Abstract
The human cerebral and systemic amyloidoses and prion-associated spongiform encephalopathies are acquired or inherited protein folding disorders in which normally soluble proteins or peptides are converted into fibrillar aggregates. This is a nucleation-dependent process that can be initiated or accelerated by fibril seeds formed from homologous or heterologous amyloidogenic precursors that serve as an amyloid enhancing factor (AEF) and has pathogenic significance in that disease may be transmitted by oral ingestion or parenteral administration of these conformationally altered components. Except for infected brain tissue, specific dietary sources of AEF have not been identified. Here we report that commercially available duck- or goose-derived foie gras contains birefringent congophilic fibrillar material composed of serum amyloid A-related protein that acted as a potent AEF in a transgenic murine model of secondary (amyloid A protein) amyloidosis. When such mice were injected with or fed amyloid extracted from foie gras, the animals developed extensive systemic pathological deposits. These experimental data provide evidence that an amyloid-containing food product hastened the development of amyloid protein A amyloidosis in a susceptible population. On this basis, we posit that this and perhaps other forms of amyloidosis may be transmissible, akin to the infectious nature of prion-related illnesses.
Keywords: amyloid protein A amyloidosis, amyloid-enhancing factor, protein aggregation, rheumatoid arthritis, transmissibility
Amyloid protein A amyloidosis (AA) occurs in patients with rheumatoid arthritis and other chronic inflammatory diseases and results from a sustained elevation of the apolipoprotein serum amyloid A (SAA) protein produced by hepatocytes under regulation by interleukin (IL)-1, IL-6, and tumor necrosis factor (1). This acute-phase reactant is cleaved into an ≈76-residue N-terminal fragment deposited as amyloid predominately in the kidneys, liver, and spleen. The disorder also can be induced experimentally in susceptible strains of mice by inflammatory stimuli that result in an >1,000-fold increase in SAA concentration (2). Further, the lag phase of this process is greatly decreased by injecting or feeding animals extracts of amyloid-laden spleens of affected mice (2–5).
To determine whether amyloid-containing food products exhibit amyloid enhancing factor (AEF) activity, we used a more robust in vivo murine model of AA amyloidosis involving mice carrying the human IL-6 (hIL-6) gene under control of either the murine metallothionein-1 (MT-1) (MT-1/hIL-6) or histocompatibility H2-Ld (H2/hIL-6) promoter (6). Typically, AA amyloid develops in these animals at ≈5 mo of age and is initially located predominately in the perifollicular regions of the spleen. Over the next 2–3 mo, the deposits spread rapidly into the liver parenchyma, renal glomerular and intertubular regions, cardiac muscle, tongue, and gastrointestinal tract and lead to death at ≈8–9 mo. However, by injection into 8-wk-old transgenic mice of a single 100-μg i.v. dose of an exogenous source of AA fibrils, amyloid deposits are formed within 3 wk, and severe systemic disease (akin to that found in 8-mo-old animals) occurs within 2 mo, at which time the resultant pathology is lethal (7).
AA amyloid deposits are commonly found in waterfowl, particularly Pekin ducks, in which the liver is predominately involved (8–10). This pathological alteration is noticeably increased in birds subjected to stressful environmental conditions as well as to the forced feeding that is used to produce foie gras (8). This culinary product, derived from massively enlarged fatty livers results from gorging young ducks or geese up to three times daily over a 4-wk period with corn-based feed.
We now report the results of our studies that have shown that AA-containing fibrils extracted from duck or goose foie gras have potent AEF activity when administered by i.v. injection or gavage into our IL-6 transgenic mice.
Results and Discussion
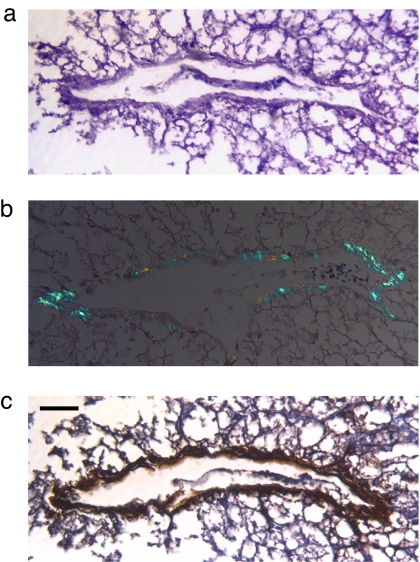
We analyzed several commercial sources of foie gras histochemically and found amyloid to be present. Microscopic examination of hematoxylin/eosin- and Congo red-stained sections cut from formalin-fixed, paraffin-embedded specimens revealed virtual replacement of the normal hepatic parenchyma by fat; additionally, green birefringent congophilic areas in residual vasculature were noted by polarizing microscopy (Fig. 1 a and b). Further, these deposits were immunostained by a specific anti-AA antiserum (Fig. 1c). Similar material was found in marketed pâtés prepared from duck or goose liver (Fig. 2).
Fig. 1.
AA deposition in foie gras. (a) Large venule surrounded by residual, extensively vacuolated fatty hepatic tissue (hematoxylin/eosin stain). (b) Green birefringent amyloid deposits in the blood vessel wall (Congo red stain). (c) Immunohistochemical identification of vascular AA amyloid. (Scale bar, 62 μm.)
Fig. 2.
Tissue fragment with amyloid in duck pâté. Congo red stain.
The AA composition of the hepatic amyloid deposits was confirmed chemically through analysis of material derived from acetone-defatted specimens extracted first with 0.15 M NaCl and then distilled water. The isolates were strongly congophilic, and, when examined by transmission electron microscopy, contained fibrils with the typical ultrastructural features of amyloid; namely, ≈10-μm-thick unbranched structures (Fig. 3a). Electrophoresis of the water-suspended product on a SDS/polyacrylamide gel in the presence of 0.1 M DTT and 8 M urea revealed, after Coomassie blue staining, a protein band with a Mr of ∼6,000, comparable to that of amyloid extracted from the spleen of a mouse with AA amyloidosis (Fig. 3b). After transfer to a PVDF membrane, this component was subjected to automated Edman degradation with which 14 residues identical in amino acid sequence to that of the N-terminal portion of duck SAA were detected. In a similar study of tryptic digests obtained from cleavage of this molecule after reduction and alkylation, six peptides that included 45 of the first 60 residues of duck SAA were identified by MS/MS (Fig. 3c) (9).
Fig. 3.
Ultrastructural and chemical characterization of amyloid extracted from foie gras. (a) Fibrillar nature of proteins contained in the pellet (electron micrograph, negative uranyl acetate stain). (Scale bar, 200 nm.) (b) SDS/PAGE of congophilic components extracted from duck foie gras and the spleen of a mouse with AA amyloidosis (Coomassie blue stain). The Mr values of the standard proteins are given; arrows show the location of AA-containing protein bands. (c) Comparison of the amino acid sequence of duck foie gras AA amyloid with that of duck SAA (9). Homologous residues are indicated by dashes.
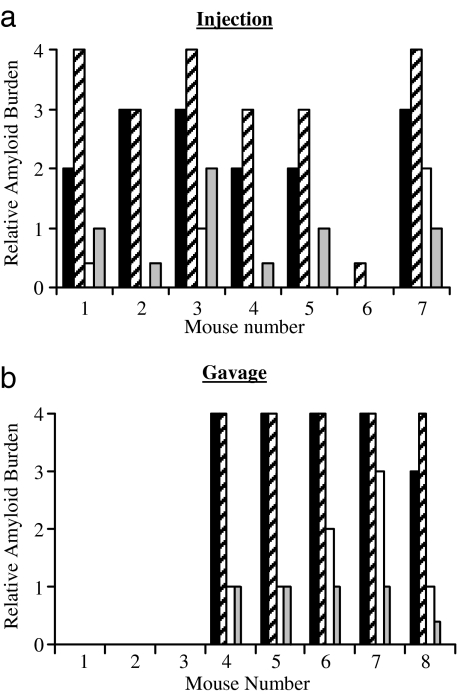
To determine whether amyloid-containing duck- or goose-derived foie gras had AEF activity, groups of up to nine MT-1/hIL-6 or H2/hIL-6 mice received tail vein injections of either 100 μg of extract suspended in 0.1 ml of PBS or the equivalent volume of PBS alone. Both sets were euthanized 8 wk later and multiple organs (liver, spleen, kidney, pancreas, heart, lung, tongue, and intestines) were obtained at necropsy for histochemical analysis. Examination by polarizing microscopy of Congo red-stained sections revealed the presence of varying amounts of amyloid deposits in one or more tissues of virtually all of the treated mice; most affected were the liver, spleen, and to a lesser extent, the kidneys and pancreas (Fig. 4a). In contrast, control animals that received PBS had no detectable amyloid.
Fig. 4.
AEF activity of foie gras. Hepatic and splenic amyloid deposits found in hIL-6 transgenic mice 8 wk after they were injected (a) or gavaged (b) with AA fibrils extracted from foie gras. The extent of amyloid burden in the liver (■), spleen (▨), kidney (□), and pancreas (▩) is as indicated. (Scale bars, 100 μm.)
Similar results were obtained in the conventional murine model of AA amyloidosis in which SAA overexpression was induced by an inflammatory stimulus (4). Two groups of wild-type BALB/c mice were given two 0.5-ml s.c. injections of aqueous 1% AgNO3 (days 1 and 10), and one set also were injected i.v. with 100 μg of the foie gras extract; the others (controls) received PBS only. At the time of euthanizing (day 21), 8 of 10 mice from the first group had detectable amyloid in the liver and spleen. In contrast, no amyloid was found in the control animals.
The amyloid induced by administration of fibril-containing foie gras into both the wild-type and transgenic mice was immunostained by an anti-AA antibody. Further, the deposits were AA in nature as confirmed by MS of protein extracted from the spleen of a recipient animal. MS/MS analyses of tryptic peptides generated from an HPLC-purified reduced and alkylated water pellet identified residues 19–56 of murine SAA.
AA-containing foie gras extracts also had AEF activity when administered orally to the hIL-6 transgenic animals. Five of eight mice that were gavaged for 5 consecutive days with 100 μg of material suspended in 50 μl of PBS were found 8 wk later to have amyloid deposits in virtually all organs examined, and, as in the case of animals injected i.v. with this material, this effect was most pronounced in the liver and spleen (Fig. 4b).
The AEF activity of foie gras was reduced, but not abolished, by cooking, as specified by the supplier. Intravenous injections into nine hIL-6 transgenic mice of 100-μg doses of extracts prepared from liver that had been heated to ≈95°C for 20 min in an oven resulted in 4+, 2+, and 1+ hepatic and/or splenic amyloid deposits in two, one, and two animals, respectively (in four cases, no amyloid was found). In contrast, when this material was dissolved in 6 M guanidine HCl, incubated at 37°C for 24 h, dialyzed against PBS, and injected into six transgenic mice, only traces of splenic amyloid were found in two mice. A summary of the results of all of the above studies is presented in Table 1 and supporting information (SI) Fig. 5.
Table 1.
Summary of the amyloidogenic potential of foie gras preparations
| Group | Mice (n) | Treatment | Route | Positive (%) | Mean score* |
|---|---|---|---|---|---|
| 1 | H-2/hIL-6 (8) | Extract 1 | i.v. | 3 (37.5) | 3+ |
| 2 | H-2/hIL-6 (6) | Extract 2 | i.v. | 2 (33.3) | 4+ |
| 3 | H-2/hIL-6 (7) | Extract 3 | i.v. | 6 (85.7) | 1.9+ |
| 4 | H-2/hIL-6 (5) | PBS | i.v. | 0 (0) | 0 |
| 5 | H-2/hIL-6 (7) | Extract 4 | i.v. | 7 (100) | 3.1+ |
| 6 | MT-1/hIL-6 (8) | Extract 4 | Gavage | 5 (62.5) | 4+ |
| 7 | MT-1/hIL-6 (7) | Extract 5 | i.v. | 5 (71.4) | 4+ |
| 8 | H-2/hIL-6 (9) | Extract 5/cooked | i.v. | 5 (55.6) | 1.75+ |
| 9 | H-2/hIL-6 (6) | Extract 5/guanidine HCl | i.v. | 2 (33.3) | 0.5+ |
| 10 | BALB/c (10) | Extract 5 | i.v. | 8 (80) | 1.6+ |
| 11 | BALB/c (5) | PBS | i.v. | 0 (0) | 0 |
*Mean score of amyloid-positive spleens in each group.
The prevalence of AA amyloidosis in the human population is unknown. In most developed countries, sustained inflammatory processes, particularly rheumatoid and juvenile chronic arthritis (as opposed to infectious diseases), now account for the majority of such cases (11). Notably, there is a marked geographic variation in incidence among such patients, e.g., the number is relatively high in Europe compared with the United States but particularly high in parts of Papua New Guinea (12). This difference has been attributed, in part, to genetic factors, including expression of a more amyloidogenic SAA allotype (13) or other genes encoding inflammatory molecules (11). Notably, increased blood levels of SAA do not necessarily result in amyloidosis (11, 14).
Given our experimental findings, exposure to exogenous substances with AEF activity also may be an important epigenetic or environmental factor in the development of AA amyloidosis in a susceptible population. In this regard, it would seem prudent for children and adults with rheumatoid arthritis or other diseases who are at risk for this disorder to avoid foods that may be contaminated with AA fibrils (15). In addition to foie gras, meat derived from sheep (16) and seemingly healthy cattle (17) may represent other dietary sources of this material. Further, the fact that chemically heterologous fibrils can serve as AEF, as demonstrated in experimental models of AA (18–20) and AApoAII amyloidosis (21–23), suggests that it may be hazardous for individuals who are prone to develop other types of amyloid-associated disorders, e.g., Alzheimer's disease or type II diabetes, to consume such products.
Materials and Methods
Materials.
Whole fresh duck and goose liver (foie gras) was purchased from three commercial venders located in the United States and France.
Mice.
Mice carrying the hIL-6 gene under control of the mouse MT-1 or histocompatibility H2-Ld promoter were obtained from Gennaro Ciliberto and Michael Potter, respectively, and generated as described previously (6). At 4 wk the mice were weaned, and the presence of the transgene was confirmed through analysis of genomic DNA derived from tail snips. Wild-type BALB/c mice were purchased from Charles River Laboratories (Boston, MA). Animals were housed in groups of eight in a positive pressure environment with a 12-h light/dark cycle and provided filtered tap water and a standard laboratory rodent chow (Harlan Teklad, Madison, WI) ab libitum. The animals were treated in accordance with National Institutes of Health regulations under the aegis of a protocol approved by the University of Tennessee's Animal Care and Use Committee.
Histochemical and Immunohistochemical Analyses.
Samples of foie gras and mouse organs obtained at necropsy were placed in 10% buffered formalin (Fisher Scientific International, Inc., Hampton, NH) and embedded in paraffin. To detect amyloid, 6-μm-thick deparaffinized sections were stained with a freshly prepared solution of alkaline Congo red and examined under polarizing microscopy. A qualitative assessment of amyloid deposition was made by an experienced microscopist (T.R.) based on the relative extent of green birefringence seen in at least 10 fields at ×20 magnification; a value of 1+, 2+, 3+, or 4+ was assigned if such material occupied, respectively, trace, minimal, moderate, or extensive portions of the sections studied, and these values were corroborated by quantitative image analyses. Electron microscopy on Epon-embedded sections stained negatively with 1% uranyl acetate on Formvar-coated copper grids was performed with a Hitachi H-800 electron microscope (Hitachi High Technology, Pleasanton, CA). Specimens were immunostained using the avidin-biotin complex technique (Vector Laboratories, Burlingame, CA). An anti-marmoset AA murine mAb (24) and a biotinylated sheep anti-mouse Ig antiserum (BioGenex, San Ramon, CA) were used as the primary and secondary reagents, respectively, and the reactions were visualized with the Super Sensitive Link-labeled HRP Detection System (BioGenex), under conditions specified by the manufacturer.
Amyloid Extraction and Characterization.
Thirty- to 80-gram portions of foie gras were cut into 0.5-cm3 pieces and, after defatting by a series of four acetone washes, were homogenized in 0.15 M NaCl using an Omni-Mixer blender (Omni International, Marietta, GA) and centrifuged (15,000 × g) for 30 min at 4°C. This step was repeated until the OD280 of the supernatant was <0.10. The saline-extracted sediment was similarly homogenized in cold distilled water, and the resultant pellet was lyophilized. After reextraction with chloroform and ether, the protein was dissolved in 0.25 M Tris·HCl buffer, pH 8.0, containing 6 M guanidine HCl, reduced and alkylated, and purified by reverse-phase HPLC (25). The peak UV-absorbing fractions were dried in a vacuum centrifuge, reconstituted in sample loading buffer, and, after electrophoresis on 10% NuPage SDS/PAGE gels (Invitrogen, Carlsbad, CA), transferred to a PVDF membrane. Coomassie blue-stained bands were excised and subjected to automated sequence analysis by Edman degradation using an ABI model 494 pulsed liquid sequencer (Applied BioSystems, Foster City, CA). The amino acid sequences of tryptic peptides generated from HPLC-purified fibrillar tissue extracts were determined by MS/MS using an ion-trap instrument (Thermo Finnigan, Waltham, MA), as described previously (25).
Supplementary Material
Acknowledgments
We thank T. K. Williams, S. D. Macy, C. Wooliver, S. Wang, and J. Dunlap for technical assistance; A. Lehberger for manuscript preparation; and R. Wetzel for critical input. This work was supported in part by Public Health Research Grant CA 10056 from the National Cancer Institute, by the Aslan Foundation, and by the Swedish Research Council and Torsten and Ragnar Söderberg's Foundations. A.S. is an American Cancer Society Clinical Research Professor.
Abbreviations
- AA
amyloid protein A amyloidosis
- AEF
amyloid enhancing factor
- IL
interleukin
- SAA
serum amyloid A protein.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0700848104/DC1.
References
- 1.Benson MD. In: The Metabolic and Molecular Bases of Inherited Disease. Scriver CR, Beaudet AL, Sly WS, Valle D, editors. New York: McGraw–Hill; 2001. pp. 5345–5378. [Google Scholar]
- 2.Kisilevsky R, Boudreau L. Lab Invest. 1983;48:53–59. [PubMed] [Google Scholar]
- 3.Hardt F, Ranløv PJ. Int Rev Exp Pathol. 1976;16:273–334. [PubMed] [Google Scholar]
- 4.Johan K, Westermark GT, Engström U, Gustavsson Å, Hultman P, Westermark P. Proc Natl Acad Sci USA. 1998;95:2558–2563. doi: 10.1073/pnas.95.5.2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lundmark K, Westermark GT, Nyström S, Murphy CL, Solomon A, Westermark P. Proc Natl Acad Sci USA. 2002;99:6979–6984. doi: 10.1073/pnas.092205999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Solomon A, Weiss DT, Schell M, Hrncic R, Murphy CL, Wall J, McGavin MD, Pan HJ, Kabalka GW, Paulus MJ. Am J Pathol. 1999;154:1267–1272. doi: 10.1016/S0002-9440(10)65378-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wall JS, Kennel SJ, Paulus MJ, Gleason S, Gregor J, Baba J, Schell M, Richey T, O'Nuallain B, Donnell R, et al. Amyloid. 2005;12:149–156. doi: 10.1080/13506120500222359. [DOI] [PubMed] [Google Scholar]
- 8.Cowan DF, Johnson WC. Lab Invest. 1970;23:551–555. [PubMed] [Google Scholar]
- 9.Guo JT, Aldrich CE, Mason WS, Pugh JC. Proc Natl Acad Sci USA. 1996;93:14548–14553. doi: 10.1073/pnas.93.25.14548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Landman WJM, Gruys E, Gielkens ALJ. Avian Pathol. 1998;27:437–449. doi: 10.1080/03079459808419367. [DOI] [PubMed] [Google Scholar]
- 11.Hazenberg BP, van Rijswijk MH. Ann Rheum Dis. 2000;59:577–579. doi: 10.1136/ard.59.8.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McAdam KPWJ, Raynes JG, Alpers MP, Westermark GT, Westermark P. Papua New Guinea Med J. 1996;39:284–296. [PubMed] [Google Scholar]
- 13.Booth DR, Booth SE, Gillmore JD, Hawkins PN, Pepys MB. Amyloid. 1998;5:262–265. doi: 10.3109/13506129809007299. [DOI] [PubMed] [Google Scholar]
- 14.Scheinberg MA, Benson MD. J Rheumatol. 1980;7:724–726. [PubMed] [Google Scholar]
- 15.Cathcart ES, Elliott-Bryant R. Amyloid. 1999;6:107–113. doi: 10.3109/13506129909007310. [DOI] [PubMed] [Google Scholar]
- 16.Mensua C, Carrasco L, Bautista MJ, Biescas E, Fernandez A, Murphy CL, Weiss DT, Solomon A, Lujan L. Vet Pathol. 2003;40:71–80. doi: 10.1354/vp.40-1-71. [DOI] [PubMed] [Google Scholar]
- 17.Tojo K, Tokuda T, Hoshii Y, Fu X, Higuchi K, Matsui T, Kametani F, Ikeda S-I. Amyloid. 2005;12:103–108. doi: 10.1080/13506120500107097. [DOI] [PubMed] [Google Scholar]
- 18.Ganowiak K, Hultman P, Engström U, Gustavsson Å, Westermark P. Biochem Biophys Res Commun. 1994;199:306–312. doi: 10.1006/bbrc.1994.1229. [DOI] [PubMed] [Google Scholar]
- 19.Cui D, Kawano H, Takahashi M, Hoshii Y, Setoguchi M, Gondo T, Ishihara T. Pathol Int. 2002;52:40–45. doi: 10.1046/j.1440-1827.2002.01309.x. [DOI] [PubMed] [Google Scholar]
- 20.Lundmark K, Westermark GT, Olsén A, Westermark P. Proc Natl Acad Sci USA. 2005;102:6098–6102. doi: 10.1073/pnas.0501814102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fu X, Korenaga T, Fu L, Xing Y, Guo Z, Matsushita T, Hosokawa M, Naiki H, Baba S, Kawata Y, et al. FEBS Lett. 2004;563:179–184. doi: 10.1016/S0014-5793(04)00295-9. [DOI] [PubMed] [Google Scholar]
- 22.Korenaga T, Fu X, Xing Y, Matsusita T, Kuramoto K, Syumiya S, Hasegawa K, Niaiki H, Ueno M, Ishihara T, et al. Am J Pathol. 2004;164:1597–1606. doi: 10.1016/S0002-9440(10)63718-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Korenaga T, Yan J, Sawashita J, Matsushita T, Naiki H, Hosokawa M, Mori M, Higuchi K, Fu X. Am J Pathol. 2006;168:898–906. doi: 10.2353/ajpath.2006.050350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ludlage E, Murphy CL, Davern SM, Solomon A, Weiss DT, Glenn-Smith D, Dworkin S, Mansfield KG. Vet Pathol. 2005;42:117–124. doi: 10.1354/vp.42-2-117. [DOI] [PubMed] [Google Scholar]
- 25.Murphy CL, Eulitz M, Hrncic R, Sletten K, Westermark P, Williams T, Macy SD, Wooliver C, Wall J, Weiss DT, et al. Am J Clin Path. 2001;116:135–142. doi: 10.1309/TWBM-8L4E-VK22-FRH5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.