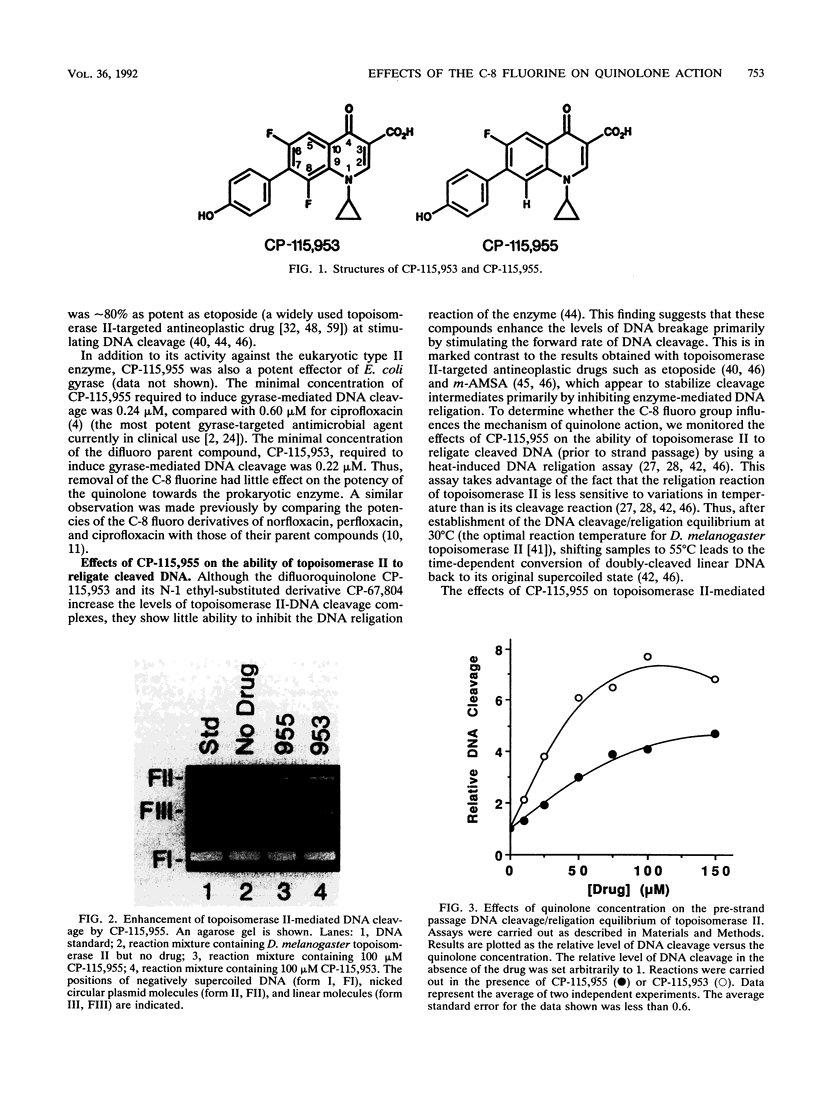
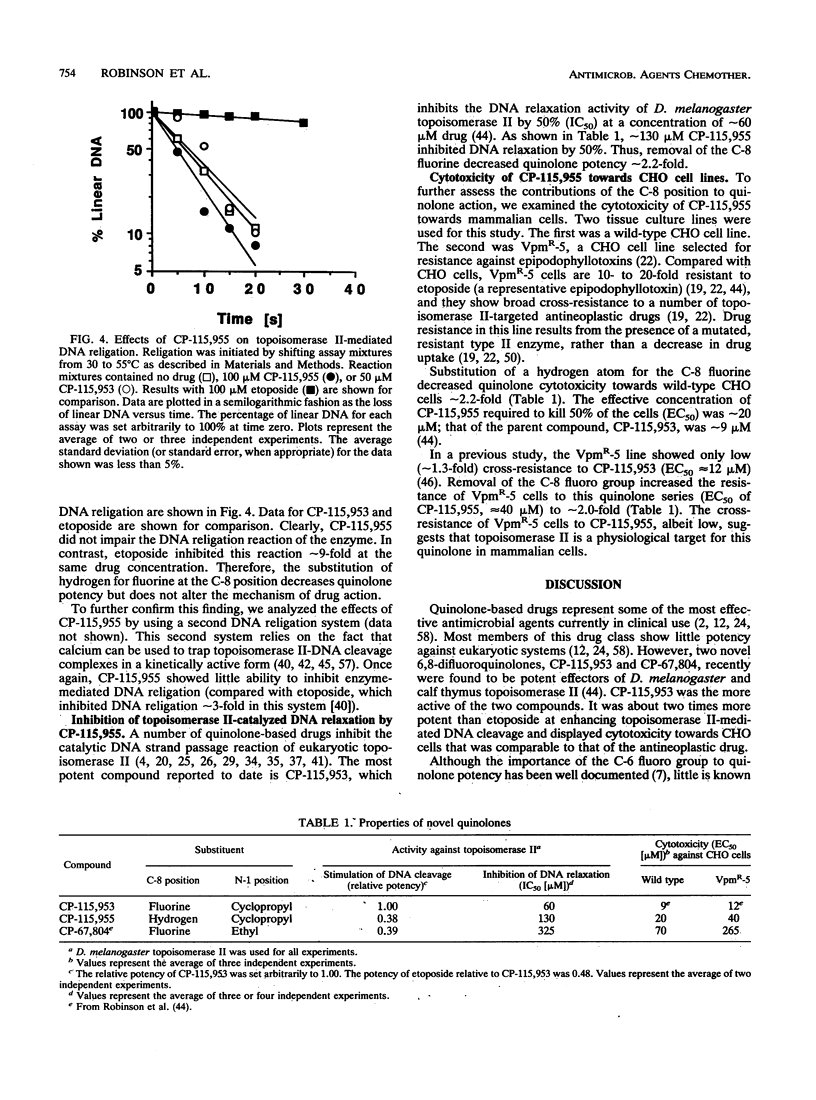
Abstract
A previous study (M.J. Robinson, B.A. Martin, T.D. Gootz, P.R. McGuirk, M. Moynihan, J.A. Sutcliffe, and N. Osheroff, J. Biol. Chem. 266:14585-14592, 1991) demonstrated that novel 6,8-difluoroquinolones were potent effectors of eukaryotic topoisomerase II. To determine the contribution of the C-8 fluorine to drug potency, we compared the effects of CP-115,955 [6-fluoro-7-(4-hydroxyphenyl)-1-cyclopropyl-4-quinolone-3-carboxylic acid] on the enzymatic activities of Drosophila melanogaster topoisomerase II with those of CP-115,953 (the 6,8-difluoro parent compound of CP-115,955). Removal of the C-8 fluoro group decreased the ability of the quinolone to enhance enzyme-mediated DNA cleavage approximately 2.5-fold. Like its difluorinated counterpart, CP-115,955 increased the levels of cleavage intermediates without impairing the DNA religation reaction of the enzyme. Removal of the C-8 fluorine reduced the ability of the quinolone to inhibit topoisomerase II-catalyzed DNA relaxation. In addition, the cytotoxicity of CP-115,955 towards Chinese hamster ovary cells was decreased compared with that of CP-115,953. These results demonstrate that the C-8 fluorine increases the potency of quinolone derivatives against eukaryotic topoisomerase II and mammalian cells. Further comparisons of CP-115,955 with CP-115,953 and CP-67,804 (the N-1 ethyl-substituted derivative of the difluoro parent compound) indicate that the two intrinsic activities of quinolone-based drugs towards topoisomerase II (i.e., enhancement of DNA cleavage and inhibition of catalytic strand passage) can be differentially influenced by alteration of ring substituents. Finally, correlations between the biochemical and cytological activities of these drugs suggest that the ability to inhibit catalytic strand passage enhances the cytotoxic potential of quinolones towards eukaryotic cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi Y., Luke M., Laemmli U. K. Chromosome assembly in vitro: topoisomerase II is required for condensation. Cell. 1991 Jan 11;64(1):137–148. doi: 10.1016/0092-8674(91)90215-k. [DOI] [PubMed] [Google Scholar]
- Bae Y. S., Kawasaki I., Ikeda H., Liu L. F. Illegitimate recombination mediated by calf thymus DNA topoisomerase II in vitro. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2076–2080. doi: 10.1073/pnas.85.7.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett J. F., Gootz T. D., McGuirk P. R., Farrell C. A., Sokolowski S. A. Use of in vitro topoisomerase II assays for studying quinolone antibacterial agents. Antimicrob Agents Chemother. 1989 Oct;33(10):1697–1703. doi: 10.1128/aac.33.10.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrios M., Osheroff N., Fisher P. A. In situ localization of DNA topoisomerase II, a major polypeptide component of the Drosophila nuclear matrix fraction. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4142–4146. doi: 10.1073/pnas.82.12.4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christman M. F., Dietrich F. S., Fink G. R. Mitotic recombination in the rDNA of S. cerevisiae is suppressed by the combined action of DNA topoisomerases I and II. Cell. 1988 Nov 4;55(3):413–425. doi: 10.1016/0092-8674(88)90027-x. [DOI] [PubMed] [Google Scholar]
- Chu D. T., Fernandes P. B. Structure-activity relationships of the fluoroquinolones. Antimicrob Agents Chemother. 1989 Feb;33(2):131–135. doi: 10.1128/aac.33.2.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiNardo S., Voelkel K., Sternglanz R. DNA topoisomerase II mutant of Saccharomyces cerevisiae: topoisomerase II is required for segregation of daughter molecules at the termination of DNA replication. Proc Natl Acad Sci U S A. 1984 May;81(9):2616–2620. doi: 10.1073/pnas.81.9.2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillehay L. E., Jacobson-Kram D., Williams J. R. DNA topoisomerases and models of sister-chromatid exchange. Mutat Res. 1989 Nov;215(1):15–23. doi: 10.1016/0027-5107(89)90213-3. [DOI] [PubMed] [Google Scholar]
- Domagala J. M., Bridges A. J., Culbertson T. P., Gambino L., Hagen S. E., Karrick G., Porter K., Sanchez J. P., Sesnie J. A., Spense F. G. Synthesis and biological activity of 5-amino- and 5-hydroxyquinolones, and the overwhelming influence of the remote N1-substituent in determining the structure-activity relationship. J Med Chem. 1991 Mar;34(3):1142–1154. doi: 10.1021/jm00107a039. [DOI] [PubMed] [Google Scholar]
- Domagala J. M., Hanna L. D., Heifetz C. L., Hutt M. P., Mich T. F., Sanchez J. P., Solomon M. New structure-activity relationships of the quinolone antibacterials using the target enzyme. The development and application of a DNA gyrase assay. J Med Chem. 1986 Mar;29(3):394–404. doi: 10.1021/jm00153a015. [DOI] [PubMed] [Google Scholar]
- Drlica K., Franco R. J. Inhibitors of DNA topoisomerases. Biochemistry. 1988 Apr 5;27(7):2253–2259. doi: 10.1021/bi00407a001. [DOI] [PubMed] [Google Scholar]
- Earnshaw W. C., Halligan B., Cooke C. A., Heck M. M., Liu L. F. Topoisomerase II is a structural component of mitotic chromosome scaffolds. J Cell Biol. 1985 May;100(5):1706–1715. doi: 10.1083/jcb.100.5.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earnshaw W. C., Heck M. M. Localization of topoisomerase II in mitotic chromosomes. J Cell Biol. 1985 May;100(5):1716–1725. doi: 10.1083/jcb.100.5.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale K. C., Osheroff N. Uncoupling the DNA cleavage and religation activities of topoisomerase II with a single-stranded nucleic acid substrate: evidence for an active enzyme-cleaved DNA intermediate. Biochemistry. 1990 Oct 16;29(41):9538–9545. doi: 10.1021/bi00493a007. [DOI] [PubMed] [Google Scholar]
- Gasser S. M., Laemmli U. K. The organisation of chromatin loops: characterization of a scaffold attachment site. EMBO J. 1986 Mar;5(3):511–518. doi: 10.1002/j.1460-2075.1986.tb04240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasser S. M., Laroche T., Falquet J., Boy de la Tour E., Laemmli U. K. Metaphase chromosome structure. Involvement of topoisomerase II. J Mol Biol. 1986 Apr 20;188(4):613–629. doi: 10.1016/s0022-2836(86)80010-9. [DOI] [PubMed] [Google Scholar]
- Glisson B., Gupta R., Smallwood-Kentro S., Ross W. Characterization of acquired epipodophyllotoxin resistance in a Chinese hamster ovary cell line: loss of drug-stimulated DNA cleavage activity. Cancer Res. 1986 Apr;46(4 Pt 2):1934–1938. [PubMed] [Google Scholar]
- Gootz T. D., Barrett J. F., Sutcliffe J. A. Inhibitory effects of quinolone antibacterial agents on eucaryotic topoisomerases and related test systems. Antimicrob Agents Chemother. 1990 Jan;34(1):8–12. doi: 10.1128/aac.34.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto T., Wang J. C. Yeast DNA topoisomerase II is encoded by a single-copy, essential gene. Cell. 1984 Apr;36(4):1073–1080. doi: 10.1016/0092-8674(84)90057-6. [DOI] [PubMed] [Google Scholar]
- Gupta R. S. Genetic, biochemical, and cross-resistance studies with mutants of Chinese hamster ovary cells resistant to the anticancer drugs, VM-26 and VP16-213. Cancer Res. 1983 Apr;43(4):1568–1574. [PubMed] [Google Scholar]
- Holm C., Goto T., Wang J. C., Botstein D. DNA topoisomerase II is required at the time of mitosis in yeast. Cell. 1985 Jun;41(2):553–563. doi: 10.1016/s0092-8674(85)80028-3. [DOI] [PubMed] [Google Scholar]
- Hooper D. C., Wolfson J. S. Fluoroquinolone antimicrobial agents. N Engl J Med. 1991 Feb 7;324(6):384–394. doi: 10.1056/NEJM199102073240606. [DOI] [PubMed] [Google Scholar]
- Hoshino K., Sato K., Akahane K., Yoshida A., Hayakawa I., Sato M., Une T., Osada Y. Significance of the methyl group on the oxazine ring of ofloxacin derivatives in the inhibition of bacterial and mammalian type II topoisomerases. Antimicrob Agents Chemother. 1991 Feb;35(2):309–312. doi: 10.1128/aac.35.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino K., Sato K., Une T., Osada Y. Inhibitory effects of quinolones on DNA gyrase of Escherichia coli and topoisomerase II of fetal calf thymus. Antimicrob Agents Chemother. 1989 Oct;33(10):1816–1818. doi: 10.1128/aac.33.10.1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiang Y. H., Jiang J. B., Liu L. F. Topoisomerase II-mediated DNA cleavage by amonafide and its structural analogs. Mol Pharmacol. 1989 Sep;36(3):371–376. [PubMed] [Google Scholar]
- Hsiang Y. H., Liu L. F. Evidence for the reversibility of cellular DNA lesion induced by mammalian topoisomerase II poisons. J Biol Chem. 1989 Jun 15;264(17):9713–9715. [PubMed] [Google Scholar]
- Hussy P., Maass G., Tümmler B., Grosse F., Schomburg U. Effect of 4-quinolones and novobiocin on calf thymus DNA polymerase alpha primase complex, topoisomerases I and II, and growth of mammalian lymphoblasts. Antimicrob Agents Chemother. 1986 Jun;29(6):1073–1078. doi: 10.1128/aac.29.6.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim R. A., Wang J. C. A subthreshold level of DNA topoisomerases leads to the excision of yeast rDNA as extrachromosomal rings. Cell. 1989 Jun 16;57(6):975–985. doi: 10.1016/0092-8674(89)90336-x. [DOI] [PubMed] [Google Scholar]
- Kreuzer K. N., Cozzarelli N. R. Escherichia coli mutants thermosensitive for deoxyribonucleic acid gyrase subunit A: effects on deoxyribonucleic acid replication, transcription, and bacteriophage growth. J Bacteriol. 1979 Nov;140(2):424–435. doi: 10.1128/jb.140.2.424-435.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lock R. B., Ross W. E. DNA topoisomerases in cancer therapy. Anticancer Drug Des. 1987 Oct;2(2):151–164. [PubMed] [Google Scholar]
- Miller K. G., Liu L. F., Englund P. T. A homogeneous type II DNA topoisomerase from HeLa cell nuclei. J Biol Chem. 1981 Sep 10;256(17):9334–9339. [PubMed] [Google Scholar]
- Moreau N. J., Robaux H., Baron L., Tabary X. Inhibitory effects of quinolones on pro- and eucaryotic DNA topoisomerases I and II. Antimicrob Agents Chemother. 1990 Oct;34(10):1955–1960. doi: 10.1128/aac.34.10.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newport J., Spann T. Disassembly of the nucleus in mitotic extracts: membrane vesicularization, lamin disassembly, and chromosome condensation are independent processes. Cell. 1987 Jan 30;48(2):219–230. doi: 10.1016/0092-8674(87)90425-9. [DOI] [PubMed] [Google Scholar]
- Osheroff N. Biochemical basis for the interactions of type I and type II topoisomerases with DNA. Pharmacol Ther. 1989;41(1-2):223–241. doi: 10.1016/0163-7258(89)90108-3. [DOI] [PubMed] [Google Scholar]
- Osheroff N. Effect of antineoplastic agents on the DNA cleavage/religation reaction of eukaryotic topoisomerase II: inhibition of DNA religation by etoposide. Biochemistry. 1989 Jul 25;28(15):6157–6160. doi: 10.1021/bi00441a005. [DOI] [PubMed] [Google Scholar]
- Osheroff N. Eukaryotic topoisomerase II. Characterization of enzyme turnover. J Biol Chem. 1986 Jul 25;261(21):9944–9950. [PubMed] [Google Scholar]
- Osheroff N., Shelton E. R., Brutlag D. L. DNA topoisomerase II from Drosophila melanogaster. Relaxation of supercoiled DNA. J Biol Chem. 1983 Aug 10;258(15):9536–9543. [PubMed] [Google Scholar]
- Osheroff N., Zechiedrich E. L. Calcium-promoted DNA cleavage by eukaryotic topoisomerase II: trapping the covalent enzyme-DNA complex in an active form. Biochemistry. 1987 Jul 14;26(14):4303–4309. doi: 10.1021/bi00388a018. [DOI] [PubMed] [Google Scholar]
- Osheroff N., Zechiedrich E. L., Gale K. C. Catalytic function of DNA topoisomerase II. Bioessays. 1991 Jun;13(6):269–273. doi: 10.1002/bies.950130603. [DOI] [PubMed] [Google Scholar]
- Robinson M. J., Martin B. A., Gootz T. D., McGuirk P. R., Moynihan M., Sutcliffe J. A., Osheroff N. Effects of quinolone derivatives on eukaryotic topoisomerase II. A novel mechanism for enhancement of enzyme-mediated DNA cleavage. J Biol Chem. 1991 Aug 5;266(22):14585–14592. [PubMed] [Google Scholar]
- Robinson M. J., Osheroff N. Effects of antineoplastic drugs on the post-strand-passage DNA cleavage/religation equilibrium of topoisomerase II. Biochemistry. 1991 Feb 19;30(7):1807–1813. doi: 10.1021/bi00221a012. [DOI] [PubMed] [Google Scholar]
- Robinson M. J., Osheroff N. Stabilization of the topoisomerase II-DNA cleavage complex by antineoplastic drugs: inhibition of enzyme-mediated DNA religation by 4'-(9-acridinylamino)methanesulfon-m-anisidide. Biochemistry. 1990 Mar 13;29(10):2511–2515. doi: 10.1021/bi00462a012. [DOI] [PubMed] [Google Scholar]
- Rose D., Thomas W., Holm C. Segregation of recombined chromosomes in meiosis I requires DNA topoisomerase II. Cell. 1990 Mar 23;60(6):1009–1017. doi: 10.1016/0092-8674(90)90349-j. [DOI] [PubMed] [Google Scholar]
- Schneider E., Hsiang Y. H., Liu L. F. DNA topoisomerases as anticancer drug targets. Adv Pharmacol. 1990;21:149–183. doi: 10.1016/s1054-3589(08)60342-7. [DOI] [PubMed] [Google Scholar]
- Shelton E. R., Osheroff N., Brutlag D. L. DNA topoisomerase II from Drosophila melanogaster. Purification and physical characterization. J Biol Chem. 1983 Aug 10;258(15):9530–9535. [PubMed] [Google Scholar]
- Sullivan D. M., Latham M. D., Rowe T. C., Ross W. E. Purification and characterization of an altered topoisomerase II from a drug-resistant Chinese hamster ovary cell line. Biochemistry. 1989 Jun 27;28(13):5680–5687. doi: 10.1021/bi00439a051. [DOI] [PubMed] [Google Scholar]
- Sutcliffe J. A., Gootz T. D., Barrett J. F. Biochemical characteristics and physiological significance of major DNA topoisomerases. Antimicrob Agents Chemother. 1989 Dec;33(12):2027–2033. doi: 10.1128/aac.33.12.2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura T., Ohkura H., Adachi Y., Morino K., Shiozaki K., Yanagida M. DNA topoisomerase II is required for condensation and separation of mitotic chromosomes in S. pombe. Cell. 1987 Sep 11;50(6):917–925. doi: 10.1016/0092-8674(87)90518-6. [DOI] [PubMed] [Google Scholar]
- Uemura T., Tanagida M. Mitotic spindle pulls but fails to separate chromosomes in type II DNA topoisomerase mutants: uncoordinated mitosis. EMBO J. 1986 May;5(5):1003–1010. doi: 10.1002/j.1460-2075.1986.tb04315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura T., Yanagida M. Isolation of type I and II DNA topoisomerase mutants from fission yeast: single and double mutants show different phenotypes in cell growth and chromatin organization. EMBO J. 1984 Aug;3(8):1737–1744. doi: 10.1002/j.1460-2075.1984.tb02040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. C. DNA topoisomerases. Annu Rev Biochem. 1985;54:665–697. doi: 10.1146/annurev.bi.54.070185.003313. [DOI] [PubMed] [Google Scholar]
- Wood E. R., Earnshaw W. C. Mitotic chromatin condensation in vitro using somatic cell extracts and nuclei with variable levels of endogenous topoisomerase II. J Cell Biol. 1990 Dec;111(6 Pt 2):2839–2850. doi: 10.1083/jcb.111.6.2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer C., Störl K., Störl J. Microbial DNA topoisomerases and their inhibition by antibiotics. J Basic Microbiol. 1990;30(3):209–224. doi: 10.1002/jobm.3620300312. [DOI] [PubMed] [Google Scholar]
- Zwelling L. A. Topoisomerase II as a target of antileukemia drugs: a review of controversial areas. Hematol Pathol. 1989;3(3):101–112. [PubMed] [Google Scholar]




