1123.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dearden C., al-Nakib W., Andries K., Woestenborghs R., Tyrrell D. A. Drug resistant rhinoviruses from the nose of experimentally treated volunteers. Arch Virol. 1989;109(1-2):71–81. doi: 10.1007/BF01310519. [DOI] [PubMed] [Google Scholar]
- Hayden F. G., Gwaltney J. M., Jr Intranasal interferon alpha 2 for prevention of rhinovirus infection and illness. J Infect Dis. 1983 Sep;148(3):543–550. doi: 10.1093/infdis/148.3.543. [DOI] [PubMed] [Google Scholar]
- McBride T. P., Doyle W. J., Hayden F. G., Gwaltney J. M., Jr Alterations of the eustachian tube, middle ear, and nose in rhinovirus infection. Arch Otolaryngol Head Neck Surg. 1989 Sep;115(9):1054–1059. doi: 10.1001/archotol.1989.01860330044014. [DOI] [PubMed] [Google Scholar]
- Otto M. J., Fox M. P., Fancher M. J., Kuhrt M. F., Diana G. D., McKinlay M. A. In vitro activity of WIN 51711, a new broad-spectrum antipicornavirus drug. Antimicrob Agents Chemother. 1985 Jun;27(6):883–886. doi: 10.1128/aac.27.6.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pevear D. C., Fancher M. J., Felock P. J., Rossmann M. G., Miller M. S., Diana G., Treasurywala A. M., McKinlay M. A., Dutko F. J. Conformational change in the floor of the human rhinovirus canyon blocks adsorption to HeLa cell receptors. J Virol. 1989 May;63(5):2002–2007. doi: 10.1128/jvi.63.5.2002-2007.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillpotts R. J., Wallace J., Tyrrell D. A., Freestone D. S., Shepherd W. M. Failure of oral 4',6-dichloroflavan to protect against rhinovirus infection in man. Arch Virol. 1983;75(1-2):115–121. doi: 10.1007/BF01314131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossmann M. G. The structure of antiviral agents that inhibit uncoating when complexed with viral capsids. Antiviral Res. 1989 Feb;11(1):3–13. doi: 10.1016/0166-3542(89)90016-8. [DOI] [PubMed] [Google Scholar]
- Sperber S. J., Hayden F. G. Chemotherapy of rhinovirus colds. Antimicrob Agents Chemother. 1988 Apr;32(4):409–419. doi: 10.1128/aac.32.4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerial A., Werner G. H., Phillpotts R. J., Willmann J. S., Higgins P. G., Tyrrell D. A. Studies on 44 081 R.P., a new antirhinovirus compound, in cell cultures and in volunteers. Antimicrob Agents Chemother. 1985 May;27(5):846–850. doi: 10.1128/aac.27.5.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- al-Nakib W., Higgins P. G., Barrow G. I., Tyrrell D. A., Andries K., Vanden Bussche G., Taylor N., Janssen P. A. Suppression of colds in human volunteers challenged with rhinovirus by a new synthetic drug (R61837). Antimicrob Agents Chemother. 1989 Apr;33(4):522–525. doi: 10.1128/aac.33.4.522. [DOI] [PMC free article] [PubMed] [Google Scholar]


