Abstract
Polygenic sex determination, although suspected in several species, is thought to be evolutionarily unstable and has been proven in very few cases. In the European sea bass, temperature is known to influence the sex ratio. We set up a factorial mating, producing 5.893 individuals from 253 full-sib families, all reared in a single batch to avoid any between-families environmental effects. The proportion of females in the offspring was 18.3%, with a large variation between families. Interpreting sex as a threshold trait, the heritability estimate was 0.62 ± 0.12. The observed distribution of family sex ratios was in accordance with a polygenic model or with a four-sex-factors system with environmental variance and could not be explained by any genetic model without environmental variance. We showed that there was a positive genetic correlation between weight and sex (rA = 0.50 ± 0.09), apart from the phenotypic sex dimorphism in favor of females. This supports the hypothesis that a minimum size is required for sea bass juveniles to differentiate as females. An evolution of sex ratio by frequency-dependent selection is expected during the domestication process of Dicentrarchus labrax populations, raising concern about the release of such fish in the wild.
IN gonochoric species with genetically determined sex, a one-to-one sex ratio is known to be optimal in an infinite population of diploid individuals with random mating and Mendelian segregation (Fisher 1930; Charnov 1975). The observation of skewed sex ratios may imply, among other things, non-Mendelian segregation as in Drosophila (Vaz and Carvalho 2004), nonrandom mating (Hamilton 1967), or environmental sex determination (ESD; Bull 1985). In the latter case, the sex of an individual is not fixed at conception, but is influenced by environmental conditions during its early life. ESD is expected to be favored when the offspring lives in patchy environments, which may confer advantages to being male or female, and neither the offspring nor the parent have control and/or predictive ability on the type of patch in which the offspring will live (Charnov and Bull 1977). Temperature (e.g., Bull and Vogt 1979; Baroiller and D'Cotta 2001) seems to be the main environmental factor implied, but density (Ellenby 1954) and social status (Francis and Barlow 1993) have been shown to be possible sex-determining environmental factors. In species with ESD, in many cases there is also a genetic variation (Bull et al. 1982; Conover and Heins 1987a; Janzen 1992; Baroiller and D'Cotta 2001), which in some cases has been described as polygenic (Bull et al. 1982; Janzen 1992). Polygenic sex determination, however, is considered to be evolutionarily unstable (Rice 1986) and its maintenance is still poorly understood. It is thought by some authors to be the ancestral type of sex determinism in fish (Kirpichnikov 1981), but organisms where it is accepted that sex has a polygenic component are indeed very few: the parasitic wasp Nasonia vitripennis (Orzack and Gladstone 1994), the turtles Graptemys ouachitensis (Bull et al. 1982) and Chelydra serpentina (Janzen 1992), and probably the swordtail fish Xiphophorus helleri (Kosswig 1964).
The European sea bass (Dicentrarchus labrax) is a gonochoristic teleost fish distributed in the northeastern Atlantic, the Mediterranean, and the Black Sea (Pickett and Pawson 1994). They live in shallow, coastal waters, estuaries, lagoons, and harbors, moving to deeper waters (up to 100 m deep) as they grow. Although they can live in waters <5°, they seek temperatures >10°, and even 15° in their first year (Kelley 1988). They spawn in open waters from late winter to early spring, depending on the latitude. The eggs hatch in 4–9 days, and the young fish move inshore in their first month toward the warmest waters, especially in estuaries (Pickett and Pawson 1994). Sex remains undifferentiated for a long period: differentiation occurs between 128 and 250 days postfertilization (dpf; Saillant et al. 2003a). Records of sea bass sex ratio in wild populations are scarce. They show balanced sex ratios (Saillant et al. 2003a) and an excess of males (B. Menu, personal communication) or of females (Arias 1980), but as a whole do not contradict the hypothesis of balanced sex ratios in the wild. The sea bass is an important species in Mediterranean aquaculture, and it appears that, in all aquaculture populations, sex ratios are strongly biased toward males (75–95%, e.g., Blazquez et al. 1998; Saillant et al. 2002, 2003a), which is a problem for farmers as males mature earlier and grow less than females. Temperature has been shown to have a major effect on sex determination in sea bass (Blazquez et al. 1998; Pavlidis et al. 2000; Saillant et al. 2002; Mylonas et al. 2005). The effect of temperature is not fully understood, as two studies show an increased proportion of males with cold temperature (15°: Blazquez et al. 1998; 13°: Saillant et al. 2002) while the other two studies show an increased proportion of females at 13° and 15° (Pavlidis et al. 2000; Mylonas et al. 2005). The current hypothesis is that low temperatures early in development (<100 dpf) may favor female sex differentiation, but that long-lasting low temperatures, through a negative effect on growth, may preclude female differentiation and result in an increased proportion of males (Piferrer et al. 2005). Thus, for productivity reasons, the excess of males observed in culture would be due to the use of temperatures higher than in nature. From the genetic point of view, in addition to the environmental effect on sex, simple female homogamety can be excluded, as the sex ratios of normal diploid and gynogenetic offspring are equivalent (Felip et al. 2002; Peruzzi et al. 2004). The sex ratio of the offspring from masculinized females is not female biased and would rule out both XX–XY (female homogamety) and ZW–ZZ (male homogamety) systems (Blazquez et al. 1999). In this latter study, however, the possible male bias induced by high rearing temperatures (22.5°), and the impossibility of ascertaining the genetic sex of the sex-reversed parents, makes the demonstration a little weak. Therefore, male homogamety with environmentally male-biased sex ratios would still be a possibility. Additionally, parental influence on the sex ratio of progeny has also been demonstrated, in very limited experimental settings, however (Saillant et al. 2002; Gorshkov et al. 2003), showing that there is a genetic component of the progeny sex ratio. Although it is clear that the sex of sea bass is determined both by genetic factors and by the environment (mostly temperature), the sex determination system of this species remains basically unknown (Piferrer et al. 2005).
In this study, our aim was to describe the genetic component influencing sex ratio in sea bass, using a large number of families in classical aquaculture conditions, and to determine which genetic models could describe it best. We described sex using a threshold model with an underlying variable (sex tendency), as this type of model integrates both genetic and environmental effects, which are known to influence sex ratio in the sea bass.
MATERIALS AND METHODS
A partly factorial mating design:
The brood fish used were from a group of 33 males and 51 females of wild Atlantic origin, collected in 2000 on the coasts of Brittany, France. Each brood fish was individually tagged and fin clipped for DNA extraction. The sperm of males was cryopreserved in 250 μl straws (Fauvel et al. 1998). In January 2001, 51 females were injected with 10 μg/kg luteinizing hormone-releasing hormone (Sigma, D-TRP6-LHRH), and eggs were stripped 72 hr later. Twenty-three females gave a sufficient quantity of good quality oocytes. From these spawns, we produced a mating design combining 33 males and 23 females in three full factorial sets of 11♂ × 9♀, 11♂ × 7♀, and 11♂ × 7♀, for a total of 253 families. All full-sib families were fertilized individually and then eggs were grouped by female for incubation (48 hr at 13°), after which 2 ml of viable eggs per female (∼2.000 eggs/female) were collected to create one batch containing all families. Standard rearing conditions were used, with temperature gradually increasing from 13° to 18° in the first 64 days. Temperature was then kept at 18° until 238 days (mean length of fish was 117 mm, and mean weight was 23.6 g) and then lowered to 14° to slow down growth until the time scheduled for tagging. Although late low temperatures are suspected of masculinizing the progeny (Piferrer et al. 2005), this does not apply at 238 days, as it was shown before that lowering the temperature from 20° to 13° at 149 days (mean length was 81 mm) had no impact on the sex ratio (Saillant et al. 2002).
Recording of traits and parentage assignment:
At 370 days, the fish had reached a mean weight of 35 g. Seven thousand of them were randomly selected, on which individual weight and length were measured. Each fish was individually tagged and fin clipped for DNA extraction. The fish were then sent to four different sites (1.750/site), where they were reared until reaching ∼400 g mean weight. This rearing in different sites was designed for estimating genetic parameters and genotype–environment interactions for growth and quality traits, in parallel with this study. Still, it was not expected to have any impact on the sex ratio, as the differentiation period is over well before 370 days. At 400 g, the remaining fish (5.988) were slaughtered and sex was recorded by visual observation of the gonads after dissection; 5.960 had an identifiable sex phenotype. In all sites, the difference between males and females was straightforward (female gonads were orange, and male gonads pink/white), and only 28 fish in total could not be determined with certainty. Parentage assignment was done by Landcatch Natural Selection (Alloa, UK) using six microsatellite markers on both parents and offspring. Of the 5.960 offspring with a sex phenotype, 5.896 (98.9%) could be assigned to a single parental pair.
Statistical methods:
Sire 23 (set 3) gave only 3 offspring, probably due to bad sperm quality. It was removed from the analysis, as it created a major disequilibrium in the data, thus reducing the number of families studied from 253 to 246. Thereafter, the base data set comprised 5.893 offspring from 246 families. Apart from sire 23, 245 of 246 possible families had offspring. No offspring were found in the sire 9 × dam 2 family (set 1), probably due to a bad quality straw of cryopreserved sperm, as both male 9 and female 2 gave satisfactory results in all other crosses. To avoid computational problems, these missing data were replaced by simulated data corresponding to the expected numbers of male and female offspring in this family (19 males and three females, which are the average numbers of males and females per family produced by male 9 and female 2).
In a first analysis, the number of females was calculated in each paternal or maternal half-sib family and compared to the expected number of females with a uniform proportion corresponding to the observed proportion of females in the whole sample, using χ2 tests to test for the existence of significant genetic variation in progeny sex ratio. In a second step, the family sex ratios in each of the three full-factorial sets were analyzed by logistic regression using SAS proc Logistic, where the proportion of females was explained by a sire and dam effect. The model fit was tested using the Hosmer and Lemeshow test (Hosmer and Lemeshow 1989). In a third step, sex was considered as a threshold trait with a polygenic basis (Bulmer and Bull 1982). Sex was analyzed using a single-trait model including additive random effects for sire and dam and a residual error. Restricted maximum-likelihood estimates of variance components for the random effects in the model were obtained on the underlying liability scale using the ASREML software (Gilmour et al. 2002). Both sire and dam heritabilities were estimated, using standard formulae (Becker 1984). Genetic correlations between sex and growth were estimated with a trivariate (sex, weight, and length at 370 days) animal model, with sex coded on the observed scale (0 or 1), using the VCE5.0 software (Kovac and Groeneveld 2003). This model included an animal additive genetic effect for all traits and for length and weight, a fixed effect of sex, which was necessary due to sex dimorphism on length and weight (females are larger than males). We used the observed scale since it produces unbiased genetic correlations as long as the threshold trait does not have both low heritability and low incidence (Olausson and Ronningen 1975). We also estimated the heritability of sex dimorphism for growth using classic formulas of (co)variance for a difference (Chapuis et al. 1996).
Then, we used the estimates generated to simulate samples of 5.893 fish from 23 dams and 32 males in three sets of 9 × 11, 7 × 10, and 7 × 11, using a heritability of sex of 0.62 on the underlying scale, and a mean proportion of females of 18.3%. We also generated simulated samples of the same size using a threshold model where the underlying sex tendency m would be determined, instead of polygenes, by one or two biallelic loci with the effect size f, such that genotype aa had a genetic effect of −f on m, genotype Aa had a genetic effect of 0, and genotype AA had a genetic effect of +f. The allelic frequencies were 0.5 for each allele, except for the one locus case where allelic frequencies 0.2, 0.4, 0.5, 0.6, and 0.8 were tested. The effect size f was tuned so that the proportion of genetic variance over phenotypic variance was 0.62, and a random residual effect representing 38% of the total phenotypic variance was added. This model is an extension of the two-factor model with environmental variance, adapted to populations with skewed sex ratios (Bull 1983). Purely genetic threshold models were also tested, either polygenic with h2 = 1 or with one to five biallelic loci, but no residual environmental variance. For each model, we generated 10.000 samples and compared the simulated distribution of full- and half-sib family sex ratios with the observed one, to test for the coherence of the model with the observed data set.
RESULTS
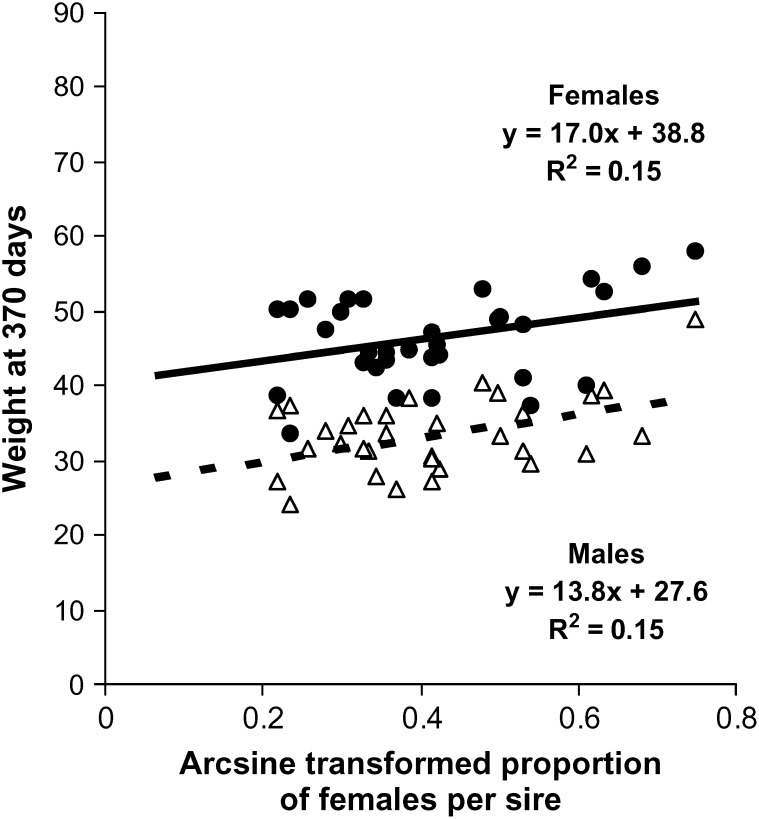
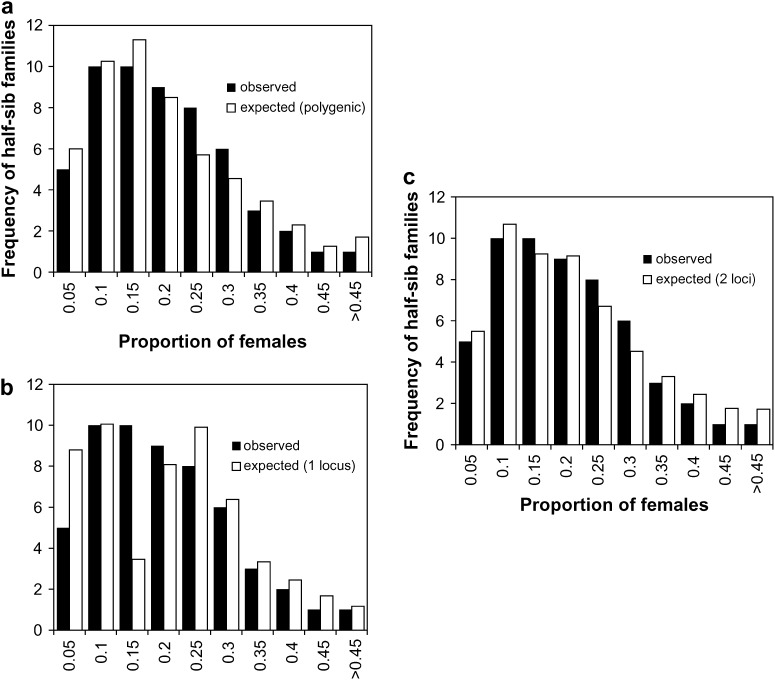
The overall proportion of females in the population was 18.3%, and there were no sex-ratio differences among fish from each of the four growing sites (17.4–19.4%, χ2 = 4.62, 3 d.f., P > 0.20). Family sex ratios are given in appendixes a–c. Comparison of observed values to expected values under the hypothesis of equal sex ratio in all families shows a strong inequality of contributions between half-sib families (χ2 = 350.6, 31 d.f., P < 0.0001 for paternal half-sib families and χ2 = 327.6, 22 d.f., P < 0.0001 for maternal half-sib families). Proportions of females ranged from 4.7 to 46.3% in paternal half-sib families and from 0.5 to 40.3% in maternal half-sib families. The logistic regression analysis showed that both sire and dam had a highly significant effect on the progeny sex ratio (P < 0.0001) and that this model without interaction was enough to explain the observed data set, the Hosmer and Lemeshow χ2 tests being far from significance (χ2 = 6.04, 8 d.f., P > 0.6 in set 1, χ2 = 1.94, 8 d.f., P > 0.9 in set 2, and χ2 = 6.04, 8 d.f., P > 0.6 in set 3). The estimated heritability of sex on the underlying scale was 0.52 ± 0.13 (sire heritability), 0.72 ± 0.20 (dam heritability), or 0.62 ± 0.12 (sire and dam heritability). The maternal-effect ratio (nongenetic maternal variance/phenotypic variance) that would explain the difference between sire and dam heritability is m2 = 0.05 ± 0.06, which is clearly nonsignificant. The heritability of growth sex dimorphism was low (0.15 for weight and 0.09 for length at 370 days). The estimated genetic correlation was 0.50 ± 0.09 between sex and weight and 0.48 ± 0.09 between sex and length. An illustration of this can be seen in Figure 1, which shows that, apart from the fixed effect of sex on weight (females being on average 40.8% heavier than males at 370 days), larger fish tended to be found in the families with the highest proportion of females. The distribution of proportions of females among the 55 half-sib families scored (32 paternal half-sib families, 23 maternal half-sib families) is plotted in Figure 2 and compared with simulated distributions in the same experimental setting. Detailed information about model fit can be found in Table 1. Additive polygenes with h2 = 0.62 could explain the data with excellent fit. For a single locus with environmental variance, the best fit was observed with allelic frequencies of 0.6 for the male-orienting allele and 0.4 for the female-orienting allele, but this could not account for the observed half-sib data. On the contrary, a two-locus system with environmental variance could fit both half-sib and full-sib observed data. No purely genetic model (without environmental variance) could explain the observed data, whatever the number of loci implied (from monogenic to polygenic).
APPENDIX A.
Males and females (M:F) in 99 European sea bass families: set 1 (11 sires × 9 dams)
| Dams |
Mean proportion of females | Total no. of offspring | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sires | D34 | D35 | D36 | D37 | D38 | D39 | D40 | D41 | D42 | ||
| S01 | 19:2 | 21:4 | 20:5 | 20:12 | 20:11 | 19:12 | 30:9 | 19:8 | 30:5 | 0.256 | 266 |
| S02 | 17:0 | 13:0 | 16:0 | 14:3 | 35:4 | 26:6 | 33:3 | 33:5 | 21:0 | 0.092 | 229 |
| S03 | 16:1 | 22:0 | 25:1 | 14:3 | 25:1 | 36:2 | 30:9 | 26:8 | 31:2 | 0.107 | 252 |
| S04 | 7:3 | 15:0 | 12:3 | 10:7 | 27:9 | 17:12 | 36:11 | 17:7 | 18:3 | 0.257 | 214 |
| S05 | 14:4 | 21:2 | 19:5 | 5:5 | 11:7 | 15:6 | 20:4 | 27:7 | 13:3 | 0.229 | 188 |
| S06 | 16:0 | 9:0 | 21:2 | 10:2 | 18:3 | 33:4 | 22:4 | 27:8 | 18:1 | 0.121 | 198 |
| S07 | 14:8 | 18:3 | 24:10 | 12:8 | 22:8 | 12:17 | 29:15 | 15:10 | 7:3 | 0.349 | 235 |
| S08 | 9:1 | 11:1 | 11:2 | 6:0 | 22:0 | 18:0 | 31:0 | 6:2 | 7:0 | 0.047 | 127 |
| S09 | 16:2 | a | 14:0 | 21:1 | 37:4 | 24:3 | 22:4 | 15:5 | 17:0 | 0.103 | 185 |
| S10 | 3:7 | 14:3 | 8:3 | 4:12 | 14:7 | 12:14 | 18:16 | 15:12 | 13:13 | 0.463 | 188 |
| S11 | 11:8 | 24:0 | 20:5 | 20:21 | 17:11 | 19:16 | 28:13 | 22:14 | 30:6 | 0.330 | 285 |
| Mean proportion of females | 0.202 | 0.072 | 0.159 | 0.352 | 0.208 | 0.285 | 0.227 | 0.279 | 0.149 | ||
| Total no. of offspring | 178 | 181 | 226 | 210 | 313 | 323 | 387 | 308 | 241 | 2367 | |
No offspring were observed in this family.
APPENDIX B.
Males and females (M:F) in 77 European sea bass families: set 2 (11 sires × 7 dams)
| Dams |
Mean proportion of females | Total no. of offspring | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Sires | D43 | D44 | D45 | D46 | D47 | D48 | D49 | ||
| S12 | 29:4 | 17:3 | 15:0 | 20:1 | 19:0 | 27:0 | 12:0 | 0.054 | 147 |
| S13 | 15:8 | 34:8 | 30:4 | 47:2 | 29:4 | 35:1 | 11:6 | 0.141 | 234 |
| S14 | 11:17 | 27:2 | 12:0 | 28:0 | 26:4 | 26:2 | 7:3 | 0.170 | 165 |
| S15 | 18:3 | 28:3 | 18:1 | 19:0 | 24:0 | 18:0 | 16:0 | 0.047 | 148 |
| S16 | 9:16 | 21:11 | 26:4 | 28:3 | 38:12 | 20:4 | 13:6 | 0.265 | 211 |
| S17 | 25:18 | 34:15 | 27:1 | 37:1 | 39:6 | 34:1 | 25:2 | 0.166 | 265 |
| S18 | 11:10 | 29:5 | 31:1 | 26:1 | 41:1 | 35:2 | 9:1 | 0.103 | 203 |
| S19 | 11:10 | 22:2 | 13:1 | 21:0 | 26:3 | 27:1 | 18:2 | 0.121 | 157 |
| S20 | 19:10 | 24:9 | 14:1 | 28:1 | 29:1 | 31:1 | 14:1 | 0.131 | 183 |
| S21 | 18:10 | 11:5 | 11:2 | 25:1 | 18:1 | 26:3 | 14:2 | 0.163 | 147 |
| S22 | 16:17 | 11:15 | 24:8 | 22:1 | 14:8 | 16:3 | 11:5 | 0.333 | 171 |
| Mean proportion of females | 0.403 | 0.232 | 0.094 | 0.035 | 0.117 | 0.058 | 0.157 | ||
| Total no. of offspring | 305 | 336 | 244 | 312 | 343 | 313 | 178 | 2031 | |
APPENDIX C.
Males and females (M:F) in 70 European sea bass families: set 3 (10 sires × 7 dams)
| Dams |
Mean proportion of females | Total no. of offspring | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Sires | D50 | D51 | D52 | D53 | D54 | D55 | D56 | ||
| S24 | 3:1 | 4:0 | 5:0 | 5:0 | 5:1 | 7:0 | 13:2 | 0.087 | 46 |
| S25 | 13:3 | 24:9 | 12:2 | 29:0 | 24:9 | 18:1 | 43:20 | 0.213 | 207 |
| S26 | 34:9 | 9:5 | 10:1 | 32:0 | 12:2 | 11:0 | 15:7 | 0.163 | 147 |
| S27 | 22:4 | 17:6 | 9:0 | 27:0 | 22:2 | 6:0 | 21:4 | 0.114 | 140 |
| S28 | 44:4 | 19:2 | 8:0 | 23:0 | 24:4 | 9:0 | 18:2 | 0.076 | 157 |
| S29 | 13:8 | 9:9 | 4:3 | 10:1 | 6:4 | 5:1 | 6:9 | 0.398 | 88 |
| S30 | 37:10 | 21:9 | 17:2 | 33:0 | 38:7 | 16:0 | 20:7 | 0.161 | 217 |
| S31 | 33:6 | 20:9 | 14:1 | 25:0 | 18:11 | 14:1 | 19:15 | 0.231 | 186 |
| S32 | 29:3 | 31:4 | 16:0 | 18:0 | 28:2 | 6:0 | 30:0 | 0.054 | 167 |
| S33 | 26:0 | 27:3 | 9:0 | 12:0 | 22:1 | 11:0 | 24:5 | 0.064 | 140 |
| Mean proportion of females | 0.159 | 0.236 | 0.080 | 0.005 | 0.178 | 0.028 | 0.254 | ||
| Total no. of offspring | 302 | 237 | 113 | 215 | 242 | 106 | 280 | 1495 | |
Figure 1.—
Relationship between the arcsine-transformed proportion of females in sire half-sib families of European sea bass and mean weight at 370 days of male and female offspring in the same families.
Figure 2.—
Observed frequencies of females in 55 half-sib families of European sea bass and expected frequencies under a threshold model with 18.3% females in the offspring and 38% environmental variance, where the genetic component of the underlying variable is (a) polygenic; (b) one biallelic locus with p = 0.6 for the masculinizing allele, q = 0.4 for the feminizing allele; and (c) two biallelic loci with p = q = 0.5.
TABLE 1.
Comparison of family sex-ratio distributions with simulated distributions from various sex-determination models
| Sex-ratio distribution of half-sib families (d.f. = 6) |
Sex-ratio distribution of full-sib families (d.f. = 11) |
|||
|---|---|---|---|---|
| Model | χ2 | P-value | χ2 | P-value |
| Polygenic, h2 = 0.62 | 2.07 | >0.9 | 10.9 | >0.4 |
| One locus with environmental variance | ||||
| p = 0.2 | 58.1 | <0.001 | 171 | <0.001 |
| p = 0.4 | 59.6 | <0.001 | 18.4 | >0.05 |
| p = 0.5 | 22.9 | <0.001 | 6.2 | >0.8 |
| p = 0.6 | 14.8 | <0.05 | 10.2 | >0.5 |
| p = 0.8 | 18.2 | <0.01 | 28.1 | <0.01 |
| Two loci with environmental variance, p = 0.5 | 1.42 | >0.9 | 12.6 | >0.3 |
| One locus without environmental variance | 383 | <0.001 | 865 | <0.001 |
| Two loci without environmental variance | 23.9 | <0.001 | 139 | <0.001 |
| Three loci without environmental variance | 12.8 | <0.05 | 63.3 | <0.001 |
| Five loci without environmental variance | 6.33 | >0.4 | 38.2 | <0.001 |
| Polygenic without environmental variance (h2 = 1) | 8.04 | >0.2 | 40.1 | <0.001 |
P-value < 0.05 shows incompatibility between simulated and observed data. p, frequency of the male-orienting allele.
DISCUSSION
The genetic influence on sex in sea bass:
Our results are fully in accordance with a polygenic model, as described by Bulmer and Bull (1982), where the sex of an offspring is determined by the fact that an underlying sex tendency (determined by both polygenes and environmental effects) is greater or less than a threshold value. In our experiment, both sires and dams have an effect on the sex ratio of their progeny, and the effects are similar in size, pleading for an additive genetic effect on sex ratio. The heritability is high (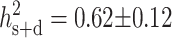 ) and clearly different from zero. It may not be biased by nonadditive genetic factors, as the mating design is factorial and all fish were in the same environment at the time of sex determination. Moreover, we verified that each dam (χ2 = 73, 66 d.f., P > 0.2) and each sire (χ2 = 104, 93 d.f., P > 0.2) contributed the same proportion of offspring in all sites and that the same sex ratios were observed in all sites, so even unexpected late actions of the environment on the sex ratio would not bias the between-family data. The “classical” vertebrate chromosomal sex determination model is expected to give 50% males and 50% females and clearly cannot explain the data obtained, even with eventual sex reversal of genetic females to males due to temperature. This holds as long as sex reversal is homogeneous among families (i.e., not genetically determined). If sex reversal was not homogeneous among families, this would imply the existence of a secondary genetic component of sex ratio, in addition to the chromosomal system. Pure ESD can also be excluded, as it is not supposed to yield any between-families differences in sex ratios. This was already suggested by previous results (Blazquez
et al. 1999; Saillant
et al. 2002; Gorshkov
et al. 2003; Peruzzi
et al. 2004). As it is clear that environment can influence sex ratio in sea bass (Piferrer
et al. 2005), the genetic component that we evidence can be considered either as a genetic variation of the primary sex ratio or as a genetic sensitivity to the environmental effects. As we tested only one environmental condition, however, we cannot distinguish between both. The observed occurrence of intratesticular oocytes in many (up to 62%) young sea bass males (Gorshkov
et al. 1999; Saillant
et al. 2003a) could be in favor of a polygenic primary sex ratio, with sex reversal occurring as a consequence of environmental effects. However, the existence of genetic-by-environment interactions, which would favor the second hypothesis, has already been evidenced (Saillant
et al. 2002). Nevertheless, we show that, whatever its true nature, there is a genetic effect that leads to a continuous distribution of family sex ratios in this species, at least in one (masculinizing) environmental condition. We also show that, in addition to the global effect of temperature, which certainly skewed sex ratio toward males in this experiment, it is necessary to include environmental variance within this global environment to explain the observed distribution of sex ratio, meaning that a purely genetic model where individual sex would be uniformly influenced by the environment could not explain our data. Even with environmental variance allowed, a two-factor system can be excluded, but a four-factor system (two biallelic loci) can explain the observed data. Similarly, in the apple snail, it was concluded that a continuous variation in family sex ratios was most likely due to at least four sex factors (Yusa 2007). Unlike what was seen in the silverside fish Menidia menidia (Conover and Heins 1987b), we could not observe a multimodal distribution of family sex ratios, which was considered an indication of the existence of only a few sex factors in this species. Indeed, as pointed out by Bull (1983), it is extremely difficult to ascertain the polygenic nature of a sex-determining system when compared to a system with only a few factors and some environmental variance. Nevertheless, both systems are expected to behave in a very similar manner and may be described by the Bulmer and Bull threshold model, provided that sex factors have individually weak effects (Bull 1983). Finally, we showed that there was considerable genetic variation for sex ratio in a given environment, as half-sib family sex ratios range from 0.5 to 46.3% of females (the proportions range from 0 to 75% in full-sib families, but their small size—on average 24 individuals—makes it less significant). We can compare this range of variation in female proportions to that produced by temperature treatments: 0–27% (Blazquez
et al. 1998), 18–66% (Koumoundouros
et al. 2002), 24–74% (Pavlidis
et al. 2000), and 11–32% (Saillant
et al. 2002). This shows that, in this species, the genetic and environmental components of sex determinism are of comparable magnitude, which confirms that there are no fundamental barriers between ESD and genotypic sex determination, as proposed by Bull (1983).
) and clearly different from zero. It may not be biased by nonadditive genetic factors, as the mating design is factorial and all fish were in the same environment at the time of sex determination. Moreover, we verified that each dam (χ2 = 73, 66 d.f., P > 0.2) and each sire (χ2 = 104, 93 d.f., P > 0.2) contributed the same proportion of offspring in all sites and that the same sex ratios were observed in all sites, so even unexpected late actions of the environment on the sex ratio would not bias the between-family data. The “classical” vertebrate chromosomal sex determination model is expected to give 50% males and 50% females and clearly cannot explain the data obtained, even with eventual sex reversal of genetic females to males due to temperature. This holds as long as sex reversal is homogeneous among families (i.e., not genetically determined). If sex reversal was not homogeneous among families, this would imply the existence of a secondary genetic component of sex ratio, in addition to the chromosomal system. Pure ESD can also be excluded, as it is not supposed to yield any between-families differences in sex ratios. This was already suggested by previous results (Blazquez
et al. 1999; Saillant
et al. 2002; Gorshkov
et al. 2003; Peruzzi
et al. 2004). As it is clear that environment can influence sex ratio in sea bass (Piferrer
et al. 2005), the genetic component that we evidence can be considered either as a genetic variation of the primary sex ratio or as a genetic sensitivity to the environmental effects. As we tested only one environmental condition, however, we cannot distinguish between both. The observed occurrence of intratesticular oocytes in many (up to 62%) young sea bass males (Gorshkov
et al. 1999; Saillant
et al. 2003a) could be in favor of a polygenic primary sex ratio, with sex reversal occurring as a consequence of environmental effects. However, the existence of genetic-by-environment interactions, which would favor the second hypothesis, has already been evidenced (Saillant
et al. 2002). Nevertheless, we show that, whatever its true nature, there is a genetic effect that leads to a continuous distribution of family sex ratios in this species, at least in one (masculinizing) environmental condition. We also show that, in addition to the global effect of temperature, which certainly skewed sex ratio toward males in this experiment, it is necessary to include environmental variance within this global environment to explain the observed distribution of sex ratio, meaning that a purely genetic model where individual sex would be uniformly influenced by the environment could not explain our data. Even with environmental variance allowed, a two-factor system can be excluded, but a four-factor system (two biallelic loci) can explain the observed data. Similarly, in the apple snail, it was concluded that a continuous variation in family sex ratios was most likely due to at least four sex factors (Yusa 2007). Unlike what was seen in the silverside fish Menidia menidia (Conover and Heins 1987b), we could not observe a multimodal distribution of family sex ratios, which was considered an indication of the existence of only a few sex factors in this species. Indeed, as pointed out by Bull (1983), it is extremely difficult to ascertain the polygenic nature of a sex-determining system when compared to a system with only a few factors and some environmental variance. Nevertheless, both systems are expected to behave in a very similar manner and may be described by the Bulmer and Bull threshold model, provided that sex factors have individually weak effects (Bull 1983). Finally, we showed that there was considerable genetic variation for sex ratio in a given environment, as half-sib family sex ratios range from 0.5 to 46.3% of females (the proportions range from 0 to 75% in full-sib families, but their small size—on average 24 individuals—makes it less significant). We can compare this range of variation in female proportions to that produced by temperature treatments: 0–27% (Blazquez
et al. 1998), 18–66% (Koumoundouros
et al. 2002), 24–74% (Pavlidis
et al. 2000), and 11–32% (Saillant
et al. 2002). This shows that, in this species, the genetic and environmental components of sex determinism are of comparable magnitude, which confirms that there are no fundamental barriers between ESD and genotypic sex determination, as proposed by Bull (1983).
The genetic relationship between size and sex:
An interesting feature of our results is the relatively strong genetic correlation between sex and size (rA = 0.50 ± 0.09), which means that some of the genes acting on sex determination and growth are the same, or at least are strongly linked in our sample. This is in accordance with previous experimental evidence showing that females are larger at the time of sex differentiation (Blazquez et al. 1999; Saillant et al. 2001). Still, we have shown that, once corrected for this sex dimorphism, there was still a size advantage in families with high proportions of females (Figure 1). This strengthens a lot the connection that was established between growth and sex. Indeed, as sea bass is a group spawner, it is clear that the size of females should have a strong impact on their fitness, through their absolute fertility, whereas it may be less important for males. Therefore, the idea that a minimum size is needed at the time of sex differentiation to be able to differentiate as female is plausible and strengthened by the fact that the size advantage of females is never as large as at the time of sex differentiation (+41% weight at 1 year and +20% at 2 years in this study; +67% at 10 months and +25% from 2 years in Saillant et al. 2001). This type of determinism is observed in nematodes (Ellenby 1954) and eels (Roncarati et al. 1997), where high density (hence limited resources for growth) favors male differentiation. However, density had no effect on sex ratio in sea bass (Saillant et al. 2003b), but in this latter case the densities and rearing conditions were chosen to avoid any impact on growth, to not confound the effects of growth and density per se.
Evolutionary consequences of polygenic sex in sea bass:
Our heritability estimates for sex ratio are high (0.62), as in turtles, where high estimates (in the range of 0.5–0.8) have also been found (Bull et al. 1982; Janzen 1992). However, the estimates in turtles were likely inflated by maternal and/or dominance effects, due to the use of full-sib designs, which is not the case in our experiment. Moreover, in turtles, the impact of high-heritability estimates was considerably lowered in natural conditions by the high variance between nest temperature, which, combined with the very narrow temperature range for complete sex change, reduces a lot the potential for selection on sensitivity to ESD (Bull et al. 1982). In our case, although temperature has a large effect on sex ratio, it cannot produce 100% female progeny, and there is a wide spectrum (13°–25°) having an impact on sex determination. Still, the temperature of the coastal waters during the first year of life of the sea bass may be quite variable and thus reduce the effectiveness of natural selection on polygenic sex ratio in this species. As our experimental growing conditions are different from natural ones [fish are 13.5 cm at 1 year vs. 7–10 cm in the wild in the Atlantic (Pickett and Pawson 1994)], and with the suspected genotype–environment interactions for sex ratio in sea bass (Saillant et al. 2002), variation that may be hidden in wild (low temperature) conditions may be expressed in experimental or farm (warm) conditions. This may be even accentuated by the growth–sex genetic relationship, as growth also has a high heritability in this experiment (0.54 ± 0.08 for weight) and is expected to have a higher heritability in fast-growing conditions than in slow-growing conditions.
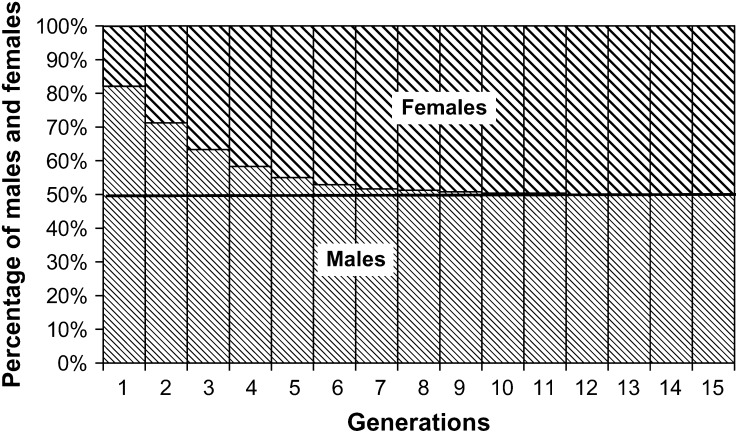
Considering the high heritability of sex ratio observed in our conditions, we could calculate the effects of frequency-dependent selection on sex using the Bulmer and Bull (1982) model (Figure 3). It shows that equilibrium sex ratio should be reached in seven to eight generations. This shift toward females, which are more interesting for aquaculture, could even be accelerated through artificial selection of female-producing families. If artificial selection on growth is also practiced, as is the case in several hatcheries, the shift in sex ratios in aquaculture populations should be even faster and may lead to predominantly female populations. As aquaculture of sea bass expands in the Mediterranean area, the impact of aquaculture escapees, with modified sex determinism, will have to be carefully evaluated, as sex ratio is doubtlessly a major determinant of fitness and may therefore have an important impact on the fitness of natural populations of this species (Lynch and O'Hely 2001).
Figure 3.—
Expected evolution of sea bass sex ratio along generations of random mating in constant environmental conditions, using the frequency-dependent model of Bulmer and Bull (1982), with heritability 0.62 and initial sex ratio 18.3% females (this study's estimates).
Conclusion:
We have demonstrated that sex determinism in the sea bass is not monogenic and is sensitive to within-tank variations in the environment and that the genetic component is essentially additive, is linked to the growth capacity of the fish, is of the same magnitude as the environmental component controlled by temperature, and can be precisely described using a polygenic threshold model with h2 = 0.62 on the underlying scale.
Selective breeding experiments are under way to explore the effective response to sex-ratio selection and the correlated sex-ratio response to selection for growth. They will also provide material for QTL search in the coming years, hopefully allowing us by that time to determine more precisely if sex determinism in this species is effectively polygenic or only oligogenic.
Acknowledgments
We thank the technical staff from the Institut National de la Recherche Agronomique (INRA), the Institut Français de Recherche pour l'Exploitation de la Mer (IFREMER), and the partner farms (listed below) for taking care of the fish for 2 years and for their active participation in data collection. Bernard Chevassus and two anonymous reviewers are thanked for constructive comments on the manuscript. This work was financed by Ardag Red Sea Mariculture (Eilat, Israël), Panittica Pugliese (Torre Canne, Italie), Viveiro Vilanova (Vila Nova de Milfontes, Portugal), and the European Union in the frame of the European project Heritabolum (Q5CR-2002-71720) and by the Research Group “Amélioration Génétique des Poissons” between INRA and IFREMER.
References
- Arias, A., 1980. Crecimento, régmen alimentario y reproduccion de la dorada (Sparus aurata L.) y del robalo (Dicentrarchus labrax L.) en los esteros de Cadiz. Invest. Pesquera 44 59–83. [Google Scholar]
- Baroiller, J. F., and H. D'Cotta, 2001. Environment and sex determination in farmed fish. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 130 399–409. [DOI] [PubMed] [Google Scholar]
- Becker, W. A., 1984. Manual of Quantitative Genetics. Academic Enterprises, Pullman, WA.
- Blazquez, M., S. Zanuy, M. Carrillo and F. Piferrer, 1998. Effects of rearing temperature on sex differentiation in the European sea bass (Dicentrarchus labrax L.). J. Exp. Zool. 281 207–216. [Google Scholar]
- Blazquez, M., M. Carrillo, S. Zanuy and F. Piferrer, 1999. Sex ratios in offspring of sex-reversed sea bass and the relationship between growth and phenotypic sex differentiation. J. Fish Biol. 55 916–930. [Google Scholar]
- Bull, J. J., 1983. Evolution of Sex-Determining Mechanisms. Benjamin/Cummings, Menlo Park, CA.
- Bull, J. J., 1985. Sex determining mechanisms: an evolutionary perspective. Experientia 41 1285–1296. [DOI] [PubMed] [Google Scholar]
- Bull, J. J., and R. C. Vogt, 1979. Temperature-dependent sex determination in turtles. Science 206 1186–1188. [DOI] [PubMed] [Google Scholar]
- Bull, J. J., R. C. Vogt and M. G. Bulmer, 1982. Heritability of sex ratio in turtles with environmental sex determination. Evolution 36 333–341. [DOI] [PubMed] [Google Scholar]
- Bulmer, M. G., and J. J. Bull, 1982. Models of polygenic sex determination and sex ratio control. Evolution 36 13–26. [DOI] [PubMed] [Google Scholar]
- Chapuis, H., V. Ducrocq, M. Tixier-Boichard and Y. Delabrosse, 1996. Multivariate restricted maximum likelihood estimation of genetic parameters for production traits in three selected strains. Genet. Sel. Evol. 28 197–215. [Google Scholar]
- Charnov, E. L., 1975. Sex-ratio selection in an age-structured population. Evolution 29 366–368. [DOI] [PubMed] [Google Scholar]
- Charnov, E. L., and J. J. Bull, 1977. When is sex environmentally determined? Nature 266 828–830. [DOI] [PubMed] [Google Scholar]
- Conover, D. O., and S. W. Heins, 1987. a Adaptive variation in environmental and genetic sex determination in a fish. Nature 326 496–498. [DOI] [PubMed] [Google Scholar]
- Conover, D. O., and S. W. Heins, 1987. b The environmental and genetic components of sex ratio in Menidia menidia (Pisces: Atherinidae). Copeia 1987 732–743. [Google Scholar]
- Ellenby, C., 1954. Environmental determination of the sex ratio of a plant parasitic nematode. Nature 174 1016–1017. [Google Scholar]
- Fauvel, C., M. Suquet, C. Dréanno, V. Zonno and B. Menu, 1998. Cryopreservation of sea bass (Dicentrarchus labrax) spermatozoa in experimental and production simulating conditions. Aquat. Living Resour. 11 387–394. [Google Scholar]
- Felip, A., F. Piferrer, M. Carrillo and S. Zanuy, 2002. Growth, gonadal development and sex ratios of meiogynogenetic diploid sea bass. J. Fish Biol. 61 347–359. [Google Scholar]
- Fisher, R. A., 1930. The Genetical Theory of Natural Selection. Oxford University Press, Oxford.
- Francis, R. C., and G. W. Barlow, 1993. Social control of primary sex differentiation in the Midas cichlid. Proc. Natl. Acad. Sci. USA 90 10673–10675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmour, A. R., B. J. Gogel, B. R. Cullis, S. J. Welham and R. Thompson, 2002. ASReml User Guide, Release 1.0. VSN International, Hemel Hepstead, UK.
- Gorshkov, S., G. Gorshkova, W. Knibb and H. Gordin, 1999. Sex ratios and growth performances of European sea bass (Dicentrarchus labrax) reared in mariculture in Eilat (Red Sea). Israeli J. Aquac.-Bamidgeh 51 91–105. [Google Scholar]
- Gorshkov, S., I. Meiri, H. Rosenfeld, S. Ben-Atia, S. Lutzki et al., 2003. Parental effects on sex ratios in progeny of the European sea bass (Dicentrarchus labrax). Israeli J. Aquac.-Bamidgeh 55 265–273. [Google Scholar]
- Hamilton, W. D., 1967. Extraordinary sex ratios. Science 156 477–488. [DOI] [PubMed] [Google Scholar]
- Hosmer, D. W., and S. Lemeshow, 1989. Applied Logistic Regression. John Wiley & Sons, New York.
- Janzen, F. J., 1992. Heritable variation for sex ratio under environmental sex determination in the common snapping turtle (Chelydra serpentina). Genetics 131 155–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley, D. F., 1988. The importance of estuaries for sea-bass Dicentrarchus labrax (L.). J. Fish Biol. 33(Suppl. A): 25–33. [Google Scholar]
- Kirpichnikov, V. S., 1981. Genetic Bases of Fish Selection. Springer-Verlag, Berlin.
- Kosswig, C., 1964. Polygenic sex determination. Experientia 20 190–199. [DOI] [PubMed] [Google Scholar]
- Koumoundouros, G., M. Pavlidis, L. Anezaki, C. Kokkari, A. Sterioti et al., 2002. Temperature sex determination in the European sea bass, Dicentrarchus labrax (L., 1758) (Teleostei, Perciformes, Moronidae): critical sensitive ontogenetic phase. J. Exp. Zool. 292 573–579. [DOI] [PubMed] [Google Scholar]
- Kovac, M., and E. Groeneveld, 2003. VCE5 User's Guide and Manual, Version 5.1. Department of Animal Sciences, University of Ljubljana, Ljubljana, Slovenia.
- Lynch, M., and M. O'Hely, 2001. Captive breeding and the genetic fitness of natural populations. Conserv. Genet. 2 363–378. [Google Scholar]
- Mylonas, C. C., L. Anezaki, P. Divanach, S. Zanuy, F. Piferrer et al., 2005. Influence of rearing temperature during the larval and nursery periods on growth and sex differentiation in two Mediterranean strains of Dicentrarchus labrax. J. Fish Biol. 67 652–668. [Google Scholar]
- Olausson, A., and K. Ronningen, 1975. Estimation of genetic parameters for threshold characters. Acta agriculturæ Scandinavica. Section A. Anim. Sci. 25 201–208. [Google Scholar]
- Orzack, S. H., and J. Gladstone, 1994. Quantitative genetics of sex ratio traits in the parasitic wasp, Nasonia vitripennis. Genetics 137 211–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlidis, M., G. Koumoundouros, A. Sterioti, S. Somarakis, P. Divanach et al., 2000. Evidence of temperature-dependent sex determination in the European sea bass (Dicentrarchus labrax L.). J. Exp. Zool. 287 225–232. [DOI] [PubMed] [Google Scholar]
- Peruzzi, S., B. Chatain, E. Saillant, P. Haffray, B. Menu et al., 2004. Production of meiotic gynogenetic and triploid sea bass, Dicentrarchus labrax L. 1. Performances, maturation and carcass quality. Aquaculture 230 41–64. [Google Scholar]
- Pickett, G. D., and M. G. Pawson, 1994. Seabass: Biology, Exploitation and Conservation. Chapman & Hall, London.
- Piferrer, F., M. Blazquez, L. Navarro and A. Gonzalez, 2005. Genetic, endocrine, and environmental components of sex determination and differentiation in the European sea bass (Dicentrarchus labrax L.). Gen. Comp. Endocrinol. 142 102–110. [DOI] [PubMed] [Google Scholar]
- Rice, W. R., 1986. On the instability of polygenic sex determination: the effect of sex-specific selection. Evolution 40 633–639. [DOI] [PubMed] [Google Scholar]
- Roncarati, A., P. Melotti, O. Mordenti and L. Gennari, 1997. Influence of stocking density of European eel (Anguilla anguilla, L.) elvers on sex differentiation and zootechnical performances. J. Appl. Ichthyol. 13 131–136. [Google Scholar]
- Saillant, E., A. Fostier, B. Menu, P. Haffray and B. Chatain, 2001. Sexual growth dimorphism in sea bass Dicentrarchus labrax. Aquaculture 202 371–387. [Google Scholar]
- Saillant, E., A. Fostier, P. Haffray, B. Menu, J. Thimonier et al., 2002. Temperature effects and genotype-temperature interactions on sex determination in the European sea bass (Dicentrarchus labrax L.). J. Exp. Zool. 292 494–505. [DOI] [PubMed] [Google Scholar]
- Saillant, E., B. Chatain, B. Menu, C. Fauvel, M. O. Vidal et al., 2003. a Sexual differentiation and juvenile intersexuality in the European sea-bass (Dicentrarchus labrax). J. Zool. 260 53–63. [Google Scholar]
- Saillant, E., A. Fostier, P. Haffray, B. Menu, S. Laureau et al., 2003. b Effects of rearing density, size grading and parental factors on sex ratios of the sea bass (Dicentrarchus labrax L.) in intensive aquaculture. Aquaculture 221 183–206. [Google Scholar]
- Vaz, S. C., and A. B. Carvalho, 2004. Evolution of autosomal suppression of the sex-ratio trait in Drosophila. Genetics 166 265–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusa, Y., 2007. Nuclear sex-determining genes cause large sex ratio variation in the apple snail Pomacea canaliculata. Genetics 175 179–184. [DOI] [PMC free article] [PubMed] [Google Scholar]