Abstract
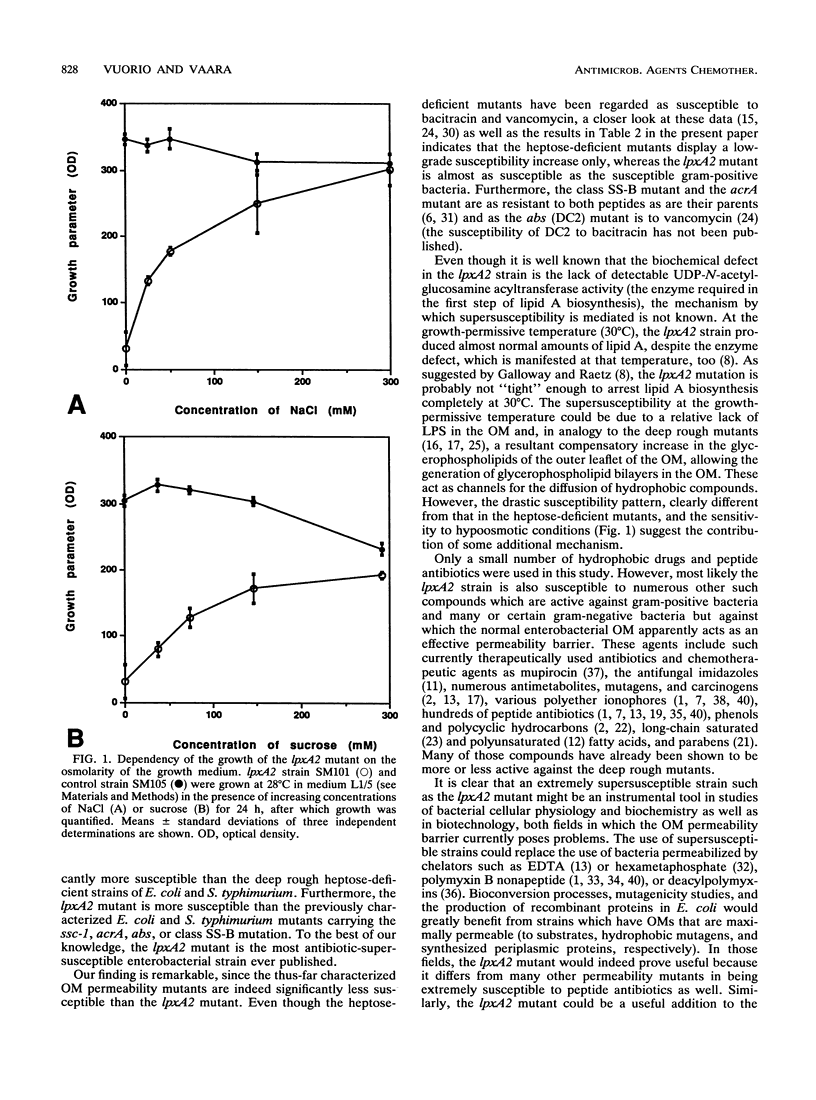
The conditionally lethal lpxA2 mutant of Escherichia coli, which lacks detectable UDP-N-acetylglucosamine acyltransferase activity and which produces greatly reduced amounts of lipid A after a shift to 42 degrees C (S. Galloway and C. R. H. Raetz, J. Biol. Chem. 265:6394-6402, 1990), was found to be, at conditions which promote normal growth, remarkably susceptible to a number of antibiotics. The MICs of hydrophobic antibiotics, such as rifampin, erythromycin, clindamycin, and fusidic acid, were 32- to greater than 128-fold lower for the lpxA2 strain than for the parent type strain, and those of the peptide antibiotics vancomycin and bacitracin were 32- and 256-fold lower, respectively. Futhermore, the lpxA2 strain was found to be sensitive to hypoosmotic conditions. Comparisons with the other characterized outer membrane permeability mutants, such as the heptose-deficient strains of E. coli and Salmonella typhimurium, the acrA and abs mutants of E. coli, and the ssc-1 and class SS-B mutants of S. typhimurium, indicated that the lpxA2 mutant had characteristically the most antibiotic-supersusceptible phenotype. These findings advocate the possible use of the lpxA2 strain as a tool in various fields of basic and applied bacterial research in which the impermeability of the outer membrane currently poses problems.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames B. N., Lee F. D., Durston W. E. An improved bacterial test system for the detection and classification of mutagens and carcinogens. Proc Natl Acad Sci U S A. 1973 Mar;70(3):782–786. doi: 10.1073/pnas.70.3.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boman H. G., Monner D. A. Characterization of lipopolysaccharides from Escherichia coli K-12 mutants. J Bacteriol. 1975 Feb;121(2):455–464. doi: 10.1128/jb.121.2.455-464.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman W. G., Jr, Leive L. Two mutations which affect the barrier function of the Escherichia coli K-12 outer membrane. J Bacteriol. 1979 Sep;139(3):899–910. doi: 10.1128/jb.139.3.899-910.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galloway S. M., Raetz C. R. A mutant of Escherichia coli defective in the first step of endotoxin biosynthesis. J Biol Chem. 1990 Apr 15;265(11):6394–6402. [PubMed] [Google Scholar]
- Hirvas L., Koski P., Vaara M. Identification and sequence analysis of the gene mutated in the conditionally lethal outer membrane permeability mutant SS-C of Salmonella typhimurium. EMBO J. 1991 Apr;10(4):1017–1023. doi: 10.1002/j.1460-2075.1991.tb08036.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones B. M., Geary I., Lee M. E., Duerden B. I. Comparison of the in vitro activities of fenticonazole, other imidazoles, metronidazole, and tetracycline against organisms associated with bacterial vaginosis and skin infections. Antimicrob Agents Chemother. 1989 Jun;33(6):970–972. doi: 10.1128/aac.33.6.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp H. R., Melly M. A. Bactericidal effects of polyunsaturated fatty acids. J Infect Dis. 1986 Jul;154(1):84–94. doi: 10.1093/infdis/154.1.84. [DOI] [PubMed] [Google Scholar]
- Leive L. The barrier function of the gram-negative envelope. Ann N Y Acad Sci. 1974 May 10;235(0):109–129. doi: 10.1111/j.1749-6632.1974.tb43261.x. [DOI] [PubMed] [Google Scholar]
- Miller H. Practical aspects of preparing phage and plasmid DNA: growth, maintenance, and storage of bacteria and bacteriophage. Methods Enzymol. 1987;152:145–170. doi: 10.1016/0076-6879(87)52016-x. [DOI] [PubMed] [Google Scholar]
- Nikaido H., Nakae T. The outer membrane of Gram-negative bacteria. Adv Microb Physiol. 1979;20:163–250. doi: 10.1016/s0065-2911(08)60208-8. [DOI] [PubMed] [Google Scholar]
- Nikaido H. Outer membrane of Salmonella typhimurium. Transmembrane diffusion of some hydrophobic substances. Biochim Biophys Acta. 1976 Apr 16;433(1):118–132. doi: 10.1016/0005-2736(76)90182-6. [DOI] [PubMed] [Google Scholar]
- Nikaido H., Vaara M. Molecular basis of bacterial outer membrane permeability. Microbiol Rev. 1985 Mar;49(1):1–32. doi: 10.1128/mr.49.1.1-32.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raetz C. R. Biochemistry of endotoxins. Annu Rev Biochem. 1990;59:129–170. doi: 10.1146/annurev.bi.59.070190.001021. [DOI] [PubMed] [Google Scholar]
- Russell A. D., Gould G. W. Resistance of Enterobacteriaceae to preservatives and disinfectants. Soc Appl Bacteriol Symp Ser. 1988;17:167S–195S. [PubMed] [Google Scholar]
- Schmidt G., Schlecht S., Westphal O. Untersuchungen zur Typisierung von Salmonella-R-Formen. 3. Typisierung von S. minnesota-Mutanten mittels chemischer Agenzien. Zentralbl Bakteriol Orig. 1969 Dec;212(1):88–96. [PubMed] [Google Scholar]
- Sheu C. W., Freese E. Lipopolysaccharide layer protection of gram-negative bacteria against inhibition by long-chain fatty acids. J Bacteriol. 1973 Sep;115(3):869–875. doi: 10.1128/jb.115.3.869-875.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shlaes D. M., Shlaes J. H., Davies J., Williamson R. Escherichia coli susceptible to glycopeptide antibiotics. Antimicrob Agents Chemother. 1989 Feb;33(2):192–197. doi: 10.1128/aac.33.2.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit J., Kamio Y., Nikaido H. Outer membrane of Salmonella typhimurium: chemical analysis and freeze-fracture studies with lipopolysaccharide mutants. J Bacteriol. 1975 Nov;124(2):942–958. doi: 10.1128/jb.124.2.942-958.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonntag I., Schwarz H., Hirota Y., Henning U. Cell envelope and shape of Escherichia coli: multiple mutants missing the outer membrane lipoprotein and other major outer membrane proteins. J Bacteriol. 1978 Oct;136(1):280–285. doi: 10.1128/jb.136.1.280-285.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker B. A., Nurminen M., Mäkelä P. H. Mutants defective in the 33K outer membrane protein of Salmonella typhimurium. J Bacteriol. 1979 Aug;139(2):376–383. doi: 10.1128/jb.139.2.376-383.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukupolvi S., Vaara M., Helander I. M., Viljanen P., Mäkelä P. H. New Salmonella typhimurium mutants with altered outer membrane permeability. J Bacteriol. 1984 Aug;159(2):704–712. doi: 10.1128/jb.159.2.704-712.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukupolvi S., Vaara M. Salmonella typhimurium and Escherichia coli mutants with increased outer membrane permeability to hydrophobic compounds. Biochim Biophys Acta. 1989 Dec 6;988(3):377–387. doi: 10.1016/0304-4157(89)90011-7. [DOI] [PubMed] [Google Scholar]
- Tamaki S., Sato T., Matsuhashi M. Role of lipopolysaccharides in antibiotic resistance and bacteriophage adsorption of Escherichia coli K-12. J Bacteriol. 1971 Mar;105(3):968–975. doi: 10.1128/jb.105.3.968-975.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaara M. Antimicrobial susceptibility of Salmonella typhimurium carrying the outer membrane permeability mutation SS-B. Antimicrob Agents Chemother. 1990 May;34(5):853–857. doi: 10.1128/aac.34.5.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaara M., Jaakkola J. Sodium hexametaphosphate sensitizes Pseudomonas aeruginosa, several other species of Pseudomonas, and Escherichia coli to hydrophobic drugs. Antimicrob Agents Chemother. 1989 Oct;33(10):1741–1747. doi: 10.1128/aac.33.10.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaara M., Vaara T. Polycations sensitize enteric bacteria to antibiotics. Antimicrob Agents Chemother. 1983 Jul;24(1):107–113. doi: 10.1128/aac.24.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaara M., Vaara T. Sensitization of Gram-negative bacteria to antibiotics and complement by a nontoxic oligopeptide. Nature. 1983 Jun 9;303(5917):526–528. doi: 10.1038/303526a0. [DOI] [PubMed] [Google Scholar]
- Viljanen P., Matsunaga H., Kimura Y., Vaara M. The outer membrane permeability-increasing action of deacylpolymyxins. J Antibiot (Tokyo) 1991 May;44(5):517–523. doi: 10.7164/antibiotics.44.517. [DOI] [PubMed] [Google Scholar]
- Ward A., Campoli-Richards D. M. Mupirocin. A review of its antibacterial activity, pharmacokinetic properties and therapeutic use. Drugs. 1986 Nov;32(5):425–444. doi: 10.2165/00003495-198632050-00002. [DOI] [PubMed] [Google Scholar]
- Wilkinson R. G., Gemski P., Jr, Stocker B. A. Non-smooth mutants of Salmonella typhimurium: differentiation by phage sensitivity and genetic mapping. J Gen Microbiol. 1972 May;70(3):527–554. doi: 10.1099/00221287-70-3-527. [DOI] [PubMed] [Google Scholar]
- Yamaguchi A., Ohmori H., Kaneko-Ohdera M., Nomura T., Sawai T. Delta pH-dependent accumulation of tetracycline in Escherichia coli. Antimicrob Agents Chemother. 1991 Jan;35(1):53–56. doi: 10.1128/aac.35.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]


