Abstract
In Arabidopsis thaliana, DICER-LIKE 1 and DICER-LIKE 3 are involved in the generation of small RNAs. Double mutants between dicer-like 1 and dicer-like 3 exhibit a delay in flowering that is caused by increased expression of the floral repressor FLOWERING LOCUS C. This delayed-flowering phenotype is similar to that of autonomous-pathway mutants, and the flowering delay can be overcome by vernalization.
THE transition from vegetative to reproductive development is a highly regulated event in the plant life cycle. In Arabidopsis there are several pathways that influence time to flowering including the photoperiod, vernalization, autonomous, and FRIGIDA pathways. The photoperiod and vernalization pathways promote flowering in response to day length and the prolonged cold of winter, respectively. The vernalization pathway functions to epigenetically silence the strong floral repressor FLOWERING LOCUS C (FLC) (Bastow et al. 2004; Sung and Amasino 2004). The autonomous pathway acts to constitutively promote flowering by repressing FLC expression (for review see Simpson 2004), whereas FRIGIDA delays the floral transition by creating a vernalization requirement via upregulating FLC expression (Michaels and Amasino 1999; Sheldon et al. 1999). Because precise regulation of FLC is essential for proper timing of the floral transition, it is not surprising that FLC expression is controlled by multiple pathways.
Forward genetic screens have unveiled a large number of genes required for the floral transition (Sung and Amasino 2005). Components of the photoperiod pathway have been identified as mutants that flower at the same developmental stage regardless of the day length. Genes required for vernalization have been identified in screens for mutants that fail to flower rapidly after an extended exposure to cold temperatures. FRIGIDA pathway genes have been identified in screens for early-flowering mutants that block the ability of FRIGIDA to promote FLC expression. Finally, genes in the autonomous pathway have been identified as mutants that flower later than wild type in both inductive and noninductive photoperiods.
In higher plants, it is common for members of gene families to be functionally redundant. For such gene families, forward genetic screens are less likely to reveal the role of a single gene in a particular developmental process. Recently, Gasciolli et al. (2005) used a reverse genetics approach to determine possible functional redundancy among the four member DICER-LIKE (DCL) gene family. DCLs are ribonucleases that generate small RNA species from double-stranded RNA (Bernstein et al. 2001; Hutvagner et al. 2001). Each of the DCL enzymes generates predominantly a particular class of small RNA species. DCL1 is required for microRNA (miRNA) biogenesis (Park et al. 2002; Reinhart et al. 2002; Kurihara and Watanabe 2004), DCL2 generates viral small interfering RNAs (siRNA) (Xie et al. 2004), DCL3 forms heterochromatic siRNAs (Xie et al. 2004), and DCL4 is required for transactivating siRNA (ta-siRNA) biogenesis (Dunoyer et al. 2005; Gasciolli et al. 2005; Xie et al. 2005; Yoshikawa et al. 2005). Although DCL1–4 have predominant roles in generating specific small RNA species, these DCLs can also have compensating functions (Gasciolli et al. 2005; Blevins et al. 2006; Deleris et al. 2006). For example, TAS1-3 mRNA levels in dcl4 mutants are lower than those in Columbia (Col), whereas levels in dcl2 or dcl3 are indistinguishable from those in wild type. However, double mutants between dcl4 and either dcl2 or dcl3 result in a further loss of TAS1-3 mRNA expression, indicating that in the absence of DCL4, DCL2 and DCL3 process siRNAs that are otherwise primarily processed by DCL4 (Gasciolli et al. 2005). Thus, double and triple dcl mutant combinations, created in the Col accession, result in phenotypes not observed in the single mutants (Gasciolli et al. 2005). For example, a double mutant between a weak dcl1 allele (dcl1 null alleles are lethal; Schauer et al. 2002) and dcl3 results in defective floral structures, an extreme delay in flowering time, and sterility (Gasciolli et al. 2005).
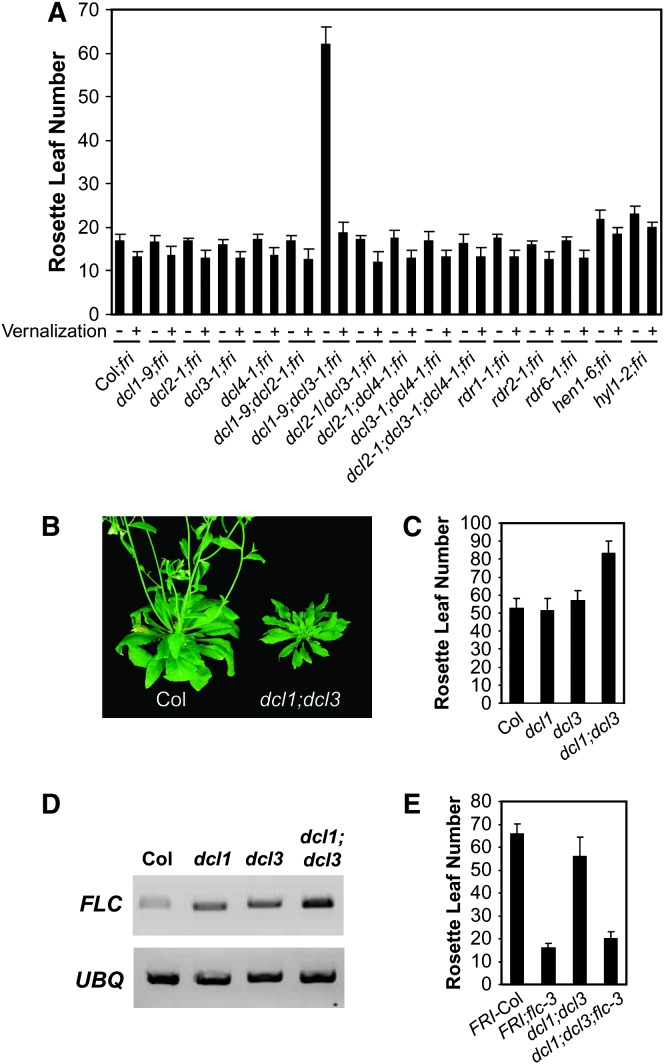
We explored the basis of the delayed-flowering phenotype of dcl1;dcl3 double mutants in Col. dcl1;dcl3 double mutants form many more rosette leaves than wild type from the primary shoot apical meristem in inductive photoperiods (Figure 1, A and B) as well as in noninductive photoperiods (Figure 1C). However, in a range of single mutants with lesions in genes required for small RNA production, there were no substantial effects on flowering behavior (Figure 1A). At a molecular level, dcl1;dcl3 double mutants contain increased levels of FLC mRNA (Figure 1D). Therefore, dcl1;dcl3 double mutants resemble mutants in the autonomous pathway. The delayed-flowering phenotype of autonomous-pathway mutants can be overcome by a vernalizing cold treatment or genetically by loss of FLC function (Michaels and Amasino 2001). To determine if the late-flowering phenotype of the dcl1;dcl3 double mutant is responsive to vernalization, seedlings were exposed to 40 days of cold (4°) and transferred to long days for assessment of flowering time. dcl1;dcl3 double mutants flowered rapidly after an extended exposure to cold (Figure 1A). In addition, the dcl1;dcl3 delayed-flowering phenotype was suppressed by the flc-3 mutation, as dcl1;dcl3;flc-3 triple mutants flowered with the same number of rosette leaves as flc-3 single mutants (Figure 1E). Therefore, DCL1 and DCL3 share a functionally redundant role in FLC repression and together could be considered a new component of the autonomous pathway.
Figure 1.—
dcl1;dcl3 double mutants result in phenotypes similar to autonomous-pathway mutants. (A) Mutations in genes encoding components required for small RNA biogenesis do not result in a strong effect on flowering time except for the dcl1;dcl3 double mutant. The dcl1;dcl3 double mutant has a late-flowering phenotype that responds to vernalization. (B) The dcl1;dcl3 double mutant phenotype includes late flowering, altered leaf shape, and a smaller rosette size. Data and images in A and B are from plants grown in inductive long-day photoperiods (16 hr light/8 hr dark). (C) The dcl1;dcl3 double mutant flowers later than Col wild type in noninductive photoperiods (8 hr light/16 hr dark) indicating that there is not a defect in detecting a noninductive photoperiod. (D) Steady-state levels of FLC mRNA are increased in dcl1;dcl3 double mutants. RNA was isolated from the shoot apices of plants after they had formed a total of six visible leaves. (E) The late-flowering phenotype of the dcl1;dcl3 double mutant is completely suppressed by the flc-3 mutation.
In the Landsberg accession, mutations in DCL1 alone result in a slight delay in flowering time (Ray et al. 1996; Liu et al. 2004). This is in contrast to Col, which displays no clear flowering delay caused by the dcl1 single mutant under our growth conditions (Figure 1, A and C). The flowering delay in dcl1 in Landsberg may be due to a failure to direct siRNA-mediated heterochromatin formation to a transposon located in the first intron of FLC (Liu et al. 2004); both the parental Landsberg accession and the derived erecta strain have a transposon insertion in FLC, but the Col allele of FLC does not have this transposon insertion (Michaels et al. 2003). Therefore, a measurable affect on flowering time of a dcl1 single mutant may be unique to accessions in which FLC expression is attenuated by a transposon insertion. Despite the lack of a flowering phenotype in the Col dcl1 single mutant, there appears to be a slight increase in FLC mRNA levels in dcl1 and dcl3 single mutants, although the increase is much less than that in the dcl1;dcl3 double mutant (Figure 1D). Swiezewski et al. (2007) also noted an increase in FLC mRNA levels in the dcl3 single mutant in Col, but did not report any change of flowering behavior in dcl3 vs. wild type.
dcl1;dcl3 double mutants exhibit altered developmental timing. For example, abaxial trichomes (often used as a marker of the juvenile to adult transition; Willmann and Poethig 2005) appear on the second to third true leaves of dcl1;dcl3 double mutants, whereas abaxial trichomes do not appear until the fifth and sixth leaves of wild-type Col. In addition, the dcl1;dcl3 double mutants develop at a slower rate than wild type (for example, when leaf 13 becomes visible in wild type, leaf 7 is emerging in the dcl1;dcl3 double mutant). These results are consistent with previous reports of the involvement of miRNAs in regulating phase change in Arabidopsis (Willmann and Poethig 2005).
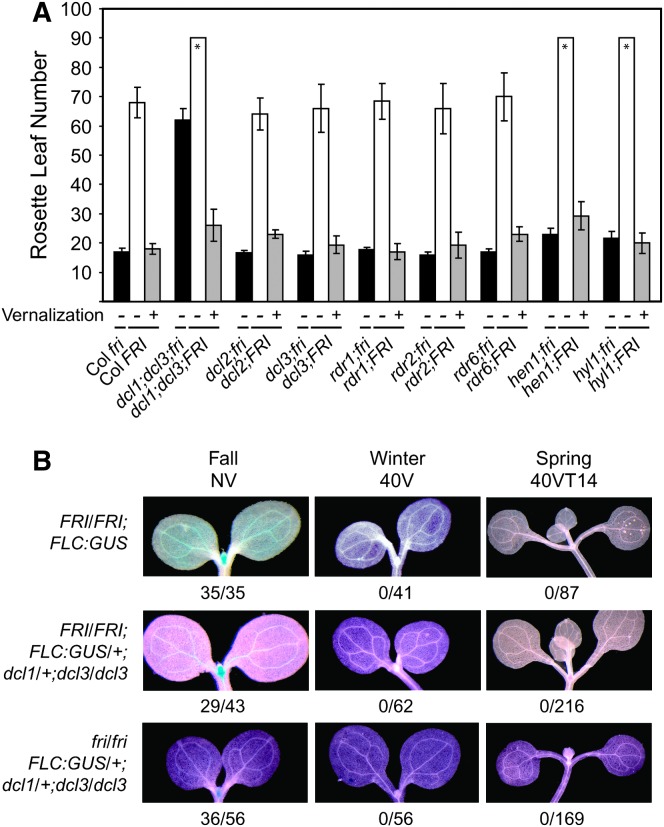
Given the role of small RNAs in repression of gene activity, we further explored the possibility of their involvement in epigenetic silencing of FLC by vernalization. We genetically introduced a range of mutants affected in either miRNA or siRNA biogenesis into the vernalization-requiring FRI–Col genetic background (Michaels and Amasino 1999) and assayed their vernalization response. All mutants tested, including the dcl1;dcl3 double mutant, responded like wild type to a vernalizing cold treatment (Figure 2A). In addition, we monitored FLC expression during a vernalization time course in dcl1;dcl3 using a reporter line. GUS expression was detected throughout the shoot and root apex as well as the vasculature prior to vernalization, but expression was repressed in seedlings immediately after 40 days of cold. This repression was maintained as plants grew in warm temperatures following cold exposure (Figure 2B). Thus, we found no evidence that known genes involved in small RNA metabolism (listed in Figure 2) play a role in initiation or maintenance of the vernalized state.
Figure 2.—
The dcl1;dcl3 double mutant does not block vernalization in a winter-annual genetic background. (A) Eight mutants with lesions in components required for small RNA biogenesis were crossed into the vernalization-requiring Col FRI genetic background (Michaels and Amasino 1999) and examined for their flowering phenotypes before and after vernalization. All mutants flowered much more rapidly after exposure to 40 days of cold. Data representing the flowering-time phenotypes in Col (which lacks FRI activity) are identical to the data presented in Figure 1A and are presented for comparison. The asterisk indicates that plants produced >90 rosette leaves. Accurate leaf counts for these genotypes were difficult to obtain due to developmental defects in combination with the late-flowering phenotype. Solid bars represent genotypes in Col without exposure to cold, open bars represent genotypes in Col FRI without vernalization, and shaded bars represent genotypes in Col FRI with vernalization. (B) An FLC:GUS reporter line was used to monitor the expression of FLC during a vernalization time course. The FLC:GUS construct consists of a 15-kb genomic region around FLC that rescues the flc-3 mutant. The GUS coding region was inserted into exon 4 of FLC within this construct (Michaels and Amasino 2000). GUS expression was examined in seedlings exposed to no cold (NV, Fall), 40 days of (4°) cold (40V, Winter), and 40 days of cold followed by 14 days of warm (40VT14, Spring). The parental genotypes of populations screened for GUS activity are listed on the left. The number of seedlings with GUS activity relative to the total examined are noted below each panel (seedlings were not selected for the GUS transgene).
Recently it was reported that DCL3, RDR2, and NRPD2 are involved in directing small RNA-mediated heterochromatin formation to a target site downstream of the region corresponding to the mature FLC transcript; mutations in these genes prevent production of a 24-nucleotide small RNA that is complementary to this 3′ site (Swiezewski et al. 2007). Additionally, a T-DNA insertion that disrupts this small RNA binding site and prevents production of this small RNA causes a slight delay in flowering (Swiezewski et al. 2007). Although dcl3, rdr2, and nrpd2 also prevent production of this small RNA, the flowering time of these mutants was not reported in Swiezewski et al. (2007). As noted above, we do not observe any change in the flowering behavior of the dcl3 or rdr2 mutant vs. wild type, although we do observe a slight increase in FLC mRNA in the dcl3 single mutant. It is possible that this increase in FLC mRNA may be below a threshold required to cause a change in flowering time or that this increase results from an expansion in the spatial expression of FLC to cells that do not contribute to the timing of flowering.
We assessed whether a range of genes required for small RNA production were required for the correct spatial expression of FLC. For these experiments we crossed mutants defective in either siRNA or miRNA biogenesis [dcl1, dcl2, dcl3, dcl1;dcl3, rdr1, rdr2, rdr6 (RNA DEPENDENT RNA POLYMERASE), hyl1 (HYPONASTIC LEAVES 1)] to a line containing an FLC:GUS reporter that includes the 3′ target site for the 24-nucleotide small RNA mentioned above. In segregating F2 populations, for the mutants listed above, no altered FLC:GUS expression patterns were detected (data shown only for the dcl1;dcl3 double mutant, Figure 2B). Considering the substantial increase in FLC mRNA observed in the dcl1;dcl3 double mutant, this would be the most likely genotype in which there might be such an expansion in spatial expression. In summary, we found no evidence for a role for these genes in controlling spatial expression of FLC using this reporter gene assay.
Except for the 24-nucleotide RNA reported in Swiezewski et al. (2007), small RNA species at the FLC locus have not yet been observed on RNA blots or identified in deep-sequencing projects (Gustafson et al. 2005; Lu et al. 2005, 2006). Nevertheless, DCL1 and DCL3 may be required to generate additional small RNAs that target FLC. Indeed, the lack of a strong flowering phenotype in lines that lack the 24-nucleotide RNA (Swiezewski et al. 2007) compared to the strong flowering phenotype of the dcl1;dcl3 double mutant indicates that, if a meaningful level of FLC repression is due to small RNAs targeted to FLC, this repression may be due to the collective effects of multiple small RNA species. Perhaps DCL1 and DCL3 are involved in the formation of different FLC-targeted small RNAs. Alternatively, DCL1 and DCL3 may be involved in down regulating an activator of FLC.
Regardless, the data presented here show that multiple DICERs are required for proper control of FLC mRNA levels. The specific role of DCL1 and DCL3 in flowering time control awaits the identification of the specific small RNA(s) that are altered in the dcl1;dcl3 double mutant.
Acknowledgments
We are grateful to Mark Doyle for his comments on this manuscript. We would also like to thank Hervé Vaucheret and James Carrington for providing dcl1;dcl3, dcl1;dcl2, dcl2;dcl3, and rdr1, rdr2, rdr6, dcl3, hyl1 seeds, respectively. Work in R.M.A's laboratory was supported by the College of Agricultural and Life Sciences and the Graduate School of the University of Wisconsin, National Institute of Health grant 1R01GM079525, and by National Science Foundation grants 0133663 and 0209786.
References
- Bastow, R., J. S. Mylne, C. Lister, Z. Lippman, R. A. Martienssen et al., 2004. Vernalization requires epigenetic silencing of FLC by histone methylation. Nature 427 164–167. [DOI] [PubMed] [Google Scholar]
- Bernstein, E., A. A. Caudy, S. M. Hammond and G. J. Hannon, 2001. Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature 409 363–366. [DOI] [PubMed] [Google Scholar]
- Blevins, T., R. Rajeswaran, P. V. Shivaprasad, D. Beknazariants, A. Si-Ammour et al., 2006. Four plant Dicers mediate viral small RNA biogenesis and DNA virus induced silencing. Nucleic Acids Res. 34 6233–6246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deleris, A., J. Gallego-Bartolome, J. Bao, K. D. Kasschau, J. C. Carrington et al., 2006. Hierarchical action and inhibition of plant Dicer-like proteins in antiviral defense. Science 313 68–71. [DOI] [PubMed] [Google Scholar]
- Dunoyer, P., C. Himber and O. Voinnet, 2005. DICER-LIKE 4 is required for RNA interference and produces the 21-nucleotide small interfering RNA component of the plant cell-to-cell silencing signal. Nat. Genet. 37 1356–1360. [DOI] [PubMed] [Google Scholar]
- Gasciolli, V., A. C. Mallory, D. P. Bartel and H. Vaucheret, 2005. Partially redundant functions of Arabidopsis DICER-like enzymes and a role for DCL4 in producing trans-acting siRNAs. Curr. Biol. 15 1494–1500. [DOI] [PubMed] [Google Scholar]
- Gustafson, A. M., E. Allen, S. Givan, D. Smith, J. C. Carrington et al., 2005. ASRP: the Arabidopsis Small RNA Project Database. Nucleic Acids Res. 33 D637–D640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutvagner, G., J. McLachlan, A. E. Pasquinelli, E. Balint, T. Tuschl et al., 2001. A cellular function for the RNA-interference enzyme Dicer in the maturation of the let-7 small temporal RNA. Science 293 834–838. [DOI] [PubMed] [Google Scholar]
- Kurihara, Y., and Y. Watanabe, 2004. Arabidopsis micro-RNA biogenesis through Dicer-like 1 protein functions. Proc. Natl. Acad. Sci. USA 101 12753–12758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, J., Y. He, R. Amasino and X. Chen, 2004. siRNAs targeting an intronic transposon in the regulation of natural flowering behavior in Arabidopsis. Genes Dev. 18 2873–2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, C., S. S. Tej, S. Luo, C. D. Haudenschild, B. C. Meyers et al., 2005. Elucidation of the small RNA component of the transcriptome. Science 309 1567–1569. [DOI] [PubMed] [Google Scholar]
- Lu, C., K. Kulkarni, F. F. Souret, R. MuthuValliappan, S. S. Tej et al., 2006. MicroRNAs and other small RNAs enriched in the Arabidopsis RNA-dependent RNA polymerase-2 mutant. Genome Res. 16 1276–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaels, S., and R. Amasino, 1999. FLOWERING LOCUS C encodes a novel MADS domain protein that acts as a repressor of flowering. Plant Cell 11 949–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaels, S., and R. Amasino, 2000. Memories of winter: vernalization and the competence to flower Plant. Cell Environ. 23 1145–1153. [Google Scholar]
- Michaels, S. D., and R. M. Amasino, 2001. Loss of FLOWERING LOCUS C activity eliminates the late-flowering phenotype of FRIGIDA and autonomous pathway mutations but not responsiveness to vernalization. Plant Cell 13 935–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaels, S. D., Y. He, K. C. Scortecci and R. M. Amasino, 2003. Attenuation of FLOWERING LOCUS C activity as a mechanism for the evolution of summer-annual flowering behavior in Arabidopsis. Proc. Natl. Acad. Sci. USA 100 10102–10107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, W., J. Li, R. Song, J. Messing and X. Chen, 2002. CARPEL FACTORY, a Dicer homolog, and HEN1, a novel protein, act in microRNA metabolism in Arabidopsis thaliana. Curr. Biol. 12 1484–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray, A., J. D. Lang, T. Golden and S. Ray, 1996. SHORT INTEGUMENT (SIN1), a gene required for ovule development in Arabidopsis, also controls flowering time. Development 122 2631–2638. [DOI] [PubMed] [Google Scholar]
- Reinhart, B. J., E. G. Weinstein, M. W. Rhoades, B. Bartel and D. P. Bartel, 2002. MicroRNAs in plants. Genes Dev. 16 1616–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauer, S. E., S. E. Jacobsen, D. W. Meinke and A. Ray, 2002. DICER-LIKE1: blind men and elephants in Arabidopsis development. Trends Plant Sci. 7 487–491. [DOI] [PubMed] [Google Scholar]
- Sheldon, C. C., J. E. Burn, P. P. Perez, J. Metzger, J. A. Edwards et al., 1999. The FLF MADS box gene: a repressor of flowering in Arabidopsis regulated by vernalization and methylation. Plant Cell 11 445–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson, G. G., 2004. The autonomous pathway: epigenetic and post-transcriptional gene regulation in the control of Arabidopsis flowering time. Curr. Opin. Plant Biol. 7 570–574. [DOI] [PubMed] [Google Scholar]
- Sung, S., and R. M. Amasino, 2004. Vernalization in Arabidopsis thaliana is mediated by the PHD finger protein VIN3. Nature 427 159–164. [DOI] [PubMed] [Google Scholar]
- Sung, S., and R. M. Amasino, 2005. REMEMBERING WINTER: toward a molecular understanding of vernalization. Annu. Rev. Plant Biol. 56 491–508. [DOI] [PubMed] [Google Scholar]
- Swiezewski, S., P. Crevillen, F. Liu, J. R. Ecker, A. Jerzmanowski et al., 2007. Small RNA-mediated chromatin silencing directed to the 3′ region of the Arabidopsis gene encoding the developmental regulator, FLC. Proc. Natl. Acad. Sci. USA 104 3633–3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willmann, M. R., and R. S. Poethig, 2005. Time to grow up: the temporal role of smallRNAs in plants. Curr. Opin. Plant Biol. 8 548–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie, Z., E. Allen, A. Wilken and J. C. Carrington, 2005. DICER-LIKE 4 functions in trans-acting small interfering RNA biogenesis and vegetative phase change in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 102 12984–12989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie, Z., L. K. Johansen, A. M. Gustafson, K. D. Kasschau, A. D. Lellis et al., 2004. Genetic and functional diversification of small RNA pathways in plants. PLoS Biol. 2 E104. [DOI] [PMC free article] [PubMed]
- Yoshikawa, M., A. Peragine, M. Y. Park and R. S. Poethig, 2005. A pathway for the biogenesis of trans-acting siRNAs in Arabidopsis. Genes Dev. 19 2164–2175. [DOI] [PMC free article] [PubMed] [Google Scholar]