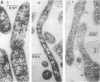
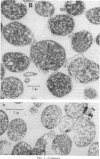
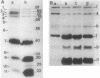
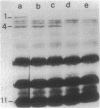
Abstract
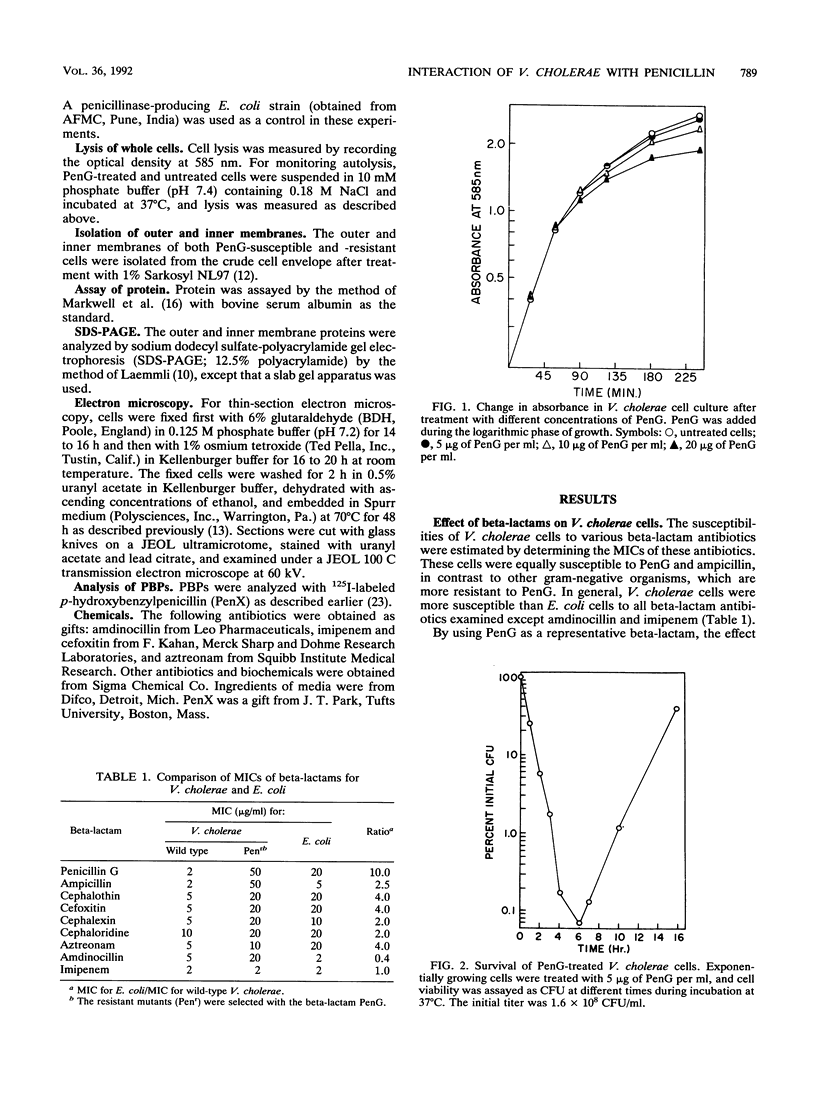
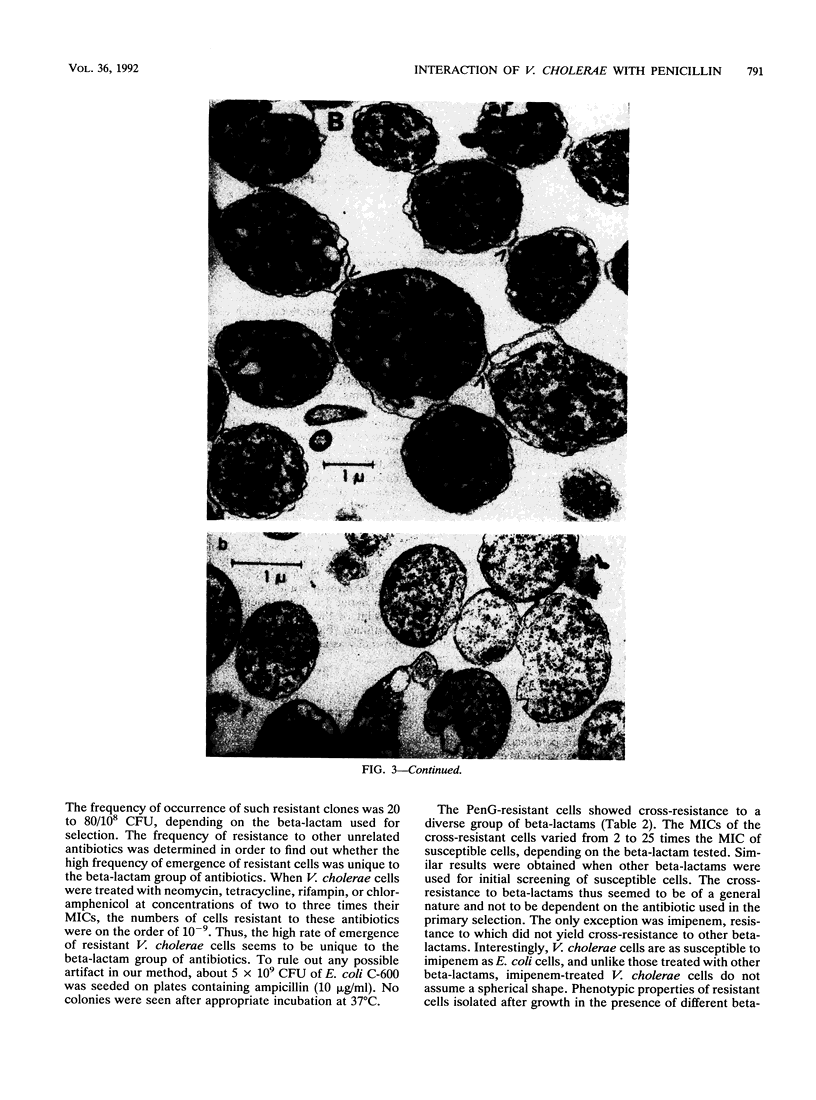
Unlike other gram-negative enteric bacteria, Vibrio cholerae cells were equally susceptible to penicillin and ampicillin and in general more susceptible than Escherichia coli to most of the beta-lactam antibiotics. The turbidity of penicillin-treated cultures contained to increase exponentially for about 3 h, although the cell viability declined rapidly within 30 min of penicillin addition. Prolonged treatment with beta-lactam antibiotics produced cells resistant to these antibiotics. A fluctuation test indicated that this resistance might be due to adaptive mutation. Cells resistant to a beta-lactam exhibited broad cross-resistance to other beta-lactam antibiotics. A new 12,000-Da outer membrane protein was detected both in beta-lactam-resistant cells and in wild-type cells growing in medium containing beta-lactam antibiotics. While the penicillin-resistant cells had all of the penicillin-binding proteins (PBPs) present in the parental cells, significant differences in the relative proportion of low-molecular-weight PBPs were seen. The low-molecular-weight PBPs from resistant cells seemed to form more stable complexes with penicillin than those from the parental strain.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aggeler R., Then R. L., Ghosh R. Reduced expression of outer-membrane proteins in beta-lactam-resistant mutants of Enterobacter cloacae. J Gen Microbiol. 1987 Dec;133(12):3383–3392. doi: 10.1099/00221287-133-12-3383. [DOI] [PubMed] [Google Scholar]
- CITRI N. DETERMINATION OF PENICILLINASE ACTIVITY. Methods Med Res. 1964;10:221–232. [PubMed] [Google Scholar]
- Cairns J., Overbaugh J., Miller S. The origin of mutants. Nature. 1988 Sep 8;335(6186):142–145. doi: 10.1038/335142a0. [DOI] [PubMed] [Google Scholar]
- Chai T. J., Foulds J. Escherichia coli K-12 tolF mutants: alterations in protein composition of the outer membrane. J Bacteriol. 1977 May;130(2):781–786. doi: 10.1128/jb.130.2.781-786.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti D., Chatterjee A. N. Studies on heterogeneous lipopolysaccharide fractions of Vibrio cholerae 569B. J Gen Microbiol. 1984 Aug;130(8):2023–2026. doi: 10.1099/00221287-130-8-2023. [DOI] [PubMed] [Google Scholar]
- Chatterjee A. N., Wong W., Young F. E., Gilpin R. W. Isolation and characterization of a mutant of Staphylococcus aureus deficient in autolytic activity. J Bacteriol. 1976 Mar;125(3):961–967. doi: 10.1128/jb.125.3.961-967.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freter R., Jones G. W. Adhesive properties of Vibrio cholerae: nature of the interaction with intact mucosal surfaces. Infect Immun. 1976 Jul;14(1):246–256. doi: 10.1128/iai.14.1.246-256.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levine M. M., Nalin D. R., Craig J. P., Hoover D., Bergquist E. J., Waterman D., Holley H. P., Hornick R. B., Pierce N. P., Libonati J. P. Immunity of cholera in man: relative role of antibacterial versus antitoxic immunity. Trans R Soc Trop Med Hyg. 1979;73(1):3–9. doi: 10.1016/0035-9203(79)90119-6. [DOI] [PubMed] [Google Scholar]
- Lohia A., Chatterjee A. N., Das J. Lysis of Vibrio cholerae cells: direct isolation of the outer membrane from whole cells by treatment with urea. J Gen Microbiol. 1984 Aug;130(8):2027–2033. doi: 10.1099/00221287-130-8-2027. [DOI] [PubMed] [Google Scholar]
- Lohia A., Majumdar S., Chatterjee A. N., Das J. Effect of changes in the osmolarity of the growth medium on Vibrio cholerae cells. J Bacteriol. 1985 Sep;163(3):1158–1166. doi: 10.1128/jb.163.3.1158-1166.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luria S. E., Delbrück M. Mutations of Bacteria from Virus Sensitivity to Virus Resistance. Genetics. 1943 Nov;28(6):491–511. doi: 10.1093/genetics/28.6.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning P. A. Involvement of cell envelope components in the pathogenesis of Vibrio cholerae: targets for cholera vaccine development. Vaccine. 1987 Jun;5(2):83–87. doi: 10.1016/0264-410x(87)90051-x. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Nguyen-Distèche M., Pollock J. J., Ghuysen J. M., Puig J., Reynolds P., Perkins H. R., Coyette J., Salton M. R. Sensitivity to ampicillin and cephalothin of enzymes involved in wall peptide crosslinking in Escherichia coli K12, strain 44. Eur J Biochem. 1974 Feb 1;41(3):457–463. doi: 10.1111/j.1432-1033.1974.tb03287.x. [DOI] [PubMed] [Google Scholar]
- Nikaido H. Proteins forming large channels from bacterial and mitochondrial outer membranes: porins and phage lambda receptor protein. Methods Enzymol. 1983;97:85–100. doi: 10.1016/0076-6879(83)97122-7. [DOI] [PubMed] [Google Scholar]
- Nikaido H., Vaara M. Molecular basis of bacterial outer membrane permeability. Microbiol Rev. 1985 Mar;49(1):1–32. doi: 10.1128/mr.49.1.1-32.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouellette M., Gerbaud G., Courvalin P. Genetic, biochemical and molecular characterization of strains of Vibrio cholerae multiresistant to antibiotics. Ann Inst Pasteur Microbiol. 1988 Jan-Feb;139(1):105–113. [PubMed] [Google Scholar]
- Sanders C. C. Chromosomal cephalosporinases responsible for multiple resistance to newer beta-lactam antibiotics. Annu Rev Microbiol. 1987;41:573–593. doi: 10.1146/annurev.mi.41.100187.003041. [DOI] [PubMed] [Google Scholar]
- Sengupta T. K., Chatterjee A. N., Das J. Penicillin binding proteins of Vibrio cholerae. Biochem Biophys Res Commun. 1990 Sep 28;171(3):1175–1181. doi: 10.1016/0006-291x(90)90808-z. [DOI] [PubMed] [Google Scholar]
- Spratt B. G. Distinct penicillin binding proteins involved in the division, elongation, and shape of Escherichia coli K12. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2999–3003. doi: 10.1073/pnas.72.8.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G. Properties of the penicillin-binding proteins of Escherichia coli K12,. Eur J Biochem. 1977 Jan;72(2):341–352. doi: 10.1111/j.1432-1033.1977.tb11258.x. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Gojobori T., Yokota T. Evolutionary origin of pathogenic determinants in enterotoxigenic Escherichia coli and Vibrio cholerae O1. J Bacteriol. 1987 Mar;169(3):1352–1357. doi: 10.1128/jb.169.3.1352-1357.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]