Abstract
Here we show that RNA interference (RNAi) machinery operates in Drosophila melanogaster 1.688 satellite transcription. Mutation in the spn-E gene, known to be involved in RNAi in the oocytes, causes an increase of satellite transcript abundance. Transcripts of both strands of 1.688 satellite repeats in germinal tissues were detected. The strength of the effects of the spn-E mutation differs for 1.688 satellite DNA subfamilies and is more pronounced for autosomal pericentromeric satellites compared to the X-linked centromeric ones. The spn-E1 mutation causes an increase of the H3-AcK9 mark and TAF1 (a component of the polymerase II transcriptional complex) occupancy in the chromatin of autosomal pericentromeric repeats. Thus, we revealed that RNAi operates in ovaries to maintain the silenced state of centromeric and pericentromeric 1.688 repeats.
RNA interference (RNAi) has been implicated in recognizing repetitive DNA elements as preferential targets for heterochromatin assembly (Volpe et al. 2002; Birchler et al. 2004; Lippman et al. 2004; Verdel et al. 2004; Kavi et al. 2005). The mechanism of transcriptional silencing of centromeric repeats in Schizosaccharomyces pombe has been extensively studied. The S. pombe centromeric repeats are transcribed in both directions, producing double-stranded RNAs (dsRNAs), which are processed into small interfering RNAs (siRNAs) by the endonuclease Dicer. RNA-induced transcriptional silencing (RITS) complexes carrying siRNAs are thought to be associated with nascent centromeric transcripts, to recruit H3-K9 methyltransferase, and to provide formation of the self-sustaining closed chromatin state (Verdel et al. 2004; Buhler et al. 2006; Sugiyama et al. 2007). The heterochromatic state of S. pombe centromeric repeats is needed for proper centromere functioning, including cohesin binding (Partridge et al. 2002). Centromeric satellite repeats in Arabidopsis and mouse are also transcribed bidirectionally, ensuring formation of dsRNAs and siRNA generation (Martens et al. 2005; May et al. 2005; Lee et al. 2006).
siRNAs corresponding to 1.688 satellite have been detected in the Drosophila siRNA library (Aravin et al. 2003). To address whether satellite DNA transcription is under RNAi control, we analyzed the effects of spn-E1, spn-Ehls-03987, and aubQC42 mutations on the transcriptional status of the 1.688 satellite DNA. The spn-E gene, encoding a DExD-box RNA helicase (Gillespie and Berg 1995), as well as the aub gene, a member of the Piwi family, are involved in dsRNA-triggered RNAi in embryos (Kennerdell et al. 2002), in transcriptional silencing of transgenes (Pal-Bhadra et al. 2004), and in the control of Drosophila retrotransposon transcript abundance in the germline (Aravin et al. 2001; Savitsky et al. 2006; Vagin et al. 2006).
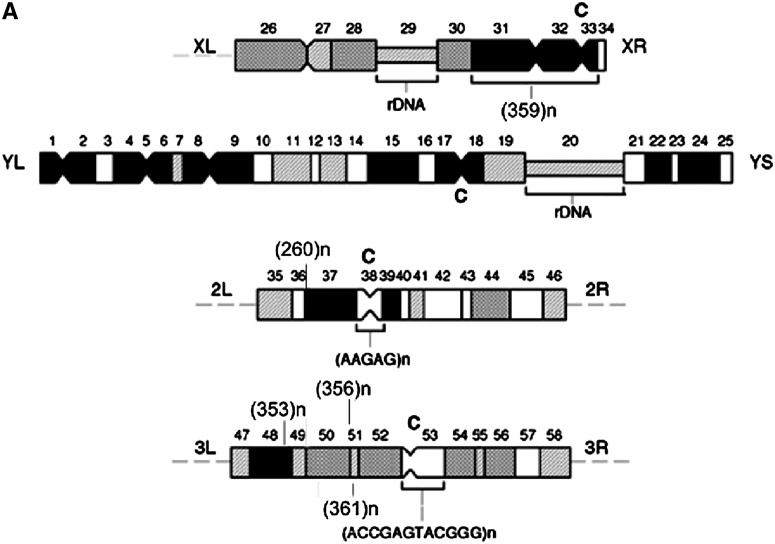
In the Drosophila melanogaster genome, each centromeric region seems to contain different sets of satellite DNA sequences (Abad et al. 1992, 2000; Lohe et al. 1993; Sun et al. 1997; Agudo et al. 1999; Lamb and Birchler 2003). The X chromosome contains a large block of complex 1.688 satellite DNA (359-bp repeat unit), located in the centromeric region and in the adjacent pericentromeric heterochromatin (Lohe et al. 1993; Abad et al. 2000) (Figure 1A), and large autosomes contain arrays of different subfamilies of 1.688 satellite DNA in the pericentromeric heterochromatin. The 260-bp arrays are located at 2L heterochromatin (Abad et al. 2000) (Figure 1A), while the 353- and 356-bp arrays are attributed to distal and proximal regions of 3L heterochromatin, respectively (Losada and Villasante 1996) (Figure 1A). The 361-bp arrays are located in a region adjacent to the 356-bp arrays (A. Villasante, unpublished results) (Figure 1A). The transient presence of the kinetochore-specific protein BubRI in all heterochromatic regions containing the 1.688 satellite has been shown by immunostaining (Abad et al. 2000). BubR1 is required for the spindle checkpoint function and for chromosome alignment (Ditchfield et al. 2003), suggesting a role of 1.688 satellites in chromosome congression. Short tandem arrays (two to four copies) of sequences homologous to the 1.688 satellite are dispersed throughout the euchromatin of the X chromosome. These 1.688 satellite-related sequences are often situated in the vicinity of genes and their requirement for some sex-chromosome-specific functions has been discussed (Waring and Pollack 1987; DiBartolomeis et al. 1992; Losada and Villasante 1996).
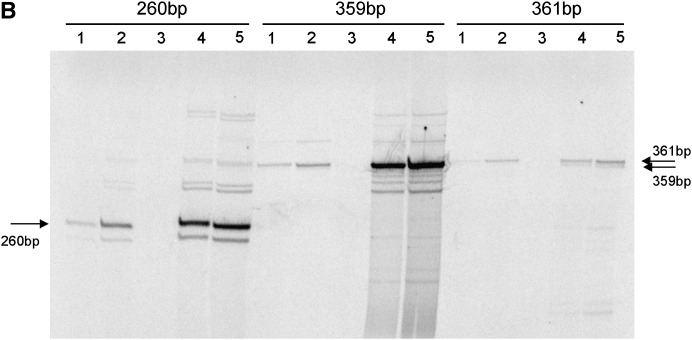
Figure 1.—
(A) Schematic of 1.688 satellite locations in heterochromatin of large D. melanogaster chromosomes. Heterochromatic segments are indicated according to Gatti et al. (1994). “C” denotes centromeres; centromere-forming and ribosomal repeats are shown. 1.688 satellite subfamilies are denoted by characteristic size of repeat unit. (B) Gel analysis of PCR-amplified products obtained with primers for 260-, 361-, and 359-bp 1.688 satellite subfamilies. The designed primers are not strictly specific and amplify many bands corresponding to nontarget satellite subfamilies or damaged satellite copies; however, major target bands may be identified. In all experiments, we have analyzed only target bands. Lanes 1 and 2, ovaries; lane 3, no-RT, spn-E1/spn-E1 ovarian RNA; lanes 4 and 5, testes; lanes 1 and 4, +/spn-E1; lanes 2 and 5, spn-E1/spn-E1.
Here we show that the 1.688 satellite DNA is transcribed bidirectionally in germinal tissues of D. melanogaster. Mutations in the spn-E and aub genes resulted in the increase of satellite transcript abundance in ovaries and the spn-E1 mutation causes the accumulation of the histone H3 acetylated at lysine 9 (H3-AcK9) mark and transcription factor TAF1 in satellite chromatin.
MATERIALS AND METHODS
Drosophila strains:
The strain bearing the spindle-E (spn-E1) mutation was ru1 st1 spn-E1 e1 ca1/TM3, Sb1 es (bearing a point mutation in the helicase domain); the aub mutant was aubQC42/CyO.
RT–PCR analyses:
RT–PCR was done according to described procedure (Aravin et al. 2001) using pairs of primers corresponding to 1.688 satellite subfamilies: 361 bp, 5′-TCAACGATGTATGACATTCC-3′ (R, right) and 5′-TGAGCTCGTAATAAAATTTCC-3′ (L, left); 260 bp, 5′-ATGAAACTGTGTTCAACAAT-3′ (R) and 5′-TGGAAATTTAATTACGAGCT-3′ (L); 359 bp, 5′-TATTCTTACATCTATGTGACC-3′ (R) and 5′-GTTTTGAGCAGCTAATTACC-3′ (L). As a loading control, we used the primers for rp49 (5′-ATGACCATCCGCCCAGCATAC-3′ and 5′-CTGCATGAGCAGGACCTCCAG-3′), Pgd (5′-AGGACTCGTGGCGCGAGGTG-3′ and 5′-GGAATGTGTGAACGGGAAAGTGGAG-3′), and adh (5′-AAACTGGCCCCCATTACCG-3′ and 5′-CAAGTCCAGTTTCCAGATG-3′). cDNA was synthesized using random (hexanucleotide) or specific primers. Primers were added to total RNA (1 μg) and heated to 70° for 10 min. First-strand cDNA synthesis was performed with SuperScript II reverse transcriptase (200 units; GIBCO BRL, Gaithersburg, MD) for 1 hr at 42°. Controls without RT were processed in parallel. The enzyme was heat inactivated at 70° for 15 min. Reverse transcription of several independently isolated RNA samples was done. Samples (2 μl) from the reverse transcription reaction were amplified with Taq polymerase in the presence of dATP-αP33. The linear range of amplification was determined in preliminary experiments, and, depending on transcript abundance, 18–30 cycles were performed. Amplification was done using touchdown PCR with a final annealing temperature of ∼40° (depending on the primer set). PCR products were separated in 5% denaturing acrylamide gel and visualized and quantified using PhosphoImager Storm-840 (Amersham, Piscataway, NJ).
Cloning and sequencing:
The fragments obtained by PCR were cloned into pGEM-T vector (Promega, Madison, WI). Sequencing was performed using big dye-termination reagents and ABI/PE 377 automated sequencers. Sequences were analyzed by BLAST searches.
Chromatin immunoprecipitation:
Chromatin immunoprecipitation (ChIP) was performed according to described procedure (Chanas et al. 2004). The analysis of DNA obtained in ChIP was done by semiquantative radioactive PCR as described above. Signals were quantified by PhosphorImager Storm-840. The ratio of signals from precipitated satellite DNA and DNA of the control Pgd gene vs. signals from input chromatin was calculated for spn-E1/spn-E1 and +/spn-E1 ovaries.
RESULTS AND DISCUSSION
1.688 satellite DNA is transcribed in germinal tissues of D. melanogaster and is under the control of RNAi in ovaries:
We have analyzed the transcriptional status of 1.688 satellite DNA in germinal tissues (ovaries and testes) of flies carrying mutations in the spn-E and aubergine genes. We compared 1.688 satellite transcript abundance in the homozygotes spn-E1/spn-E1 and aubQC42/aubQC42 and in the trans-heterozygote spn-E1/spn-Ehls-03987 with corresponding heterozygous flies. The evaluation of transcript abundance was monitored by reverse transcription using random primers followed by semiquantitative PCR with primers specific for a given 1.688 satellite DNA subfamily. PCR amplification and gel analysis revealed a major target band as well as minor bands corresponding to nontarget satellite subfamilies or damaged satellite copies (Figure 1B), since we failed to design primers strictly specific to a definite subfamily of 1.688 satellites because of a high redundancy of their sequences. Therefore, we considered only the bands corresponding to the expected size of a target satellite subfamily in RT–PCR and ChIP (see below) analysis (Figure 1B).
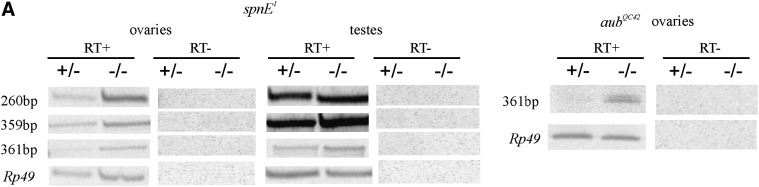
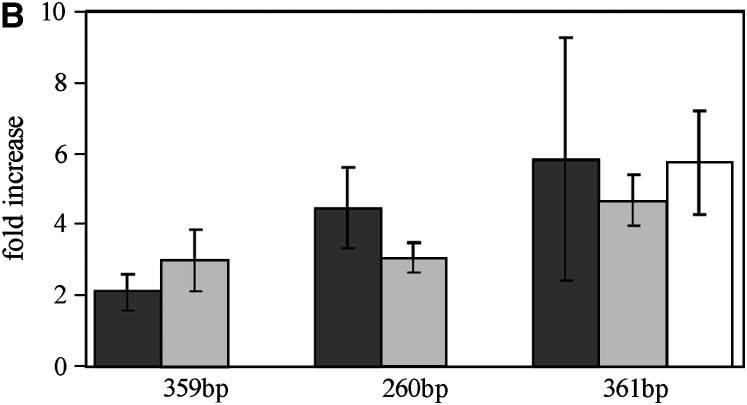
Transcription of 1.688 satellite DNAs was detected both in ovaries and in testes (Figure 2A). 1.688 satellite transcript abundance normalized to a rp49 transcript level shows its increase in spn-E1/spn-E1, spn-E1/spn-Ehls-03987, and aubQC42/aubQC42 ovaries relative to ovaries from heterozygous flies (Figure 2A). The increase of satellite expression in ovaries is twofold for the X-linked 359-bp subfamily and achieves a fivefold increase for autosomal 260- and 361-bp subfamilies (Figure 2B). In ovaries, the transcript abundances of all subfamilies are maintained on a roughly equal and lower level. This result suggests a low level of the 359-bp subfamily transcripts in ovaries compared to autosomal pericentromeric subfamilies, taking into account that the X-linked array of 359-bp repeats is represented by several thousand copies and constitutes the major part of the 1.688 satellite DNA (Hsieh and Brutlag 1979). In testes, the level of 1.688 transcripts in spn-E1/spn-E1 flies does not significantly change due to spn-E1 mutation (Figure 2A).
Figure 2.—
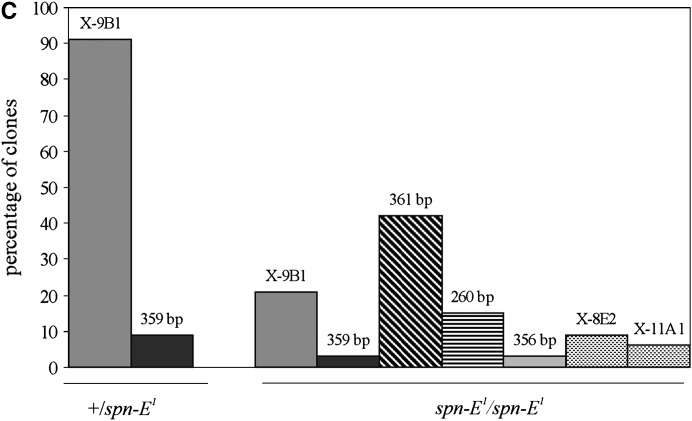
Analysis of 1.688 satellite transcription in germinal tissues. (A) RT–PCR analysis of transcription of 1.688 satellite subfamilies in ovaries and testes of homozygous (−/−) and heterozygous (+/−) spn-E1 and aubQC42 flies. Transcription of the ubiquitously expressed rp49 gene was used as a loading control. Amplification of RT controls (RT−) without reverse transcriptase gives no signals. (B) Mean ratios of satellite transcript abundance normalized to the rp49 transcript level show the increase of 1.688 satellite transcript abundance in spn-E1/spn-E1 (dark shading), spn-E1/spn-Ehls-03987(light shading), and aubQC42/aubQC42 (no shading) ovaries relative to ovaries of heterozygous flies. (C) Cloning, sequencing, and annotating of PCR-amplified cDNAs from spn-E1/spn-E1 and +/spn-E1 ovaries show transcriptional activation of various groups of 1.688 satellites in the presence of the spn-E1 mutation. PCR amplification of cDNAs was performed in exponential phase with primers for the 361-bp subfamily. However, several other subfamilies were also detected: X-linked euchromatic regions 9B1, 8E2, and 11A1; the X-linked 359-bp subfamily; and autosomal 260- and 356-bp subfamilies. The y-axis presents percentages of clones. The number of clones is 23 for +/ spn-E1 and 33 for spn-E1/spn-E1 flies. The sequences of all clones are in supplemental Table 1 (http://www.genetics.supplemental/).
To extend the analysis of the effects of spn-E1 mutation on satellite transcription, the cDNAs obtained from spn-E1/spn-E1 and +/spn-E1 ovaries were PCR amplified with primers for the 361-bp subfamily, cloned, and sequenced (Figure 2C). Owing to a high similarity of 1.688 satellite subfamilies, we also obtained clones related to the other subfamilies. We detected strong differences between the representatives of cDNA sets from spn-E1/spn-E1 and +/spn-E1 ovaries (Figure 2C). cDNA clones from +/spn-E1 ovaries are represented mainly by 1.688 satellites located at the 9B1 euchromatic region of the X chromosome (∼90%). The repertoire of the cDNA set from spn-E1/spn-E1 ovaries is more diverse. The appearence of the target 361-bp cDNAs is the most pronounced effect of the spn-E1 mutation: no 361-bp clones from +/spn-E1 ovaries were detected, whereas approximately half of the clones from spn-E1/spn-E1 ovaries corresponds to the 361-bp subfamily. Interestingly, three of four previously cloned 1.688 satellite siRNAs (ACUAAUUACCAGCGCUAACGAUCCCUAU, UUAGGGAAAUUAGUUUUGG, and UGAUGACCGAAAUUUGGAAAAACGG) (Aravin et al. 2003) originated from the 361-bp subfamily transcripts. The spn-E1 mutation leads to the increase of cDNA clones of pericentromeric autosomal satellites, including the 260- and 356-bp subfamilies. No effect of the spn-E1 mutation on the X-linked 359-bp subfamily was detected. This result may not be attributed to a low efficiency of primers used to amplify the 359-bp repeats, because the effective amplification of 359-bp satellites was attained in ChIP experiments (data not shown).
Thus, the extent of the effect of the spn-E1 mutation was more pronounced for pericentromeric autosomal subfamilies (260 and 361 bp) than for the X-linked 359-bp subfamily. These results indicate the differential transcriptional responses of euchromatic, centromeric, and pericentromeric subfamilies of 1.688 satellite DNA, owing to the disturbance of the RNAi mechanism.
Both strands of the 1.688 satellite DNA are transcribed:
It is thought that the formation of double-stranded RNA is crucial for RNAi-dependent regulation of repeats. The emergence of double-stranded RNA in S. pombe is a primary step in siRNA formation, but the subsequent self-maintenance of the siRNA pool is most likely ensured by RNA-dependent RNA polymerase (RdRP) (Volpe et al. 2002). Persistent formation of transcripts from both DNA strands ensures the maintenance of a double-stranded RNA level in humans and flies lacking RdRP and may be considered a prominent feature of heterochromatic repeats in eukaryotes lacking RdRP. It has been recently shown that cellular dsRNAs in mammals are mainly produced by tandem repeat transcription, but not as a result of the transcription of repeated interspersed elements (Martens et al. 2005).
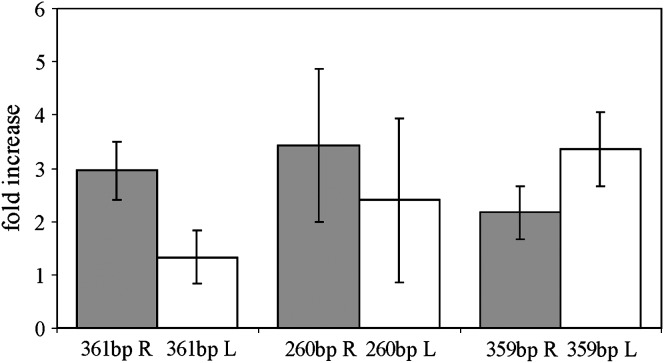
We detected transcripts corresponding to both strands of the 260-, 361-, and 359-bp subfamilies (Figure 3). The spn-E1 mutation causes the increase of transcript abundance, corresponding to both strands of satellites of the 260- and 359-bp subfamilies and to only a single strand of the 361-bp subfamily (Figure 3). The presence of transcripts from both strands of satellite DNA may lead to dsRNA formation.
Figure 3.—
RT–PCR analysis of strand-specific 1.688 satellite transcription in spn-E1/spn-E1 and +/spn-E1 ovaries revealed transcription of both strands. The diagram presents the ratios of satellite transcript abundance in homozygous spn-E1/spn-E1 ovaries to spn-E1/+ heterozygotes. Strand-specific primers (R, “right,” and L, “left”) for each subfamily are the same as in randomly primed RT–PCR analysis. Adh gene product was used as a loading control.
spn-E1 mutation leads to the increase of the H3-AcK9 mark and TAF1 amount in the chromatin of autosomal pericentromeric 1.688 satellites:
Histone lysine modifications, including lysine 9 of histone H3, mark functionally distinct chromatin regions. Dimethylation of Lys9 (H3-diMeK9) followed by binding of heterochromatic protein 1 (HP1) or its homologs is generally thought to be a heterochromatic feature (Lachner et al. 2001; Nakayama et al. 2001). Acetylation at Lys9 (H3-AcK9) is a mark of active euchromatic regions (Turner 2000; Jenuwein and Allis 2001). Volpe et al. (2002) have reported a loss of H3-diMeK9 and Swi6, the HP1 homolog, at the centromeric repeats in S. pombe as a result of mutations in the RNAi system. It has been shown that in D. melanogaster mutations in the spn-E gene and in the other genes of the RNAi system, piwi and aubergine produce a drastic effect on heterochromatin state in somatic tissues, resulting in a considerable loss of H3-diMeK9 and redistribution of heterochromatic proteins HP1 and HP2 away from the chromocenter (Pal-Bhadra et al. 2004).
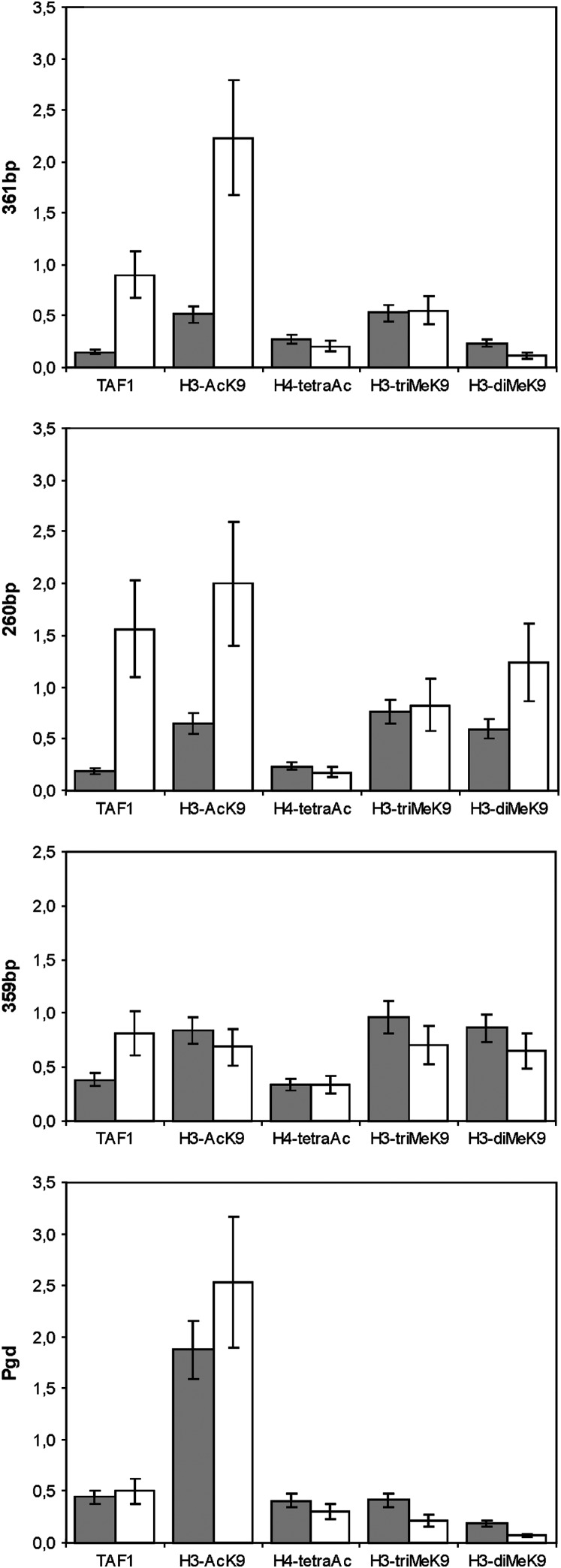
To test if spn-E1 mutation causes changes in the chromatin of 1.688 satellite DNA, we performed ChIP analysis of ovarian chromatin in spn-E1/spn-E1 and +/spn-E1 flies with antibodies against H3-diMeK9, H3-AcK9, H3-triMeK9, H4-tetraAc (H4, acetylated at lysines 5, 8, 12, 16), and TAF1, a component of the RNA polymerase II transcriptional complex (Wassarman et al. 2000) (Figure 4). ChIP data show the increase of accumulation of the H3-AcK9 mark and TAF1 on chromatin of tested autosomal pericentromeric repeats, owing to spn-E1 mutation (Figure 4). The four- to fivefold increase of TAF1 occupancy of the 361- and 260-bp subfamilies of satellites was revealed in homozygous spn-E1 flies as compared with heterozygous ones, while difference in TAF1 amount on 359-bp repeats was shown to be insignificant (Figure 4). We detected no changes in H3-MeK9 marks of 1.688 satellite chromatin. No significant effects for all tested antibodies were detected for the chromatin of the X-linked 359-bp subfamily. These data correlate with the level of transcript abundance increase, owing to spn-E1 mutation, that is stronger for pericentromeric satellites (361 and 260 bp) than for the 359-bp subfamily (Figure 2).
Figure 4.—
ChIP analysis of 1.688 satellite chromatin in +/spn-E1 and spn-E1/spn-E1 ovaries, using antibodies against H3-diMeK9, H3-AcK9, H3-triMeK9, H4-tetraAc, and TAF1. DNA purified from input ovarian chromatin or precipitated with antibodies was analyzed by semiquantitative PCR. To normalize target DNA signal, we used DNA of the transcribed region of the ubiquitously expressed housekeeping Pgd gene. Shaded columns refer to +/spn-E1 and open columns to spn-E1/spn-E1 ovaries. DNA precipitated with antibodies was PCR amplified in exponential phase with primers for the corresponding subfamily, separated on polyacrylamide gel, and quantified by PhosphorImager. Values are indicated as the percentage of precipitation relative to the input. The experiment was performed two times.
Together, these results indicate that 1.688 satellite transcription is most probably executed by RNA polymerase II and is modulated by a RNAi-dependent mechanism of silencing on a chromatin level.
Conclusion:
Transcription of the 1.688 satellite DNA in the D. melanogaster genome has not been previously shown. We detected transcription of 1.688 satellite subfamilies in the germline and the increase of their transcript abundance in ovaries of flies carrying mutations in the spn-E and aub genes. These results reveal the participation of the RNAi system in silencing of the 1.688 satellite DNA. The effect of the spn-E1 mutation is more pronounced for satellite arrays localized in pericentromeric regions of autosomes (361 and 260 bp) than for the X-linked centromeric cluster (359 bp). These observations are in accordance with the results of ChIP analysis showing the increase of TAF1 occupancy of the 361-bp subfamily but not of the 359-bp subfamily. Our results suggest that differences in the RNAi-dependent regulation of 1.688 satellite transcription may be correlated with a specificity of their chromatin organization. This difference between centromeric and pericentromeric satellites may be related to their function in kinetochore formation and chromosome cohesion, respectively. The significance of the correlation between functional peculiarities of satellite subfamilies and the dependence of their chromatin properties on RNAi needs to be unveiled in further studies.
Acknowledgments
We are indebted to V. E. Alatortsev and Yu.Ya. Shevelyov for valuable comments and suggestions. This work was supported by grants from the Russian Academy of Sciences program for Molecular and Cell Biology, Russian Foundation for Basic Research (05-04-48034), and the program of Scientific School support (6113.2006.4) to V.A.G. and by a grant from the Ministerio de Educación y Ciencia (BFU2005-07690-C02-01) to Alfredo Villasante and an institutional grant from the Fundación Ramón Areces to Centro de Biologia Molecular “Severo Ochoa”.
References
- Abad, J. P., M. Carmena, S. Baars, R. D. Saunders, D. M. Glover et al., 1992. Dodeca satellite: a conserved G+C-rich satellite from the centromeric heterochromatin of Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 89 4663–4667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abad, J. P., M. Agudo, I. Molina, A. Losada, P. Ripoll et al., 2000. Pericentromeric regions containing 1.688 satellite DNA sequences show anti-kinetochore antibody staining in prometaphase chromosomes of Drosophila melanogaster. Mol. Gen. Genet. 264 371–377. [DOI] [PubMed] [Google Scholar]
- Agudo, M., A. Losada, J. P. Abad, S. Pimpinelli, P. Ripoll et al., 1999. Centromeres from telomeres? The centromeric region of the Y chromosome of Drosophila melanogaster contains a tandem array of telomeric HeT-A- and TART-related sequences. Nucleic Acids Res. 27 3318–3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aravin, A. A., N. M. Naumova, A. V. Tulin, V. V. Vagin, Y. M. Rozovsky et al., 2001. Double-stranded RNA-mediated silencing of genomic tandem repeats and transposable elements in the D. melanogaster germline. Curr. Biol. 11 1017–1027. [DOI] [PubMed] [Google Scholar]
- Aravin, A. A., M. Lagos-Quintana, A. Yalcin, M. Zavolan, D. Marks et al., 2003. The small RNA profile during Drosophila melanogaster development. Dev. Cell 5 337–350. [DOI] [PubMed] [Google Scholar]
- Birchler, J. A., H. H. Kavi and H. R. Fernandez, 2004. Heterochromatin: RNA points the way. Curr. Biol. 14 R759–R761. [DOI] [PubMed] [Google Scholar]
- Buhler, M., A. Verdel and D. Moazed, 2006. Tethering RITS to a nascent transcript initiates RNAi- and heterochromatin-dependent gene silencing. Cell 125 873–886. [DOI] [PubMed] [Google Scholar]
- Chanas, G., S. Lavrov, F. Iral, G. Cavalli and F. Maschat, 2004. Engrailed and polyhomeotic maintain posterior cell identity through cubitus-interruptus regulation. Dev. Biol. 272 522–535. [DOI] [PubMed] [Google Scholar]
- DiBartolomeis, S. M., K. D. Tartof and F. R. Jackson, 1992. A superfamily of Drosophila satellite related (SR) DNA repeats restricted to the X chromosome euchromatin. Nucleic Acids Res. 20 1113–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditchfield, C., V. L. Johnson, A. Tighe, R. Ellston, C. Haworth et al., 2003. Aurora B couples chromosome alignment with anaphase by targeting BubR1, Mad2, and Cenp-E to kinetochores. J. Cell Biol. 161 267–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatti, M., S. Bonaccorsi and S. Pimpinelli, 1994. Looking at Drosophila mitotic chromosomes. Methods Cell Biol. 44 371–391. [DOI] [PubMed] [Google Scholar]
- Gillespie, D. E., and C. A. Berg, 1995. Homeless is required for RNA localization in Drosophila oogenesis and encodes a new member of the DE-H family of RNA-dependent ATPases. Genes Dev. 9 2495–2508. [DOI] [PubMed] [Google Scholar]
- Hsieh, T., and D. Brutlag, 1979. Sequence and sequence variation within the 1.688 g/cm3 satellite DNA of Drosophila melanogaster. J. Mol. Biol. 135 465–481. [DOI] [PubMed] [Google Scholar]
- Jenuwein, T., and C. D. Allis, 2001. Translating the histone code. Science 293 1074–1080. [DOI] [PubMed] [Google Scholar]
- Kavi, H. H., H. R. Fernandez, W. Xie and J. A. Birchler, 2005. RNA silencing in Drosophila. FEBS Lett. 579 5940–5949. [DOI] [PubMed] [Google Scholar]
- Kennerdell, J. R., S. Yamaguchi and R. W. Carthew, 2002. RNAi is activated during Drosophila oocyte maturation in a manner dependent on aubergine and spindle-E. Genes Dev. 16 1884–1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachner, M., D. O'Carroll, S. Rea, K. Mechtler and T. Jenuwein, 2001. Methylation of histone H3 lysine 9 creates a binding site for HP1 proteins. Nature 410 116–120. [DOI] [PubMed] [Google Scholar]
- Lamb, J. C., and J. A. Birchler, 2003. The role of DNA sequence in centromere formation. Genome Biol. 4 214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, H. R., P. Neumann, J. Macas and J. Jiang, 2006. Transcription and evolutionary dynamics of the centromeric satellite repeat CentO in rice. Mol. Biol. Evol. 23 2505–2520. [DOI] [PubMed] [Google Scholar]
- Lippman, Z., A. V. Gendrel, M. Black, M. W. Vaughn, N. Dedhia et al., 2004. Role of transposable elements in heterochromatin and epigenetic control. Nature 430 471–476. [DOI] [PubMed] [Google Scholar]
- Lohe, A. R., A. J. Hilliker and P. A. Roberts, 1993. Mapping simple repeated DNA sequences in heterochromatin of Drosophila melanogaster. Genetics 134 1149–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losada, A., and A. Villasante, 1996. Autosomal location of a new subtype of 1.688 satellite DNA of Drosophila melanogaster. Chromosome Res. 4 372–383. [DOI] [PubMed] [Google Scholar]
- Martens, J. H., R. J. O'Sullivan, U. Braunschweig, S. Opravil, M. Radolf et al., 2005. The profile of repeat-associated histone lysine methylation states in the mouse epigenome. EMBO J. 24 800–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May, B. P., Z. B. Lippman, Y. Fang, D. L. Spector and R. A. Martienssen, 2005. Differential regulation of strand-specific transcripts from Arabidopsis centromeric satellite repeats. PLoS Genet. 1 e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama, J., J. C. Rice, B. D. Strahl, C. D. Allis and S. I. Grewal, 2001. Role of histone H3 lysine 9 methylation in epigenetic control of heterochromatin assembly. Science 292 110–113. [DOI] [PubMed] [Google Scholar]
- Pal-Bhadra, M., B. A. Leibovitch, S. G. Gandhi, M. Rao, U. Bhadra et al., 2004. Heterochromatic silencing and HP1 localization in Drosophila are dependent on the RNAi machinery. Science 303 669–672. [DOI] [PubMed] [Google Scholar]
- Partridge, J. F., K. S. Scott, A. J. Bannister, T. Kouzarides and R. C. Allshire, 2002. cis-acting DNA from fission yeast centromeres mediates histone H3 methylation and recruitment of silencing factors and cohesin to an ectopic site. Curr. Biol. 12 1652–1660. [DOI] [PubMed] [Google Scholar]
- Savitsky, M., D. Kwon, P. Georgiev, A. Kalmykova and V. Gvozdev, 2006. Telomere elongation is under the control of the RNAi-based mechanism in the Drosophila germline. Genes Dev. 20 345–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama, T., H. P. Cam, R. Sugiyama, K. Noma, M. Zofall et al., 2007. SHREC, an effector complex for heterochromatic transcriptional silencing. Cell 128 491–504. [DOI] [PubMed] [Google Scholar]
- Sun, X., J. Wahlstrom and G. Karpen, 1997. Molecular structure of a functional Drosophila centromere. Cell 91 1007–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner, B. M., 2000. Histone acetylation and an epigenetic code. BioEssays 22 836–845. [DOI] [PubMed] [Google Scholar]
- Vagin, V. V., A. Sigova, C. Li, H. Seitz, V. Gvozdev et al., 2006. A distinct small RNA pathway silences selfish genetic elements in the germline. Science 313 320–324. [DOI] [PubMed] [Google Scholar]
- Verdel, A., S. Jia, S. Gerber, T. Sugiyama, S. Gygi et al., 2004. RNAi-mediated targeting of heterochromatin by the RITS complex. Science 303 672–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpe, T. A., C. Kidner, I. M. Hall, G. Teng, S. I. Grewal et al., 2002. Regulation of heterochromatic silencing and histone H3 lysine-9 methylation by RNAi. Science 297 1833–1837. [DOI] [PubMed] [Google Scholar]
- Waring, G. L., and J. C. Pollack, 1987. Cloning and characterization of a dispersed, multicopy, X chromosome sequence in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 84 2843–2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassarman, D. A., N. Aoyagi, L. A. Pile and E. M. Schlag, 2000. TAF250 is required for multiple developmental events in Drosophila. Proc. Natl. Acad. Sci. USA 97 1154–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]