Abstract
The ApcMin mouse model of colorectal cancer provides a discrete, quantitative measurement of tumor multiplicity, allowing for robust quantitative trait locus analysis. This advantage has previously been used to uncover polymorphic modifiers of the Min phenotype: Mom1, which is partly explained by Pla2g2a; Mom2, a spontaneous mutant modifier; and Mom3, which was discovered in an outbred cross. Here, we describe the localization of a novel modifier, Mom7, to the pericentromeric region of chromosome 18. Mom7 was mapped in crosses involving four inbred strains: C57BL/6J (B6), BTBR/Pas (BTBR), AKR/J (AKR), and A/J. There are at least two distinct alleles of Mom7: the recessive, enhancing BTBR, AKR, and A/J alleles and the dominant, suppressive B6 allele. Homozygosity for the enhancing alleles increases tumor number by approximately threefold in the small intestine on both inbred and F1 backgrounds. Congenic line analysis has narrowed the Mom7 region to within 7.4 Mb of the centromere, 28 Mb proximal to Apc. Analysis of SNP data from various genotyping projects suggests that the region could be as small as 4.4 Mb and that there may be five or more alleles of Mom7 segregating among the many strains of inbred mice. This has implications for experiments involving ApcMin and comparisons between different or mixed genetic backgrounds.
COLORECTAL cancer is the second leading cause of cancer death in the United States and the third in the world (Jemal et al. 2006). The multiple intestinal neoplasia (Min) mouse model has provided a means by which modifiers of the colorectal tumor phenotype can be readily identified. This line of mice harbors a truncating mutation at codon 850 of the adenomatous polyposis coli (Apc) gene, which is mutated in ∼80% of all human colorectal tumors (Fearnhead et al. 2001). The discrete, countable nature of ApcMin tumors has made the mouse a tractable model for quantitative studies.
Quantitative trait locus (QTL) analysis allows searches for polymorphic modifiers in either a backcross or an intercross population derived from different inbred strains. Identification of the first discovered modifier of ApcMin, Mom1, included the use of three different strains (Dietrich et al. 1993). Mom1 has two major alleles, resistant (Mom1R) and sensitive (Mom1S), and has been localized to a 5-cM region on chromosome 4. Cormier et al. (1997, 2000) showed that the gene Pla2g2a, in addition to a second locus distal to D4Mit64, is responsible for the Mom1 effect. Mom2, discovered by Silverman et al. (2002), maps distal to the Apc locus on chromosome 18. Although the region has been narrowed to 7 cM, the underlying gene(s) remain to be identified (Silverman et al. 2003). In addition, Haines et al. (2005) identified a second polymorphic modifier locus linked to Apc, Mom3, the phenotype of which is affected by pregnancy (Suraweera et al. 2006). Mom3 arose from an outbred stock, and the lack of known polymorphic markers has prevented its resolution beyond 25 cM.
Numerous other genetic modifiers have been described upon breeding known mutations onto the ApcMin/+ background. A subset of modifiers has been shown to affect the pathway leading to loss of heterozygosity (LOH) at the Apc locus, which is believed to be the initiating process for tumorigenesis in the intestine. For example, Blm-deficient mice have a defect in maintaining genomic stability, including elevated somatic recombination rates that lead to increased tumor multiplicities in ApcMin/+ mice (Luo et al. 2000; Goss et al. 2002; Suzuki et al. 2006). Haigis and Dove (2003) found that the Robertsonian translocation Rb(7.18)9Lub (Rb9) disrupted the somatic pairing of chromosome 18 homologs, thus decreasing the probability for somatic recombination and tumor multiplicity. We describe here the localization of a new Modifier of Min, Mom7, to within the first 7.4 Mb of chromosome 18. Noting its centromere-proximal position relative to Apc, we speculate that the modifier may directly regulate the loss of heterozygosity of distal elements.
MATERIALS AND METHODS
Mice:
The mice used to generate segregating populations were C57BL/6J, AKR/J, and C57BL/6-Chr 18A/J (B6.18A/J) consomics (The Jackson Laboratory, Bar Harbor, ME) and BTBR/Pas (Pasteur Institute, Paris). Congenic lines bred in our laboratory are described in the text. These strain names have been registered with the Mouse Genomic Nomenclature Committee: B6.AKR-Mom7AKR/J (B6.Mom7AKR), AKR.B6-Mom7C57BL/6J (AKR.Mom7B6), AKR.B6-(D4Mit13-D4Mit54) (abbreviated as AKR.Mom1S), BTBRPas.B6-Mom7C57BL/6 (BTBR.Mom7B6), and C57BL/6-Chr 18BTBR/Pas (B6.18BTBR). Mice were housed in a standard facility with automatic watering and access to Purina 5020 chow (Purina, St. Louis). B6.ApcMin/+ and BTBR.ApcMin/+ mice were sacrificed between 2 and 4 months of age. Since AKR.ApcMin/+ mice showed no significant difference in tumor multiplicity between 6 and 11 months of age (data not shown), tumor counts over this age range were combined. Intestinal preparation and tumor counts were performed as previously described (Haigis and Dove 2003).
Statistical analysis:
Pairwise P-values for the B6.Mom7 congenic strains were determined by a Wilcoxon rank-sum test and subjected to a Bonferroni correction for multiple comparisons (see supplemental Table 1 at http://www.genetics.org/supplemental/). LOD scores and maps were obtained using the Mapmaker3 program (Lander et al. 1987; Lincoln et al. 1992).
LOH analysis:
DNA extraction from tumors and Pyrosequencing assays (Biotage, Upsala, Sweden) were performed as described previously (Amos-Landgraf et al. 2007). Tumors taken from AKR mice were all >2 mm in diameter. Primers used were forward (TTT TGA CGC CAA TCG ACA TG) and reverse (biotinylated) (GAT GGT AAG CAC TGA GGC CAA TA); sequencing (CGT TCT GAG AAA GAC AGA AG). An LOH/maintenance of heterozygosity (MOH) cutoff value of 31.4% was determined by adapting the normal distribution mixture technique previously described (Shoemaker et al. 1998): we used the method of maximum likelihood to fit a mixture of two normal curves to data on single nucleotide polymorphism (SNP) signal intensity. Tumor data were considered to arise from either an LOH distribution normal component or an MOH normal distribution component, according to some mixing probability; control data, normal tissue from a heterozygous animal, consists of known MOH components. Using the fitted mixture model, a critical intensity value, c, was determined so that Prob[intensity > c | LOH ] = 0.05. N = 348 tumor values and n = 22 control values were used for maximum-likelihood calculations. In the estimated normal mixture, the LOH component had a mean of 17.3 and a standard deviation of 6.5; the MOH component had a mean of 43.2 and a standard deviation of 5.1. From this, the critical signal value was c = 31.4. Modes of the likelihood surface at the boundary of the parameter space were avoided. Computations were done in R software (R Development Core Team 2005).
In silico SNP analysis and original markers:
In silico SNP data from the recent Perlegen strain resequencing project covering 16 inbred strains were obtained from the Mouse Phenome Database (http://phenome.jax.org/pub-cgi/phenome/mpdcgi?rtn=snpsdvsd/door). The first 3 Mb of chromosome 18 is an unassembled centromeric sequence with no SNP data; the next 0.5 Mb (positions 3.0–3.5 Mb on MGSC Build 36) were also excluded due to a high degree of homology to regions on chromosomes 4, 6, and 16. Therefore, only the region between 3.5 and 7.4 Mb was considered, encompassing 2171 informative SNPs (∼1 SNP/2 kb). Novel primers were designed to amplify SSLPs using sequences found through the Tandem Repeat Finder program (Benson 1999).
RESULTS
An AKR.Mom1S/S congenic line demonstrates the presence of other AKR modifiers:
It was previously shown that the AKR genetic background strongly reduces tumor multiplicity compared with the B6 background (Shoemaker et al. 1998). This effect is due in part to the semidominant resistant Mom1R allele carried by AKR. Accordingly, the Mom1 effect was removed by breeding a line of AKR mice congenic for the sensitive Mom1S allele from B6. Marker-assisted selection was employed so that progeny are considered congenic by the N4 generation (Hospital et al. 1992). Genome scans were performed on mice from N2–N6, selecting for those with the highest percentage of AKR homozygosity for 55 markers, with at least two markers/chromosome. At N4, 53/55 (94.5%) markers were homozygous for AKR, not including the regions surrounding the B6-derived Mom1S allele between D4Mit13 and D4Mit54 and the B6-derived ApcMin allele between D18Mit111 and D18Mit24. All data presented here involved mice from the N6 to N9 generations to ensure congenic status. AKR.ApcMin/+;Mom1S/S mice developed an average of 16 intestinal tumors, 1.5-fold more than Mom1R/S and 4-fold more than Mom1R/R mice (Table 1A, Mom7 congenic lines), consistent with the effects of Mom1 found in previous studies with B6.Mom1R congenic mice (Gould et al. 1996a). Therefore, additional modifiers must be present in the AKR background, since tumor counts among AKR.ApcMin/+;Mom1S/S mice (16) were sevenfold less than among B6-ApcMin/+;Mom1S/S mice (118; Figure 1A).
TABLE 1.
Effect of the Mom7 alleles on ApcMin/+ small intestinal tumor counts
| Tumor count [mean ± SD (N)] |
|||
|---|---|---|---|
| Genetic background | Mom7B6/AKR | Mom7AKR/AKR | Fold effect of Mom7AKR/AKR |
| A. Effect of the AKR allele | |||
| Mapping crosses | |||
| (B6 × AKR) × AKR Mom1S/S N2 | 17 ± 10 (75) | 50 ± 22 (87) | 3.0* |
| (B6 × AKR) × (B6 × AKR) Mom1S/S F2 | 53 ± 35 (51) | 97 ± 48 (39) | 1.8* |
| Mom7 congenic lines | |||
| AKR.Mom1S/S | ND | 16 ± 7.5 (9) | ND |
| AKR.Mom1R/S | 5.4 ± 3.4 (18) | 10 ± 5.8 (23) | 1.9* |
| AKR.Mom1R/R | 1.0 ± 0.8 (8) | 3.5 ± 2.6 (42) | 3.5* |
| (B6 × AKR).Mom1S/S F1 | 37 ± 10 (10) | 116 ± 15 (4) | 3.1* |
| (B6 × AKR).Mom1R/S F1 | 19 ± 10 (15) | 69 ± 10 (4) | 3.6* |
| Tumor count [mean ± SD (N)] |
|||
| Genetic background |
Mom7B6/BTBR |
Mom7BTBR/BTBR |
Fold effect of Mom7BTBR/BTBR |
| B. Effect of the BTBR allele | |||
| Mapping cross | |||
| (B6 × BTBR) × BTBR N2 | 175 ± 74 (12) | 557 ± 165 (28) | 3.2* |
| Congenic line | |||
| BTBR.ApcMin/+ | 307 ± 116 (53) | 625 ± 104 (75) | 2.0* |
The Mom7 allele was determined using marker D18Wis1 in the crosses with AKR, and marker D18Mit110 in the crosses with BTBR. *P < 0.01, Wilcoxon rank-sum test; ND, not done.
Figure 1.—
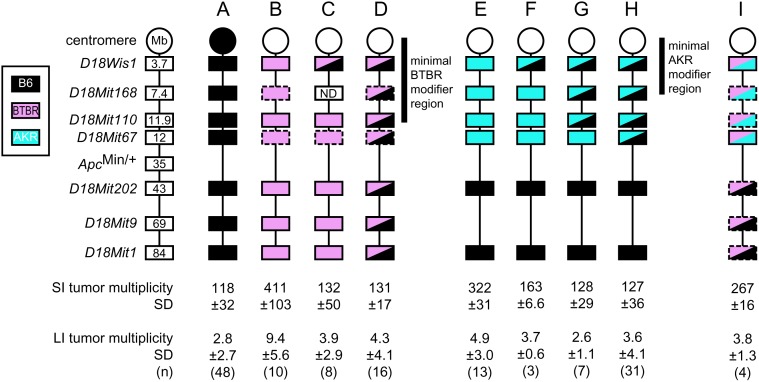
Mapping of the Mom7 modifier in B6 ApcMin/+ mice. Genotypes on chromosome 18 are shown; all other chromosomes are homozygous B6. Boxes outlined by dashes represent inferred genotypes based on either flanking markers or obligate genotypes. The congenic sublines C and F define a minimal region for the BTBR and AKR alleles of the Mom7 locus, respectively. SI, small intestinal; LI, large intestinal. For line I, D18Mit19 was used as a surrogate for D18Wis1. All mice were sacrificed between 2 and 4 months of age. ND, not done.
Identification of a modifier of intestinal tumor multiplicity near the chromosome 18 centromere in crosses between B6 and AKR:
To identify such additional polymorphic modifiers between AKR and B6, (B6 × AKR) Mom1S/S F1 progeny were backcrossed to AKR.ApcMin/+;Mom1S/S mice to generate an N2 population. The genomes of 162 phenotyped N2 animals were scanned with 71 markers, with a total of 6598 informative genotypes (see supplemental Table 2 at http://www.genetics.org/supplemental/). A LOD score of 21 (supplemental Figure 1 at http://www.genetics.org/supplemental/) corresponding to D18Wis1 indicated the presence of a strong modifier, which we have designated “Mom7,” linked to the position at 3.7 Mb (1 cM) as measured on MGSC Build 36. Although the LOD interval is maximal between D18Wis1 and D18Mit64, a more centromeric location cannot be excluded, owing to the absence of more proximal markers. Strikingly, the D18Wis1AKR/AKR class developed 3-fold more small intestinal tumors than the D18Wis1B6/AKR class (genomewide P ≪ 0.01; Table 1A, Mapping crosses). In addition, 90 F2 mice were generated by intercrossing Mom1S/S (B6 × AKR) F1 progeny. A complete genome scan was not carried out on this population; however, we found that tumor multiplicities vs. genotypes at D18Wis1 confirmed this position: the D18Wis1AKR/AKR class developed 1.8-fold more tumors than the D18Wis1B6/AKR class (Table 1A, Mapping crosses). We note that the AKR grandparent always transmitted the ApcMin allele, which is linked to D18Wis1AKR. Therefore, it was rare to obtain the D18Wis1B6/B6;ApcMin/+ class; one such animal was obtained but was omitted from statistical analysis. Thus, the AKR allele of this modifier appears to be a recessive enhancer of tumor multiplicity.
To test whether the AKR allele of this modifier region alone is sufficient to produce the modified phenotype, we bred a reciprocal pair of congenic lines in which B6 mice carried a proximal chromosome 18 segment from AKR, and AKR mice carried the same region from B6. The interval selected extends from D18Wis1 to Apc. We were aware that, if the modifier were proximal to D18Wis1, it could be lost by recombination. Therefore, we always confirmed its presence by phenotyping candidate congenic carriers.
After at least eight generations of backcrossing, these heterozygous Mom7 congenics carrying either ApcMin/+ or Apc+/+ were intercrossed. On the B6 background, all three Mom7 genotypes were analyzed. Homozygotes for the AKR Mom7 allele developed an average of 322 ± 31 small intestinal tumors (Figure 1E), 2.5-fold more than either Mom7 heterozygotes (Figure 1, G and H) or B6-ApcMin/+ controls (Figure 1A); further, there was no significant difference between the heterozygotes and the controls (supplemental Table 1 at http://www.genetics.org/supplemental/).
On the AKR background, Mom7B6/B6 animals were not generated; however, Mom7 AKR homozygotes developed two- to threefold more small intestinal tumors than heterozygotes (Table 1A, Mom7 congenic lines). This effect is independent of Mom1, suggesting that the two modifiers act independently. As reported previously, tumors did not develop in the colon (Shoemaker et al. 1998). To determine whether a fully heterozygous genetic background influenced the effects of the modifier and to confirm the lack of interaction between Mom1 and Mom7, (B6 × AKR) ApcMin/+ F1 mice with different Mom1 and Mom7 genotypes were generated by crossing B6.Mom7AKR/B6;(Mom1S/S); ApcMin/+ congenics to AKR.(Mom7AKR/AKR);Mom1R/S congenics. Again, homozygosity for the AKR allele of Mom7 enhanced tumor number more than threefold relative to AKR/B6 heterozygosity (Table 1A, Mom7 congenic lines), and this effect was independent of the Mom1 genotype. Thus, on three different genetic backgrounds—B6, AKR, and (B6 × AKR) F1—the AKR allele of Mom7 enhanced tumor multiplicity approximately more than threefold in a manner fully recessive to the B6 allele and independent of Mom1 status.
Identification of a modifier of intestinal tumor multiplicity near the chromosome 18 centromere in crosses between B6 and BTBR:
It had previously been shown that the inbred BTBR strain, like B6, carries the sensitive Mom1S allele. Further, BTBR does not appear to carry other dominant modifiers of Min (Gould et al. 1996b). However, after backcrossing the ApcMin/+ allele for >10 generations onto the BTBR background, a significant enhancing effect was observed: mice became moribund by 60 days of age and were found to have in excess of 600 tumors/animal (Table 1B, Congenic line). To identify the enhancing modifier, we used the QTL strategy described in the AKR analysis. A partial genome scan of DNA from the N2 generation identified a highly significant modifier locus linked to the D18Mit110 marker (11.9 Mb): D18Mit110BTBR/BTBR mice developed 3.2-fold more tumors than D18Mit110B6/BTBR mice (Table 1B, Mapping cross).
Using marker-assisted selection, a B6.18BTBR/BTBR consomic line was obtained with marker D18Wis1 (3.7 Mb) at the most proximal end and marker D18Mit1 (84 Mb) at the most distal end. Both ApcMin/+ and Apc+/+ were bred to produce two lines, each heterozygous for chromosome 18, that could be intercrossed to obtain animals homozygous for all the BTBR alleles in the region, except for the ApcMin allele. Animals homozygous for the BTBR alleles developed significantly more tumors in both the small intestine and the colon (Figure 1B) than either heterozygotes or B6-ApcMin/+ controls (supplemental Table 1 at http://www.genetics.org/supplemental/; Figure 1, A and D; P < 0.05 for colon); there was no significant difference between lines A and D. A congenic strain carrying the B6 Mom7 region on the BTBR background was also bred. Homozygotes for the Mom7 BTBR alleles developed twofold more tumors than heterozygotes (Table 1B, Congenic line). Thus, on both the B6 and BTBR genetic backgrounds, the BTBR allele of Mom7 is an enhancer, fully recessive to the B6 allele.
The AKR and BTBR modifiers are not statistically different from one another:
Although both the AKR and BTBR modifiers are linked to D18Wis1, perhaps they represent separate factors within the Mom7 region. To further characterize the relationship of these alleles, (BTBR × AKR) ApcMin;Mom1S/S F1 mice were backcrossed to the AKR (Mom1R/R) strain. D18Mit19 (5.0 Mb) was used as a surrogate marker for D18Wis1, which is not polymorphic between the two strains. Results from 41 N2 progeny indicated that the tumor multiplicity for Mom7 heterozygotes (18 ± 17, n = 4) is not statistically different from that of AKR homozygotes (27 ± 16, n = 36; P = 0.3). To confirm this initial result, we crossed B6.Mom7AKR congenics to B6.Mom7BTBR consomics. These results indicate that the B6.Mom7AKR/BTBR progeny (Figure 1I) develop tumor multiplicities greater than B6.ApcMin/+ controls and not significantly different from AKR or BTBR Mom7 homozygotes (supplemental Table 1 at http://www.genetics.org/supplemental/). Similarly, lines B and E did not differ significantly from each other (supplemental Table 1 at http://www.genetics.org/supplemental/). Importantly, there is an overlap in tumor distributions for lines B, E, and I (194–541, 279–392, and 232–307, respectively). Thus, it appears that the AKR and BTBR alleles are additive in trans and probably represent a single Mom7 modifier factor.
The major AKR Mom7 enhancer lies within the proximal 7.4 Mb of chromosome 18:
Several recombinant sublines were established. One (Figure 1F) is a B6 subline homozygous for AKR alleles within the Mom7 region except for loci proximal to D18Mit168 (7.4 Mb). A second subline (Figure 1G) is homozygous for the Mom7 AKR alleles except for loci proximal to D18Mit67 (12 Mb). Tumor counts on the small sample of mice from these sublines are statistically indistinguishable from B6 controls and full heterozygotes (Figure 1, A and H), yet line G is significantly different from B6.Mom7AKR/AKR congenics (Figure 1E; supplemental Table 1 at http://www.genetics.org/supplemental/). Although line F is not statistically different from the full congenic line E (supplemental Table 1 at http://www.genetics.org/supplemental/), this is likely owing to the low number of mice assayed for line F. Furthermore, the individual tumor distributions for lines E and F do not overlap (279–392 vs. 156–169, respectively), which suggests that they are indeed different. Therefore, the major Mom7 determinant most likely lies proximal to 7.4 Mb. Similar results were obtained with sublines carrying the BTBR modifier: animals that are homozygous BTBR for the complete chromosome 18 (Figure 1B) develop greater than threefold more tumors than do partial consomics (Figure 1C) that are heterozygous only for loci proximal to D18Mit110 (11.9 Mb). These proximal heterozygotes are phenotypically the same as heterozygotes for the entire chromosome (Figure 1D). Thus, the positions of both the AKR and BTBR alleles of Mom7 have been refined to the most pericentromeric region of chromosome 18.
The heterozygous B6.Mom7AKR sublines F, G, and H also allowed us to determine whether the Mom7 effect differs when the B6 allele is in cis or in trans to ApcMin. Lines F and G carry the B6 allele in cis to ApcMin and the AKR allele is in trans, while line H has the opposite orientation. Since no significant difference in multiplicity exists when F and G are combined and compared to H (P = 0.22), the B6 Mom7 allele is dominant in both a cis or trans configuration to ApcMin.
Preliminary data (see supplemental Table 3 at http://www.genetics.org/supplemental/) suggest that a fourth strain, A/J, also carries an enhancing Mom7 allele. This result is consistent with the identical proximal chromosome 18 haplotype of strains A/J, AKR, and BTBR (see Table 2 and below).
TABLE 2.
Haplotype block groups between 3.5 and 4.4 Mb seen among the 16 strains in the Perlegen mouse resequencing project
| Group 1 | Group 2a | Group 3 | Group 4b | Group 5 |
|---|---|---|---|---|
| C57BL/6J | C3H/HeJ | AKR/J | FVB/NJ | CAST/EiJ |
| NOD/LtJ | BALB/cByJ | A/J | DBA/2J | MOLF/EiJ |
| KK/HlJ | BTBR T〈+〉 tf/J | PWD/PhJ | ||
| WSB/EiJ | NZW/LacJ | CZECHII/EiJc | ||
| 129S1/SvImJ |
Actual data are given in supplemental Table 4 at http://www.genetics.org/supplemental/.
Group 2 shares a haplotype block with group 1 up through position 3827436 and then has a unique sequence beyond (see supplemental Tables 5 and 6 at http://www.genetics.org/supplemental/ for additional evidence and group members).
Group 4 differs from group 3 by only a single SNP (rs13483184) at position 3796540 (see supplemental Tables 5 and 6 at http://www.genetics.org/supplemental/ for additional evidence and group members).
Informative 537 SNPs, most of which are from other sequencing projects.
BTBR and AKR share a pericentromeric haplotype distinct from B6:
SNP data have become instrumental in reducing critical QTL intervals by identifying the regions most likely to contain relevant polymorphisms (Park and Hunter 2003). An in silico analysis was therefore performed on high-density SNP data in the minimal 7.4-Mb Mom7 region. Only the region between 3.5 and 7.4 Mb was considered (see materials and methods). Setting AKR, B6, and MOLF as reference strains, extensive sequence homology among the 16 strains identified at least five segregating haplotypes (supplemental Table 4 at http://www.genetics.org/supplemental/). In the region between 3.5 and 4.4 Mb, the strains could be classified into five broad groups (Table 2). Importantly, AKR, BTBR, and A/J grouped together and separately from B6 in this region, but not distal to 4.4 Mb, where BTBR and A/J shared a haplotype with B6 (supplemental Table 4 at http://www.genetics.org/supplemental/). We independently verified the haplotype data using seven novel SSLPs (sequences and positions given in Table 3) and D18Mit19 (5.0 Mb) for B6, AKR, BTBR, and A/J (Table 3). Thus, AKR, BTBR, and A/J share at least a 0.9-Mb haplotype block (positions 3.5–4.4 Mb), which is distinct from that of B6 and which may extend into the centromere.
TABLE 3.
Primers used to genotype the first 14 Mb of assembled pericentromeric chromosome 18 and their amplicon lengths
| Primer name | Forward primer | Reverse primer | Chromosome 18 position (bp) | AKR | A/J | BTBR | B6 |
|---|---|---|---|---|---|---|---|
| D18Wis2 | AAGTCCTGGTCCCCTACCTC | ATCCACTCATGGCACAAACA | 3478885 | ∼200–210 | ∼200–210 | ∼200–210 | 192 |
| D18Wis3 | GGCAGGCAAGACTTCAATGT | CTGGAAGCAAGGATGGGTAA | 3591908 | ∼194 | ∼194 | ∼194 | 209 |
| D18Wis1 | TAAGGCAGCAGGGAGAGAAA | CCCTGAGACAGGAGAACTGG | 3669044 | ∼155 | ∼155 | ∼155 | 171 |
| D18Wis4 | ATTGGGCGACTAGCAAGAGA | AGGACACCCAGGGCTATACA | 3670134 | ∼255–260 | ∼255–260 | ∼255–260 | 249 |
| D18Wis5 | TTCCCAAACTACGAAGGTGAA | GAAGGCTCAGGCTCTTCCAT | 4185518 | ∼245–250 | ∼245–250 | ∼245–250 | 234 |
| D18Wis6 | AAACCTGCAGAAAGGCTTGA | ATCCTTCCATCTCCCTGTCC | 4351585 | ∼235 | ∼235 | ∼235 | 243 |
| D18Wis7 | GGGGGAAAGTATAGCTGAAGG | AGAGTTGGCCCTTTGCTTTT | 4373408 | ∼145 | ∼145 | ∼145 | 162 |
| D18Mit19 | ATTGGGTGTTCAGGTGCAG | ATGCACAATAGCTCATAGCTTCT | 4941117 | 138 | 158 | ∼150 | 150 |
Sequence positions and PCR product sizes for the B6 strain were taken from MGSC Build 36 (http://www.ncbi.nlm.nih.gov). All primers except D18Mit19 are novel. Product sizes for D18Mit19 were taken from Mouse Genome Informatics (http://www.informatics.jax.org). All other products were sized in our laboratory using 3% agarose gels.
Cross-referencing the Perlegen SNP data with the 48-strain SNP survey (Pletcher et al. 2004), we found that 103 informative SNPs further supported the division between groups 3 and 4 and added 34 more putative members to the five groups (supplemental Table 5 at http://www.genetics.org/supplemental/). A smaller set of 15 informative SNPs from the Broad Institute and Applied Biosystems provided preliminary data on an additional 33 strains (supplemental Table 6 at http://www.genetics.org/supplemental/).
The rate of loss of heterozygosity in Mom7 congenics:
To determine the effects of the Mom7 locus on LOH at Apc, we developed a Pyrosequencing assay to quantify allelic ratios. On the B6 background, the percentage of tumors showing LOH did not differ significantly between any of the five B6.Mom7 classes carrying B6, AKR, or BTBR alleles (P > 0.1, Fisher's exact test; Table 4). However, it is notable that homozygotes for both the AKR and the BTBR Mom7 alleles showed LOH in every tumor tested.
TABLE 4.
LOH at the Apc locus in ApcMin/+ B6.Mom7 and AKR.Mom1;Mom7 congenic small intestinal tumors
| Genetic background | Frequency of tumors showing LOH (%) | No. of mice |
|---|---|---|
| B6.Mom7B6/B6 | 77/80 (96) | 4 |
| B6.Mom7B6/AKR | 86/86 (100) | 4 |
| B6.Mom7AKR/AKR | 85/85 (100) | 5 |
| B6.Mom7B6/BTBR | 47/53 (89) | 7 |
| B6.Mom7BTBR/BTBR | 56/56 (100) | 7 |
| AKR.Mom1S/S;Mom7AKR/AKR | 33/47 (70) | 5 |
| AKR.Mom1R/S;Mom7B6/AKR | 13/16 (81) | 3 |
| AKR.Mom1R/S;Mom7AKR/AKR | 18/21 (86) | 2 |
| AKR.Mom1R/R;Mom7AKR/AKR | 4/12 (33) | 4 |
On the AKR background, Mom7 homozygous congenics did not differ from heterozygotes in the percentage of LOH tumors (Table 4; compare AKR.Mom1R/S;Mom7AKR/AKR to AKR.Mom1R/S;Mom7B6/AKR). By contrast, one or two copies of Mom1S on AKR.Mom7AKR/AKR significantly increased the percentage of tumors with LOH from 33% (a percentage consistent with Shoemaker et al. 1998) to 86 and 70%, respectively (P < 0.05; Table 4). However, even two copies of Mom1S did not raise the AKR LOH percentage to the 100% observed in B6.ApcMin/+;Mom7AKR/AKR mice (P < 0.05).
DISCUSSION
In this study, we identify a novel modifier of the ApcMin tumor phenotype by a classical genetic approach. We used a previously described tumor-resistant mouse strain, AKR (Shoemaker et al. 1998), and the BTBR strain, which was found to be tumor sensitive. To our surprise, both the AKR and BTBR strains carry a strong recessive enhancer linked to the D18Wis1 marker (3.7 Mb) on chromosome 18. We have given one name to this factor: “Mom7” (Modifier of Min 7). Congenic and consomic sublines refined the majority of the Mom7 effect to 7.4 Mb between the centromere and D18Mit168. The enhancers were found to have a consistent effect on different inbred backgrounds and to be independent of the Mom1 modifier effect. Preliminary results indicate that the AKR and BTBR alleles are noncomplementing (Figure 1). Possible interpretations of these observations are discussed below.
In principle, a modifier can control tumor viability, growth, or initiation. For example, a modifier linked to ApcMin could reduce tumor multiplicity by inducing cellular lethality after the lethal allele undergoes somatic homozygosis. Mom1 and Dnmt have been shown to control the net growth rate of tumors (Cormier and Dove 2000). The Rb9 modifier prevents tumor initiation by disrupting the pairing of chromosome 18 homologs (Haigis and Dove 2003). Which of these modes of action applies to Mom7? The cellular lethality model is eliminated since homozygotes for each allele of Mom7 are viable. Support for the growth model awaits analysis of tumor size, which is currently underway. An effect on tumor initiation is consistent with our results, but requires confirmation at the genomic level.
Classically, the recessive nature of the Mom7 enhancer alleles would be interpreted as a loss of function of a trans-acting gene product. Similar effects are seen in knockouts for the Recql4 helicase, the normal function of which is to suppress aneuploidy by maintaining sister-chromatid cohesion (Mann et al. 2005). Alternatively, the Mom7 locus could modulate tumor phenotypes in cis by affecting the recombination substrates on chromosome 18 and the consequent rate of LOH at the distal Apc locus. In such a cis-acting model, the Mom7 alleles could represent polymorphisms that alter pericentromeric sequences required for the efficiency of somatic recombination hotspots. For example, the ribosomal DNA (rDNA) repeats on centromeric chromosome 18 could be recombinogenic due to the high fidelity and copy number of homologous sequence and to the presence of transcription termination sequences and replication fork barriers (Gerber et al. 1997; Lopez-estrano et al. 1998). If Mom7 does act in cis, it is important to note that its phenotype differs from that of the Rb9 rearrangement (Haigis and Dove 2003), which has a semidominant effect on tumor multiplicity, and from the action of sequence heterozygosity (Shao et al. 2001), which would be predicted to have an overdominant effect. Although our preliminary evidence (Table 4) does not reach statistical significance, it is at least consistent with a direct effect of Mom7 on the frequency of somatic recombination. A more sensitive assay is required to investigate this hypothesis.
It will be important to determine whether the Mom7 effect is attributable to one or many genes. At present, the first ∼3 Mb of chromosome 18 remain unassembled, owing to the highly repetitive nature of the centromere, which includes the rDNA repeats. Thus, determining the sequence identity of the Mom7 modifier(s) remains a challenging prospect. Toward this end, more recombinant sublines are being identified and analyzed to further narrow the Mom7 region before candidate testing. All congenic sublines have been backcrossed >10 generations, providing a genetic resource with which to identify the salient pericentromeric sequence associated with the Mom7 effect.
Intriguingly, the sequence of the Mom7 genetic region provides insight into its evolutionary nature. AKR, BTBR, and A/J all share the same pericentromeric haplotype, distinct from B6, providing evidence that the major Mom7 determinant likely lies proximal to the 4.4-Mb position. Although the SNP analysis indicates that there may be as many as five haplotype blocks in the Mom7 region, the Mom7 allelic status of other inbred strains remains to be determined experimentally. The Perlegen SNP analysis also indicates that there are no nonsynonymous coding region polymorphisms among AKR, BTBR, or B6 for any of the annotated genes (Crem, Cul2, Bambi, Lyzl1, Map3k8, Papd1) in the interval between 3 and 4.4 Mb. This does not rule out regulatory or splice mutations or mutations in unannotated genes. Finally, none of the genes in the minimal modifier region were found to be mutated in the recent survey of human colorectal cancer mutations (Sjoblom et al. 2006). Nevertheless, copy number variations, epigenetic or structural differences, or more proximal mutations may underlie the Mom7 determinant.
An important aspect of modifiers is that they can act either additively or synergistically (Cormier and Dove 2000). We have shown that Mom7 acts independently of Mom1; the two modifiers therefore affect tumor multiplicity through separate, noncomplementary pathways. This independence creates an even wider disparity in relative tumor multiplicity between B6 and AKR mice: upon factoring out the effects of Mom1 and Mom7, there remains a 15-fold difference. Although the N2 genome scan did identify a second polymorphic modifier on chromosome 11 with a LOD score of 3.3 (supplemental Figure 1 at http://www.genetics.org/supplemental/), congenic line analysis has not borne out a significant effect (L. N. Kwong, data not shown). Similarly, the higher tumor multiplicity of the congenic line BTBR.Mom7B6/BTBR compared to the partial consomic lines shown in Figure 1, C and D, or in (BTBR × B6) F1 mice (data not shown) suggests the presence of additional recessive enhancers in the BTBR strain.
The demonstration that different alleles of Mom7 segregate among inbred lines will have an impact on studies involving ApcMin. Currently, the decision to use particular inbred strains must take into account the status of the Mom1 alleles; future ApcMin experiments will also require knowledge of the pertinent Mom7 allelic genotypes when comparing strains or using mixed genetic backgrounds. Unlike Mom1, where screening for the Pla2g2a mutation can give information regarding an untested strain's allelic status, no specific mutation has yet been found for Mom7. However, it would be relatively simple to cross the strain of interest to a genotypically informative Mom7 congenic line (i.e., one with polymorphic markers) and determine its allelic status phenotypically.
The identification of a novel gene underlying Mom7 would add another target for chemoprevention. The demonstration of somatic recombination hotspots would also be valuable, as similar genetic determinants could exist for human colon cancer patients. What is now needed is to determine whether humans harbor a polymorphic modifier in the region orthologous to Mom7 on human chromosome 5.
Acknowledgments
We thank Michael Newton for the analysis of LOH distributions; James Amos-Landgraf and Kevin Haigis for critical discussions; Cory Hartman for assistance with Pyrosequencing assays and genotyping; Kurt Weiss, Natalie Borenstein, Kristen Meddaugh, Tegan Corrigan, and Bethany Hyduke for assistance with genotyping; Kristine Hahn for use of the PSQ96MA machine; and Richard Halberg for assistance with development of the BTBR.Min congenic line. We are grateful to the reviewers whose critical suggestions led us to reanalyze portions of this work. This research was supported by the National Cancer Institute (NCI) training grant CA009135 to L.N.K. and by NCI grant R37CA63677. This is publication 3634 from the Laboratory of Genetics, University of Wisconsin–Madison.
References
- Amos-Landgraf, J. M., L. N. Kwong, C. M. Kendziorski, M. Reichelderfer, J. Torrealba et al., 2007. A target-selected Apc-mutant rat kindred enhances the modeling of familial human colon cancer. Proc. Natl. Acad. Sci. USA 104 4036–4041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson, G., 1999. Tandem repeats finder: a program to analyze DNA sequences. Nucleic Acids Res. 27 573–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cormier, R. T., and W. F. Dove, 2000. Dnmt1N/+ reduces the net growth rate and multiplicity of intestinal adenomas in C57BL/6-Multiple intestinal neoplasia (Min)/+ mice independently of p53 but demonstrates strong synergy with the Modifier of Min 1AKR resistance allele. Cancer Res. 60 3965–3970. [PubMed] [Google Scholar]
- Cormier, R. T., K. H. Hong, R. B. Halberg, T. L. Hawkins, P. Richardson et al., 1997. Secretory phospholipase Pla2g2a confers resistance to intestinal tumorigenesis. Nat. Genet. 17 88–91. [DOI] [PubMed] [Google Scholar]
- Cormier, R. T., A. Bilger, A. J. Lillich, R. B. Halberg, K. H. Hong et al., 2000. The Mom1AKR intestinal tumor resistance region consists of Pla2g2a and a locus distal to D4Mit64. Oncogene 19 3182–3192. [DOI] [PubMed] [Google Scholar]
- Dietrich, W. F., E. S. Lander, J. S. Smith, A. R. Moser, K. A. Gould et al., 1993. Genetic identification of Mom-1, a major modifier locus affecting Min-induced intestinal neoplasia in the mouse. Cell 75 631–639. [DOI] [PubMed] [Google Scholar]
- Fearnhead, N. S., M. P. Britton and W. F. Bodmer, 2001. The ABC of APC. Hum. Mol. Genet. 10 721–733. [DOI] [PubMed] [Google Scholar]
- Gerber, J. K., E. Gogel, C. Berger, M. Wallisch, F. Muller et al., 1997. Termination of mammalian rDNA replication: polar arrest of replication fork movement by transcription termination factor TTF-I. Cell 90 559–567. [DOI] [PubMed] [Google Scholar]
- Goss, K. H., M. A. Risinger, J. J. Kordich, M. M. Sanz, J. E. Straughen et al., 2002. Enhanced tumor formation in mice heterozygous for Blm mutation. Science 297 2051–2053. [DOI] [PubMed] [Google Scholar]
- Gould, K. A., W. F. Dietrich, N. Borenstein, E. S. Lander and W. F. Dove, 1996. a Mom1 is a semi-dominant modifier of intestinal adenoma size and multiplicity in Min/+ mice. Genetics 144 1769–1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould, K. A., C. Luongo, A. R. Moser, M. K. McNeley, N. Borenstein et al., 1996. b Genetic evaluation of candidate genes for the Mom1 modifier of intestinal neoplasia in mice. Genetics 144 1777–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigis, K. M., and W. F. Dove, 2003. A Robertsonian translocation suppresses a somatic recombination pathway to loss of heterozygosity. Nat. Genet. 33 33–39. [DOI] [PubMed] [Google Scholar]
- Haines, J., V. Johnson, K. Pack, N. Suraweera, P. Slijepcevic et al., 2005. Genetic basis of variation in adenoma multiplicity in ApcMin/+ Mom1S mice. Proc. Natl. Acad. Sci. USA 102 2868–2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hospital, F., C. Chevalet and P. Mulsant, 1992. Using markers in gene introgression breeding programs. Genetics 132 1199–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemal, A., R. Siegel, E. Ward, T. Murray, J. Xu et al., 2006. Cancer statistics, 2006. CA Cancer J. Clin. 56 106–130. [DOI] [PubMed] [Google Scholar]
- Lander, E. S., P. Green, J. Abrahamson, A. Barlow, M. J. Daly et al., 1987. MAPMAKER: an interactive computer package for constructing primary genetic linkage maps of experimental and natural populations. Genomics 1 174–181. [DOI] [PubMed] [Google Scholar]
- Lincoln, S., M. Daly and E. Lander, 1992. Constructing genetic maps with MAPMAKER/EXP 3.0, Ed. 3. Whitehead Institute Technical Report. Whitehead Institute, Cambridge, MA.
- Lopez-estrano, C., J. B. Schvartzman, D. B. Krimer and P. Hernandez, 1998. Co-localization of polar replication fork barriers and rRNA transcription terminators in mouse rDNA. J. Mol. Biol. 277 249–256. [DOI] [PubMed] [Google Scholar]
- Luo, G., I. M. Santoro, L. D. McDaniel, I. Nishijima, M. Mills et al., 2000. Cancer predisposition caused by elevated mitotic recombination in Bloom mice. Nat. Genet. 26 424–429. [DOI] [PubMed] [Google Scholar]
- Mann, M. B., C. A. Hodges, E. Barnes, H. Vogel, T. J. Hassold et al., 2005. Defective sister-chromatid cohesion, aneuploidy and cancer predisposition in a mouse model of type II Rothmund-Thomson syndrome. Hum. Mol. Genet. 14 813–825. [DOI] [PubMed] [Google Scholar]
- Park, Y. G., R. Clifford, K. H. Buetow and K. W. Hunter, 2003. Multiple cross and inbred strain haplotype mapping of complex-trait candidate genes. Genome Res. 13 118–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pletcher, M. T., P. McClurg, S. Batalov, A. I. Su, S. W. Barnes et al., 2004. Use of a dense single nucleotide polymorphism map for in silico mapping in the mouse. PLoS. Biol. 2 e393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team, 2005. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna.
- Shao, C., P. J. Stambrook and J. A. Tischfield, 2001. Mitotic recombination is suppressed by chromosomal divergence in hybrids of distantly related mouse strains. Nat. Genet. 28 169–172. [DOI] [PubMed] [Google Scholar]
- Shoemaker, A. R., A. R. Moser, C. A. Midgley, L. Clipson, M. A. Newton et al., 1998. A resistant genetic background leading to incomplete penetrance of intestinal neoplasia and reduced loss of heterozygosity in ApcMin/+ mice. Proc. Natl. Acad. Sci. USA 95 10826–10831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman, K. A., R. Koratkar, L. D. Siracusa and A. M. Buchberg, 2002. Identification of the modifier of Min 2 (Mom2) locus, a new mutation that influences Apc-induced intestinal neoplasia. Genome Res. 12 88–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman, K. A., R. A. Koratkar, L. D. Siracusa and A. M. Buchberg, 2003. Exclusion of Madh2, Madh4, and Madh7 as candidates for the modifier of Min 2 (Mom2) locus. Mamm. Genome 14 119–129. [DOI] [PubMed] [Google Scholar]
- Sjoblom, T., S. Jones, L. D. Wood, D. W. Parsons, J. Lin et al., 2006. The consensus coding sequences of human breast and colorectal cancers. Science 314 268–274. [DOI] [PubMed] [Google Scholar]
- Suraweera, N., J. Haines, A. McCart, P. Rogers, A. Latchford et al., 2006. Genetic determinants modulate susceptibility to pregnancy-associated tumourigenesis in a recombinant line of Min mice. Hum. Mol. Genet. 15 3429–3435. [DOI] [PubMed] [Google Scholar]
- Suzuki, T., K. Minehata, K. Akagi, N. A. Jenkins and N. G. Copeland, 2006. Tumor suppressor gene identification using retroviral insertional mutagenesis in Blm-deficient mice. EMBO J. 25 3422–3431. [DOI] [PMC free article] [PubMed] [Google Scholar]