Abstract
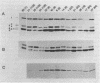
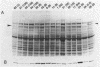
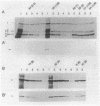
In 1989 and 1990, a large number of ampicillin-resistant strains of Enterococcus faecium were isolated from infected patients treated at intensive care units in Berlin, Germany. Twenty-five clinical isolates, including five different biotypes as classified by acid production from various sugars and a wide range of susceptibilities to ampicillin (MICs between 0.5 and 128 micrograms/ml), were selected for a detailed analysis of penicillin-binding proteins (PBPs). All strains contained a slowly reacting PBP with low penicillin affinity known to be present in enterococci. Overproduction of this PBP relative to susceptible isolates was noted, especially in all strains for which the MIC of ampicillin was 8 micrograms/ml, to a lesser degree in the more resistant strains, but not at all in the three highly resistant isolates for which the MIC was 128 micrograms/ml. In these three strains, this PBP appears to have a reduced affinity for beta-lactams. The results suggest that overproduction of PBP 6 correlates only with intermediate resistance levels and that higher resistance is mediated by yet another, still unknown mechanism, probably including reduction of beta-lactam affinity in one or more PBPs.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berger-Bächi B., Strässle A., Kayser F. H. Characterization of an isogenic set of methicillin-resistant and susceptible mutants of Staphylococcus aureus. Eur J Clin Microbiol. 1986 Dec;5(6):697–701. doi: 10.1007/BF02013308. [DOI] [PubMed] [Google Scholar]
- Bush L. M., Calmon J., Cherney C. L., Wendeler M., Pitsakis P., Poupard J., Levison M. E., Johnson C. C. High-level penicillin resistance among isolates of enterococci. Implications for treatment of enterococcal infections. Ann Intern Med. 1989 Apr 1;110(7):515–520. doi: 10.7326/0003-4819-110-7-515. [DOI] [PubMed] [Google Scholar]
- Courvalin P. Resistance of enterococci to glycopeptides. Antimicrob Agents Chemother. 1990 Dec;34(12):2291–2296. doi: 10.1128/aac.34.12.2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowson C. G., Hutchison A., Spratt B. G. Extensive re-modelling of the transpeptidase domain of penicillin-binding protein 2B of a penicillin-resistant South African isolate of Streptococcus pneumoniae. Mol Microbiol. 1989 Jan;3(1):95–102. doi: 10.1111/j.1365-2958.1989.tb00108.x. [DOI] [PubMed] [Google Scholar]
- Fontana R., Cerini R., Longoni P., Grossato A., Canepari P. Identification of a streptococcal penicillin-binding protein that reacts very slowly with penicillin. J Bacteriol. 1983 Sep;155(3):1343–1350. doi: 10.1128/jb.155.3.1343-1350.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana R., Grossato A., Rossi L., Cheng Y. R., Satta G. Transition from resistance to hypersusceptibility to beta-lactam antibiotics associated with loss of a low-affinity penicillin-binding protein in a Streptococcus faecium mutant highly resistant to penicillin. Antimicrob Agents Chemother. 1985 Nov;28(5):678–683. doi: 10.1128/aac.28.5.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackbarth C. J., Chambers H. F. Methicillin-resistant staphylococci: genetics and mechanisms of resistance. Antimicrob Agents Chemother. 1989 Jul;33(7):991–994. doi: 10.1128/aac.33.7.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakenbeck R., Briese T., Ellerbrok H. Antibodies against the benzylpenicilloyl moiety as a probe for penicillin-binding proteins. Eur J Biochem. 1986 May 15;157(1):101–106. doi: 10.1111/j.1432-1033.1986.tb09644.x. [DOI] [PubMed] [Google Scholar]
- Hakenbeck R., Ellerbrok H., Briese T., Handwerger S., Tomasz A. Penicillin-binding proteins of penicillin-susceptible and -resistant pneumococci: immunological relatedness of altered proteins and changes in peptides carrying the beta-lactam binding site. Antimicrob Agents Chemother. 1986 Oct;30(4):553–558. doi: 10.1128/aac.30.4.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman D. J., Gerding D. N. Antimicrobial resistance among enterococci. Antimicrob Agents Chemother. 1991 Jan;35(1):1–4. doi: 10.1128/aac.35.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman D. J., Gerding D. N. Screening and treatment of infections caused by resistant enterococci. Antimicrob Agents Chemother. 1991 Feb;35(2):215–219. doi: 10.1128/aac.35.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horodniceanu T., Delbos F. Les streptocoques du groupe D dans les infections humaines: identification et sensibilité aux antibiotiques. Ann Microbiol (Paris) 1980 Sep-Oct;131B(2):131–144. [PubMed] [Google Scholar]
- Klare I., Witte W. Methoden des beta-Laktamase-Nachweises bei Bakterien. Z Gesamte Hyg. 1984 Aug;30(8):442–449. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laible G., Spratt B. G., Hakenbeck R. Interspecies recombinational events during the evolution of altered PBP 2x genes in penicillin-resistant clinical isolates of Streptococcus pneumoniae. Mol Microbiol. 1991 Aug;5(8):1993–2002. doi: 10.1111/j.1365-2958.1991.tb00821.x. [DOI] [PubMed] [Google Scholar]
- Lee W. S., Komarmy L. New method for detecting in vitro inactivation of penicillins by Haemophilus influenzae and Staphlycoccus aureus. Antimicrob Agents Chemother. 1976 Sep;10(3):564–566. doi: 10.1128/aac.10.3.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowitz S. M., Wells V. D., Williams D. S., Stuart C. G., Coudron P. E., Wong E. S. Antimicrobial susceptibility and molecular epidemiology of beta-lactamase-producing, aminoglycoside-resistant isolates of Enterococcus faecalis. Antimicrob Agents Chemother. 1991 Jun;35(6):1075–1080. doi: 10.1128/aac.35.6.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami K., Tomasz A. Involvement of multiple genetic determinants in high-level methicillin resistance in Staphylococcus aureus. J Bacteriol. 1989 Feb;171(2):874–879. doi: 10.1128/jb.171.2.874-879.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray B. E., Mederski-Samoraj B., Foster S. K., Brunton J. L., Harford P. In vitro studies of plasmid-mediated penicillinase from Streptococcus faecalis suggest a staphylococcal origin. J Clin Invest. 1986 Jan;77(1):289–293. doi: 10.1172/JCI112289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Callaghan C. H., Morris A., Kirby S. M., Shingler A. H. Novel method for detection of beta-lactamases by using a chromogenic cephalosporin substrate. Antimicrob Agents Chemother. 1972 Apr;1(4):283–288. doi: 10.1128/aac.1.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oster S. E., Chirurgi V. A., Goldberg A. A., Aiken S., McCabe R. E. Ampicillin-resistant enterococcal species in an acute-care hospital. Antimicrob Agents Chemother. 1990 Sep;34(9):1821–1823. doi: 10.1128/aac.34.9.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapico F. L., Canawati H. N., Ginunas V. J., Gilmore D. S., Montgomerie J. Z., Tuddenham W. J., Facklam R. R. Enterococci highly resistant to penicillin and ampicillin: an emerging clinical problem? J Clin Microbiol. 1989 Sep;27(9):2091–2095. doi: 10.1128/jcm.27.9.2091-2095.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanakunakorn C. Enterococci from blood cultures during 1980-1989: susceptibility to ampicillin, penicillin and vancomycin. J Antimicrob Chemother. 1990 Oct;26(4):602–604. doi: 10.1093/jac/26.4.602. [DOI] [PubMed] [Google Scholar]
- Williamson R., le Bouguénec C., Gutmann L., Horaud T. One or two low affinity penicillin-binding proteins may be responsible for the range of susceptibility of Enterococcus faecium to benzylpenicillin. J Gen Microbiol. 1985 Aug;131(8):1933–1940. doi: 10.1099/00221287-131-8-1933. [DOI] [PubMed] [Google Scholar]
- Witte W., Reissbrodt R. Standardisierte Bestimmung minimaler Hemmkonzentrationen (MHK) antibakteriell wirksamer Chemotherapeutika für schnell wachsende Keime im Plattenverdünnungstest. Z Gesamte Hyg. 1983 Mar;29(3):178–180. [PubMed] [Google Scholar]
- al-Obeid S., Gutmann L., Williamson R. Modification of penicillin-binding proteins of penicillin-resistant mutants of different species of enterococci. J Antimicrob Chemother. 1990 Nov;26(5):613–618. doi: 10.1093/jac/26.5.613. [DOI] [PubMed] [Google Scholar]