Abstract
Nuclear export of tRNA is an essential eukaryotic function, yet the one known yeast tRNA nuclear exporter, Los1, is nonessential. Moreover recent studies have shown that tRNAs can move retrograde from the cytosol to the nucleus by an undefined process. Therefore, additional gene products involved in tRNA nucleus–cytosol dynamics have yet to be identified. Synthetic genetic array (SGA) analysis was employed to identify proteins involved in Los1-independent tRNA transport and in regulating tRNA nucleus–cytosol distribution. These studies uncovered synthetic interactions between los1Δ and pho88Δ involved in inorganic phopshate uptake. Further analysis revealed that inorganic phosphate deprivation causes transient, temperature-dependent nuclear accumulation of mature cytoplasmic tRNA within nuclei via a Mtr10- and retrograde-dependent pathway, providing a novel connection between tRNA subcellular dynamics and phosphate availability.
TRANSPORT of tRNA from its nuclear site of biogenesis to the cytosol is an essential eukaryotic process as tRNA must be available for translation, which occurs in the cytosol. Intracellular tRNA movement in the budding yeast Saccharomyces cerevisiae involves several steps. First, end-matured pre-tRNAs are transported from nucleus to cytosol (“primary tRNA nuclear export”). Once in the cytosol, pre-tRNAs containing introns are spliced by the splicing endonuclease complex located on the cytosolic surface of the mitochondrial outer membrane (Yoshihisa et al. 2003). Numerous nucleoside modification steps also occur in the nucleus and cytosol (Hopper and Phizicky 2003). Mature cytosolic tRNA are then available for translation. Recent work demonstrated that mature tRNA can move from cytosol to nucleus (Shaheen and Hopper 2005; Takano et al. 2005) and vice versa (“re-export”; Whitney et al. 2007). However, many of the gene products involved in the tRNA nucleus-to-cytosol and cytosol-to-nucleus transport pathways have yet to be defined.
Transport of many macromolecules between the nucleus and the cytosol requires the small GTPase, Ran, and its protein-binding partners, the β-importins. Ran is maintained in the GTP-bound state in nuclei by Ran guanosine exchange factor (RanGEF), which is encoded in yeast by PRP20 and resides in the nucleus. β-Importin family members that export cargo from the nucleus to the cytosol bind their cargo only in the presence of RanGTP and then mediate interactions with the nuclear pore complexes to allow the entire complex to move to the cytosol. Once in the cytosol, RanGTP interacts with the Ran GTPase activating protein (RanGAP), encoded by RNA1, which activates GTP hydrolysis, leading to conformational changes that cause the heterotrimeric complexes to dissociate, releasing the cargo in the cytosol (Corbett et al. 1995; Gorlich and Kutay 1999; Weis 2003). For tRNA nuclear export, Los1, a member of the β-importin family, binds tRNA in a RanGTP-dependent manner (Hellmuth et al. 1998). The mammalian homolog of Los1, Exportin-t (Xpo-t), has also been shown to bind end-processed tRNA, with or without introns, as part of a heterotrimeric complex with RanGTP (Arts et al. 1998a,b; Kutay et al. 1998).
Inhibiting the Los1-dependent tRNA transport pathway through a temperature-sensitive mutation of RNA1 (rna1-1) or mutation of LOS1 caused nuclear accumulation of tRNA (Sarkar and Hopper 1998). Similarly, blocking Xpo-t-mediated tRNA transport through microinjection of Xpo-t antibodies in Xenopus oocytes reduced nuclear export of tRNAPhe by 99% (Arts et al. 1998b). Blocking transport through mutation of RNA1, PRP20, LOS1, or some nucleopore genes also caused accumulation of intron-containing pre-tRNA (Hopper et al. 1978, 1980; Kadowaki et al. 1993; Sharma et al. 1996), which indicated that these proteins were involved in the transport of end-matured pre-tRNA to the cytosol.
Numerous mutations have been identified that affect tRNA nucleus–cytosol distribution without causing accumulation of intron-containing pre-tRNA. These include temperature-sensitive mutations of methionyl- (mes1-1), isoleucyl- (ils1-1), and tyrosyl- (tys1-1) aminoacyl-tRNA synthetases (Sarkar et al. 1999), 3′ tRNA CCA-nucleotidyl transferase (cca1-1; Grosshans et al. 2000; Feng and Hopper 2002), and the tef1Δ tef2-1 double mutation of the eukaryotic elongation factor 1A (Grosshans et al. 2000). Lack of intron-containing pre-tRNA accumulation indicates that tRNA nuclear accumulation observed in these mutants occurs via accrual of fully processed, mature tRNA from the cytosol, and not by inhibition of the primary tRNA nuclear export pathway that exports pre-tRNA to the cytosol.
One genetic approach for defining parallel pathways that accomplish essential cellular functions is the identification of new mutations that have synthetic interactions with known mutations that impair or block one of the pathways. As tRNA nuclear export is an essential process and LOS1 is a known nonessential nuclear exporter of tRNA (Hurt et al. 1987), this approach should be useful for the identification of genes that encode proteins participating in Los1-independent transport of end-matured pre-tRNA and mature tRNA and, possibly, negative regulators of tRNA retrograde transport.
Previous work employing standard synthetic lethal methodology identified several synthetic interactions with los1Δ. The genes identified affected a variety of cellular processes, including tRNA aminoacylation (Simos et al. 1996a), tRNA modification (Simos et al. 1996a), Pol III transcription (Simos et al. 1996b), and translation (Hellmuth et al. 1998; Grosshans et al. 2000). Synthetic genetic array (SGA) analysis (Tong et al. 2001) allows extensive, large-scale, systematic searches for genetic interactions. This approach was utilized with a collection of promoter replacement alleles of essential genes and los1Δ (Davierwala et al. 2005). Depletion of Taf3, a subunit of the Pol II transcription initiation complex, and depletion of Pop5, a subunit of both RNase MRP (pre-rRNA cleavage) and RNase P (pre-tRNA 5′-end processing), were found to have synthetic interactions with los1Δ (Davierwala et al. 2005). None of the previously reported studies have identified tRNA nuclear exporters that function in parallel to Los1 or regulators of tRNA nucleus–cytosol distribution.
Here, we describe the results of SGA screens of los1Δ using the yeast MATa deletion collection. Novel synthetic interactions were observed between los1Δ and snt309Δ, aro7Δ, rpl12aΔ, rpl13bΔ, lrp1Δ, gtr1Δ, gtr2Δ, or pho88Δ. Of these verified candidates, gtr1Δ, gtr2Δ, and pho88Δ caused tRNA nuclear accumulation. As gtr1Δ, gtr2Δ, and pho88Δ have been previously shown to influence inorganic phosphate (Pi) uptake (Bun-Ya et al. 1992; Yompakdee et al. 1996; Lagerstedt et al. 2005), we examined the affect of Pi starvation upon tRNA nucleus–cytosol distribution. Transient, temperature-dependent, tRNA nuclear accumulation was observed in wild-type cells grown on synthetic media lacking Pi for 1–2 hr at 23° or 30°. Northern and heterokaryon analyses of Pi-deprived cells and of pho88Δ cells indicated that the observed nuclear accumulation was due to accrual of mature tRNA, which entered the nucleus via retrograde transport. This work provides a novel connection between Pi availability and tRNA nucleus–cytosol distribution.
MATERIALS AND METHODS
Strains and media:
Strains:
Yeast strains used for these studies are listed in Table 1. All plasmid transformations were made by lithium acetate (LiOAc) transformation (Schiestl and Gietz 1989). Deletions were accomplished by LiOAc transformation of the parent yeast strain with a PCR product cassette that replaced the endogenous gene. Sequences for the oligonucleotides used are listed in supplemental Table S1 at http://www.genetics.org/supplemental/. The los1∷natMX4 cassette was generated by PCR amplification using oligonucleotide primers RLH007A and RLH007B and a natR-MX4 template (p4339; Tong et al. 2001). LOS1KO1B was derived from the transformation of Y3656 with the los1∷natMX4 cassette. The deletion of LOS1 was verified using the primers RLH010A and RLH010B. The pho88∷natMX4 cassette was generated by PCR amplification using oligonucleotide primers RLH017A and RLH017B and a natR-MX4 template (Goldstein and McCusker 1999). 8MS88ΔN2C was derived from the transformation of MS739 with the pho88∷natMX4 cassette. BY88ΔF1 was derived from the transformation of BY4741 with the pho88∷natMX4 cassette. The pho88∷hphMX4 cassette was generated by PCR amplification using the oligonucleotide primers RLH017A and RLH017B and the hphR-MX4 template (Goldstein and McCusker 1999). BY88ΔD1 and BY88ΔG4 were derived from independent transformations of BY4741 with the pho88∷hphMX4 cassette. 8MS88ΔH2D was derived from the transformation of MS739 with the pho88∷hphMX4 cassette. YD05055 (los1∷kanMX) was transformed with YCpLOS1 (Hurt et al. 1987) and then with the pho88∷hphMX4 cassette to form strain los1Δ pho88Δ + YCpLOS1. The deletion of PHO88 was verified using primers RLH017C, RLH017D, and RLH017E. The strain los1Δ pho88Δ + YCpLOS1 was grown on nonselective media to allow plasmid loss from which los1Δ pho88Δ was derived.
TABLE 1.
Yeast strains employed
| Strain(s) | Genotype | Source |
|---|---|---|
| Y3656 | MATα can1Δ∷MFA1pr-HIS3-MFα1pr-LEU2 his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 | Tonget al. (1999) |
| LOS1KO1B | MATα can1Δ:MFA1pr-HIS3-MFα1pr-LEU2 his3Δ1 leu2Δ0 los1∷natMX4 lys2Δ0 ura3Δ0 | This study |
| BY4741 | MATahis3Δ1 leu2Δ0 met15Δ0 ura3Δ0 | Open Biosystems (Huntsville, AL) |
| MATa deletion collection | MATaorf∷kanMX his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 TRP1 LYS2 | Winzeler et al. (1999); Open Biosystems |
| YD05055 | MATalos1∷kanMX his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 TRP1 LYS2 | MATa deletion collection |
| los1Δpho88Δ | MATalos1∷kanMX pho88∷hphMX his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 TRP1 LYS2 | This study |
| arc1ΔMATa | MATaarc1∷kanMX his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 TRP1 LYS2 | MATa deletion collection |
| pho88ΔMATa | MATapho88∷kanMX his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 TRP1 LYS2 | MATa deletion collection |
| rpl13bΔMATa | MATarpl13b∷kanMX his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 TRP1 LYS2 | MATa deletion collection |
| npr1ΔMATa | MATanpr1∷kanMX his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 TRP1 LYS2 | MATa deletion collection |
| BY88ΔD1 | MATapho88∷hphMX his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 TRP1 LYS2 | This study |
| BY88ΔF1 | MATapho88∷natMX his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 TRP1 LYS2 | This study |
| BY88ΔG4 | MATapho88∷hphMX his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 TRP1 LYS2 | This study |
| BY4742 | MATα his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 | Open Biosystems |
| MATα deletion collection | MATα orf∷kanMX his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 | Winzeler et al. (1999); Open Biosystems |
| MS739 | MATα ade2-101 kar1-1 leu2-3,112 ura3-52 | M. Rose, Princeton University |
| MS739 + tRNAGluD | MATα ade2-101 kar1-1 leu2-3,112 ura3-52 pRS416tRNAGlu-D | Shaheen and Hopper (2005) |
| 8MS88ΔN2C | MATα ade2-101 kar1-1 leu2-3,112 pho88∷natMX4 ura3-52 | This study |
| 8MS88ΔH2D | MATα ade2-101 kar1-1 leu2-3,112 pho88∷hphMX4 ura3-52 | This study |
| 8MS88ΔH2D + tRNAGluD | MATα ade2-101 kar1-1 leu2-3,112 pho88∷hphMX4 ura3-52 pRS416tRNAGlu-D | This study |
Media:
Yeast strains were maintained on YEPD [yeast extract, trypticase peptone, dextrose, supplemented with adenine (0.04 g/liter), uracil (0.04 g/liter)] medium; SC (synthetic complete) defined medium; Difco yeast nitrogen base without amino acids (6.7 g/liter; Becton Dickinson, Sparks, MD) supplemented with amino acids, dextrose (2%), adenine (0.04 g/liter), and uracil (0.04 g/liter). SC–Pi liquid medium is synthetic defined medium lacking K2PO4 [the source of Pi in SC; yeast nitrogen base lacking amino acids and K2PO4 (5.7 g/liter; ForMedium, Norwich, UK) supplemented with amino acids, uracil, and 1.0 g/liter KCl]. Solid YEPD medium supplemented with 2 g/liter KH2PO4 (Fisher) was utilized for the “wild-type” heterokaryon fluorescence in situ hybridization (FISH) analysis. To select for natMX cassette integration, the strains were grown on YEPD + clonNAT (100 mg/liter; Werner BioAgents, Jena, Germany) solid medium. To select for hphMX cassette integration, the strains were grown on YEPD + hygromycin B (300 mg/liter; Calbiochem, La Jolla, CA) solid medium.
Genetic analysis:
SGA was performed and scored as previously described (Tong et al. 2001) using LOS1KO1B as the bait strain. The final replica plates were grown at 22° or 37°. Tetrad dissection was done by standard procedures.
Growth assay:
Serial dilutions were made of the indicated yeast cultures. Aliquots (5 μl) of each dilution were spotted onto each plate of the indicated solid medium. The plates were incubated for 2–3 days at the indicated temperature. Images of the plates were captured using Eagle Eye II (Stratagene, La Jolla, CA), digital camera, or scanner (ScanMaker 8700 by Microtek, Carson, CA). Adobe Photoshop 5.0 was used for image assembly.
Fluorescence in situ hybridization:
FISH was performed as previously described (Sarkar and Hopper 1998) with the modifications detailed in Stanford et al. (2004). Heterokaryons for FISH were mated and grown as described in Shaheen and Hopper (2005) except the solid YEPD medium used for the “wild-type” mating was medium supplemented with 2 g/liter KH2PO4 (Fisher) to prevent premature induction of the PHO pathway, as low levels of PHO5 expression have been observed when cells are grown in YEPD medium (Yoshida et al. 1989a,b). Oligonucleotides used as probes are listed in supplemental Table S1 at http://www.genetics.org/supplemental/. Each slide contained positive and negative controls for tRNA nuclear accumulation. When adjectives describing the relative amount of tRNA nuclear accumulation are used, the probes were hybridized under the same conditions. All critical experiments were independently viewed and scored by at least two people, one of whom was unaware of the experimental details. A Nikon Microphot-FX microscope was used to observe fluorescence and a Sensys charge-coupled device camera (Photometrics, Tucson AZ) using QED software (QED Imaging, Pittsburgh) was used to capture the images. Adobe Photoshop 5.0 was used for image assembly.
Northern analysis:
Small RNAs were extracted from yeast cultures grown to densities similar to those used for FISH as previously described (Hopper et al. 1980). Samples (10 μg of RNA) were electrophoretically separated at 4° in 10% polyacrylamide gel containing 8 m urea and 1× TBE (0.09 m Tris, 0.09 m borate, 0.001 m ethylenediaminetetraacetic acid). RNAs were electrophoretically transferred onto Hybond N+ membranes (Amersham Pharmacia) using a Hoefer TE42 Transphor apparatus (Hoefer Scientific) filled with 1× TAE buffer (40 mm Tris, 20 mm acetate, 1 mm EDTA). Membranes were prehybridized at 37° in 4× SSC (1× is 0.15 m NaCl, 0.015 m sodium citrate), 2.5× Denhart's solution [1× is 0.02% Ficoll (type 400), 0.02% polyvinylpyrrolidone, 0.02% bovine serum albumin], 50 μg/ml single-stranded salmon sperm DNA, and 0.05% sodium dodecyl sulfate. Probe hybridization was at 37° in the same buffer with the addition of oligonucleotide probes (supplemental Table S1 at http://www.genetics.org/supplemental/) that were 5′ end labeled with [32P]ATP using T4 polynucleotide kinases (Promega, Madison, WI). After hybridization, membranes were UV crosslinked, washed in 2× SSC, and used to expose Kodak BioMax MS film (Eastman Kodak, Rochester, NY).
Acid phosphatase assay:
Acid phoshatase activity was determined as previously described (Byrne et al. 2004) except that yeast cultures were grown to early log phase and the volume of cells was increased to compensate for the reduced number of cells.
RESULTS
SGA analysis of los1Δ uncovers eight novel interactions:
SGA was performed twice using LOS1KO1B (los1∷natR) as bait. All of the incubation steps for the first screen were conducted at 22°, the standard temperature for SGA. The final replica plates of the second screen were incubated at 37°, a temperature that exaggerates the los1Δ phenotype in some strains (Hopper et al. 1980). The first screen provided 18 novel candidate interactions, whereas the second screen uncovered 118 candidates. As only six candidates appeared in both screens—deletions of ARC1 (YGL105W), LDB16 (YCL005W), LRP1 (YHR081W), RCY1 (YJL204C), WHI3 (YNL197C), and RPL13B (YMR142C)—the total number of candidates was 131 (supplemental Table S2 at http://www.genetics.org/supplemental/). Candidates of the first screen did not necessarily overlap with the candidates found in the second screen, possibly due to changes in the phenotype of individual deletion strains at different temperatures, manipulation errors, false positives, or false negatives. ARC1, a known synthetic lethal interaction (Simos et al. 1996b), was found in both screens, indicating that the assay was functioning as predicted.
Tetrad analysis and growth assays of selected haploid progeny were utilized to detect and verify synthetic lethal, slow, or enhanced growth interactions. Tetrad analysis of 97 candidate interactions revealed eight bona fide los1Δ interactions (Table 2); only three of these appeared as candidates in both searches. Synthetic enhanced growth interaction was observed between los1Δ and snt309Δ (YPR101W). Synthetic slow-growth interactions were observed between los1Δ and aro7Δ (YPR060C), rpl12a (YEL054C), rpl13bΔ (YMR142C), lrp1Δ (YHR081W), gtr1Δ (YML121W), or gtr2Δ (YGR163W).
TABLE 2.
Bona fide los1Δ interactions
| ORF | Gene | Interaction | tRNA distribution |
|---|---|---|---|
| YGL105W | ARC1 | Lethal | Nuclear accumulation/even distributiona |
| YPR101W | SNT309 | Enhanced growth | Not determined |
| YPR060C | ARO7 | Slow growth | Not determined |
| YEL054C | RPL12A | Slow growth | Even distribution |
| YMR142C | RPL13B | Slow growth | Even distribution |
| YHR081W | LRP1 | Slow growth | Not determined |
| YML121W | GTR1 | Slow growth | Nuclear accumulation |
| YGR163W | GTR2 | Slow growth | Nuclear accumulation |
| YBR106W | PHO88 | Lethal/slow growth | Nuclear accumulation |
Nuclear accumulation of tRNAMet, even distribution of tRNATyr and tRNAIle.
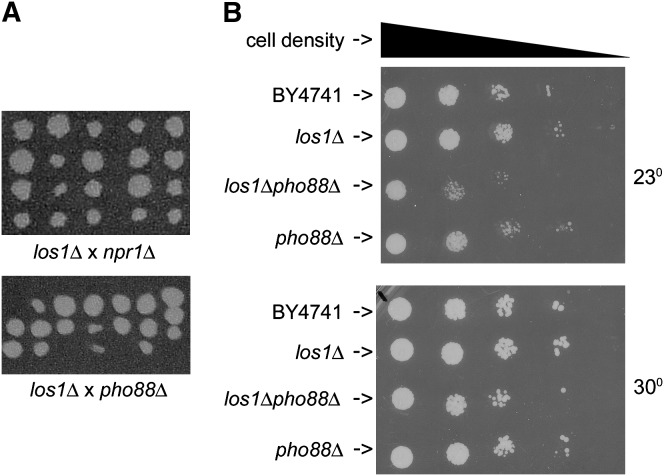
A synthetic lethal interaction between los1Δ and pho88Δ (YBR106W) was documented upon tetrad dissection at 23° by the absence of nonparental ditypes and the absence of los1Δ pho88Δ containing progeny in tetratype asci as predicted by two-gene synthetic lethality (Figure 1A). However, we were able construct a viable los1Δ pho88Δ strain via direct mutagenesis (see materials and methods). The los1Δ pho88Δ strain exhibited slower growth at 23° than strains containing either individual mutation (Figure 1B). The slow growth vs. synthetic lethal interactions observed between los1Δ and pho88Δ are addressed in the discussion.
Figure 1.—
Analysis of spore viability and growth of cells with PHO88 and/or LOS1 deletions. (A) Tetrad dissection of los1Δ (LOS1KO1B) × pho88Δ (pho88ΔMATa) and of los1Δ (LOS1KO1B) × npr1Δ (npr1ΔMATa) on YEPD. (B) BY4741, YD05055 (los1Δ MATa), los1Δpho88Δ, and pho88Δ MATa were grown to saturation, and then equal amounts of cells from each strain were used to make two series of dilutions. Aliquots (5 μl) of each dilution were placed on solid synthetic complete medium. Plates were incubated for 2 days at the indicated temperatures.
SGA analysis uncovered genes influencing tRNA nucleus–cytosol distribution:
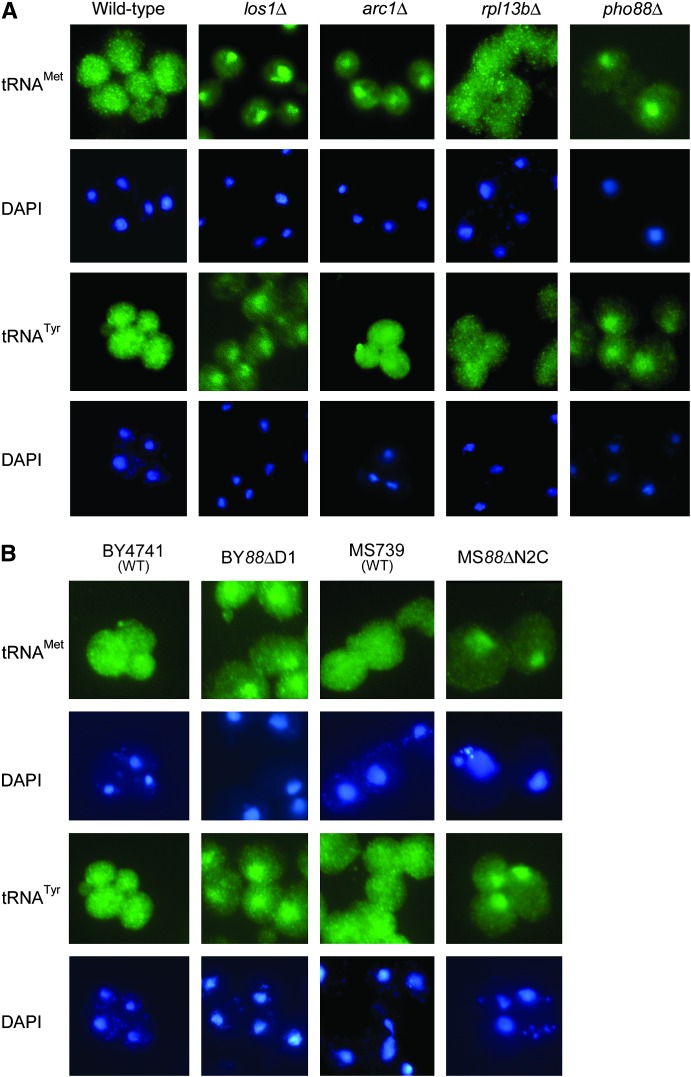
The subcellular distributions of tRNA between the nucleus and the cytosol of selected synthetic interactors were evaluated using fluorescence in situ hybridization (Sarkar and Hopper 1998). Gene deletions that had synthetic slow-growth phenotypes were expected to cause tRNA nuclear accumulation if the growth defect was due to impairment of tRNA export. Nuclear accumulation of both tRNAMet and tRNATyr, as indicated by the increased FITC signal that colocalizes with the DAPI-stained DNA, was observed in los1Δ (Figure 2A, column 2), pho88Δ (Figure 2A, column 5), gtr1Δ (supplemental Figure S1 at http://www.genetics.org/supplemental/ and data not shown), and gtr2Δ (supplemental Figure S1 at http://www.genetics.org/supplemental/ and data not shown) strains (Table 2). Several independent pho88Δ strains were generated, and all exhibited tRNA nuclear accumulation (Figure 2B, columns 2 and 4; also see supplemental Figure S1 at http://www.genetics.org/supplemental/). In contrast, arc1Δ cells exhibited nuclear accumulation of tRNAMet while the cellular distribution of tRNATyr was similar to wild-type cells (Figure 2A, column 3). Deletion of arc1Δ influencing the nucleus–cytosol distribution of tRNAMet, but not tRNATyr, is consistent with the function of Arc1 as a cofactor of the methionyl- and glutamyl-tRNA synthetases (Simos et al. 1996a). However, not all deletions that had synthetic slow-growth interactions with los1Δ caused tRNA nuclear accumulation. For example, wild-type, rpl13bΔ, and rpl12aΔ cells had a similar cellular distribution of tRNAMet and tRNATyr (Figure 2A, columns 1 and 4, and supplemental Figure S1 at http://www.genetics.org/supplemental/, respectively; Table 2).
Figure 2.—
Subcellular distribution of tRNA in selected SGA candidate deletions. (A) Location of tRNA within candidate deletion strains identified by SGA was determined by FISH analysis of BY4741 (wild type), YD05055 (los1Δ), arc1ΔMATa, rpl13bΔMATa, and pho88ΔMATa cells. (Row 1) tRNAMet; (row 3) tRNATyr; (rows 2 and 4) DAPI stain of row 1 and 3 cells, respectively. (B) FISH analysis of BY4741 (wild type), BY88ΔD1 (pho88∷hphR in BY4741), MS739 (kar1-1), and 8MS88ΔN2C (pho88∷natR in MS739) cells. (Row 1) tRNAMet. (Row 3) tRNATyr. (Rows 2 and 4) DAPI stain of row 1 and row 3 cells, respectively.
Candidate deletions that caused tRNA nuclear accumulation had been implicated in uptake of Pi. Gtr1 and Gtr2 formed a small GTPase complex (Nakashima et al. 1999) that negatively regulated the Ran cycle (Nakashima et al. 1996) and was required for efficient Pi uptake in response to Pi starvation (Bun-Ya et al. 1992; Lagerstedt et al. 2005). Deletion of PHO88 caused impaired Pi uptake and slowed growth (Yompakdee et al. 1996). PHO88 was selected for further study because it had strong synthetic interactions with los1Δ, its deletion caused tRNA nuclear accumulation, and it has been shown to impact the regulation of the PHO pathway (Yompakdee et al. 1996).
Pi deprivation of wild-type cells caused tRNA nuclear accumulation:
We obtained a plasmid encoding an N-terminal GST-tagged Pho88 (Martzen et al. 1999) and found that GST-Pho88 was located in the endoplasmic reticulum (ER; data not shown). This location is consistent with the results of the genomewide protein localization study that employed C-terminal GFP tags (Huh et al. 2003) and the observed membrane association of Pho88 (Yompakdee et al. 1996). As Pho88 was located in the ER, it was unlikely to directly bind and transport tRNA from the nucleus to the cytosol. Given that deletion of PHO88 caused a defect in Pi uptake from media (Yompakdee et al. 1996), it was possible that the low intracellular Pi levels caused the tRNA nuclear accumulation. This possibility had precedent as amino acid starvation has been shown to cause tRNA nuclear accumulation (Shaheen and Hopper 2005). If pho88Δ cells redistributed tRNA in response to low intracellular Pi levels, then wild-type cells that cannot import Pi due to lack of substrate should also have tRNA nuclear accumulation.
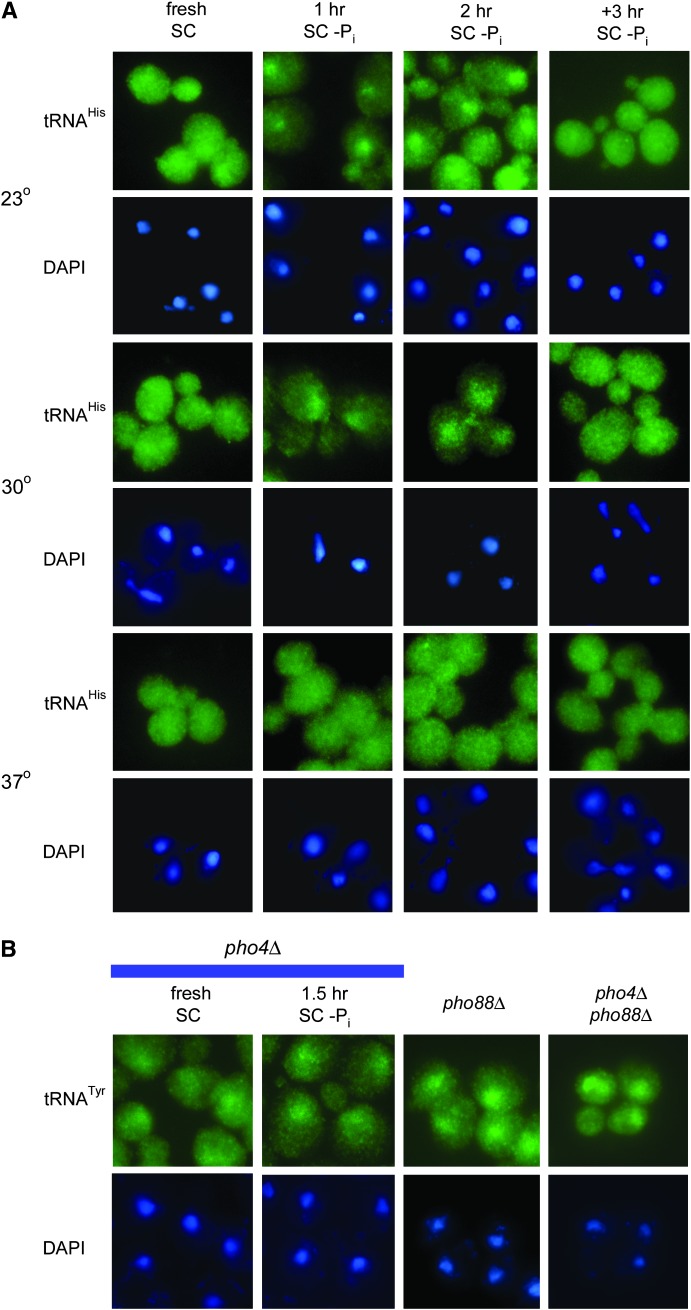
To address this prediction, wild-type cells (strain BY4742) were grown on synthetic complete media lacking KH2PO4 (SC–Pi) for various lengths of time. This approach allowed a controlled starvation period and minimized the risk of suppressor appearance. The cellular distributions of tRNA were then determined by FISH. As expected, tRNAHis was evenly distributed in wild-type cells grown in fresh SC media for 2 hr (Figure 3A, column 1). An even distribution of tRNAHis was also visible in cells grown for 30 min in SC–Pi (data not shown). Cells grown at 23° in SC–Pi for 1 hr displayed nuclear accumulation of tRNAHis (Figure 3A, column 2, row 1) as did cells grown at 30° in SC–Pi for 1 hr (Figure 3A, column 2, row 3). Thus, cells redistributed tRNA in response to Pi removal.
Figure 3.—
tRNA location within Pi-deprived cells. (A) Pi starvation time course of wild-type cells grown at 23°, 30°, and 37°. BY4742 cells grown in SC (column 1) or SC–Pi (columns 2–4) for the indicated amount of time at 23° (rows 1 and 2), 30° (rows 3 and 4), or 37° (rows 5 and 6) were analyzed by FISH. The cellular distribution of tRNAHis is shown (rows 1, 3, and 5). The same cells were stained with DAPI (rows 2, 4, and 6). (B) Pi-starved pho4Δ cells and YEPD-grown pho88Δ pho4Δ and pho88Δ cells. Cellular distributions of tRNATyr (row 1) determined by FISH, of pho4Δ MATa cells grown in fresh SC or SC–Pi for 1.5 hr, and of pho88Δ MATa and pho4Δ pho88Δ. Cells were stained with DAPI to reveal the location of DNA (row 2).
Unexpectedly, tRNA nuclear accumulation in response to Pi deprivation demonstrated temperature- and time-dependent components. While FISH is primarily a qualitative technique, the tRNAHis nuclear accumulation in cells grown at 23° reproducibly appeared to be more intense than in cells grown at 30° (Figure 3A, columns 2 and 3, rows 1 and 3). In contrast, cells grown at 37° in SC–Pi did not exhibit nuclear accumulation of tRNAHis (Figure 3A, columns 1–4, row 5). When cells were grown at 23° or 30° for 2 hr in SC–Pi, tRNA nuclear accumulation appeared diminished in comparison to the 1-hr time point. Moreover, cells grown for 3.5 or 12 hr in SC–Pi had tRNAHis nucleus–cytosol distributions similar to cells grown in fresh SC. The redistribution of tRNATyr and tRNAMet in response to growth in SC–Pi mirrored the demonstrated redistribution of tRNAHis (data not shown). The data indicated that denying wild-type cells readily available sources of Pi caused temperature-dependent, transient tRNA nuclear accumulation, in contrast to pho88Δ cells, which constitutively accumulated tRNA in the nucleus.
Activation of the PHO pathway is a well-studied response to Pi deprivation (Carroll and O'Shea 2002). The subcellular location and activity of Pho4p, the master transcription factor of the Pi starvation response, is regulated through phosphorylation at four sites. Pho4 is cytosolic, inactive, and highly phosphorylated when cells are grown in media rich in Pi as the Pho80/Pho85 cyclin–CDK complex is fully active and maintains Pho4 in a highly phosphorlyated state (Carroll and O'Shea 2002). When cells are grown in low Pi medium, the Pho80/Pho85 cyclin–CDK complex is partially inhibited by Pho81, causing Pho4 to be only partially phosphorylated. In this partially phosphorylated state, Pho4 is nuclear and activates the transcription of genes such as Pho84, a high-affinity Pi transporter (50% of maximal expression). Partially phosphorylated Pho4 has a minimal effect upon the transcription of other phosphate-responsive genes, including the acid phosphatase (rAPase), encoded by PHO5, which is expressed at 10% of maximum levels (Springer et al. 2003). Full inhibition of Pho80/Pho85 by Pho81 in response to Pi deprivation causes Pho4 to become fully dephosphorylated and competent in activating transcription of all its target genes, including Pho5 (Springer et al. 2003).
To ascertain when tRNA nuclear accumulation occurs with respect to the PHO pathway-mediated gene expression, we determined the enzymatic activity of secreted Pho5. As the amount of the p-nitrophenylphosphate converted to p-nitrophenolate is proportional to the amount of Pho5 (Toh-e et al. 1973), determining production of p-nitrophenolate provides an indirect measurement of Pho5 expression. The rAPase activity was assayed (Byrne et al. 2004) using intact, early log-phase wild-type cells (strain BY4742) grown in SC–Pi, which duplicated the growth conditions of 23° cell cultures used for FISH analysis. Cells grown for 2 hr in SC–Pi had 145% of basal activity, whereas cells grown for 3.5 hr in SC–Pi had 278% of basal activity (Table 3). After 10 hr of growth in SC–Pi, cells had 1100% of basal activity (Table 3). The rAPase activity indicates that Pho5 has low levels of expression at the time points when tRNA nuclear accumulation occurs (1–2 hr of Pi deprivation).
TABLE 3.
Acid phosphatase activity
| Pi deprivation (hr) | Averagea (A420/A600) | Standard deviation | % rAPase activity of unstarved cells |
|---|---|---|---|
| 0 | 0.845 | 0.146 | 100 |
| 2 | 1.223 | 0.035 | 145 |
| 3.5 | 2.350 | 0.396 | 278 |
| 5 | 3.707 | 0.909 | 439 |
| 7 | 6.827 | 1.112 | 808 |
| 10 | 9.294 | 0.244 | 1100 |
Average of four data points.
Nuclear accumulation of tRNA appears to be an early response to Pi deprivation as it occurs slightly after Pho4p translocation from the cytosol to the nucleus (Kaffman et al. 1998) in contrast to the later, strong induction of Pho5p expression (Table 3). To address the possibility that the tRNA nuclear accumulation in response to Pi deprivation could be dependent on the PHO pathway, the subcellular tRNA distributions in Pi-deprived and replete pho4Δ or pho81Δ cells were determined. Unstarved pho4Δ cells grown at 23° in fresh SC have a slight accumulation of tRNATyr (Figure 3B, column 1). As pho4Δ and pho81Δ cells grown at 23° in SC–Pi for 1.5 hr also exhibited nuclear accumulation of tRNA (Figure 3B, columns 2 and data not shown), an intact PHO pathway is not required for tRNA nuclear accumulation induced by Pi deprivation. In addition, pho4Δ pho88Δ cells displayed tRNA nuclear accumulation that is similar to the accumulation within pho88Δ cells (Figure 3B, columns 4 and 3, respectively). Therefore, Pi deprivation-induced tRNA nuclear accumulation is an early, signal-mediated response to Pi deprivation that does not require the PHO pathway.
Mature tRNA accumulated within nuclei in response to Pi deprivation enter via the retrograde transport pathway:
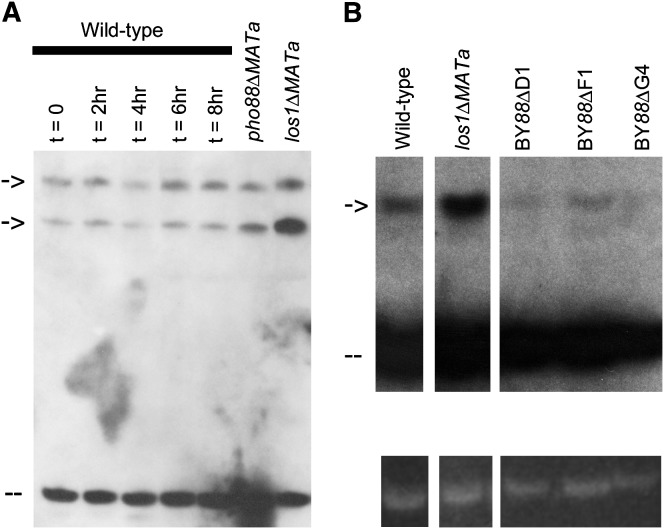
The current model of tRNA nucleus–cytosol transport has two modes for tRNA nuclear egress: primary nuclear export of newly synthesized tRNAs and re-export of mature tRNA. Blocking the primary nuclear export of tRNAs by mutation of LOS1, or any other members of the Ran pathway, causes accumulation of intron-containing pre-tRNAs. If Pi deprivation or deletion of PHO88 caused a block in the primary tRNA nuclear export pathway, then accumulation of intron-containing pre-tRNAs should have occurred. To determine whether tRNA nuclear accumulation observed in response to Pi deprivation and in pho88Δ cells occurs due to an inhibition of the primary export pathway or the re-export pathway, tRNA from wild-type cells grown in SC–Pi and pho88Δ cells were analyzed by Northern blot analysis. Depriving wild-type cells of Pi did not cause accumulation of intron-containing pre-tRNAs (Figure 4A). The independently derived pho88Δ strains did not exhibit accumulation of intron-containing pre-tRNAs (Figure 4). Therefore, mature tRNA had accumulated within nuclei of pho88Δ cells and Pi-deprived wild-type cells.
Figure 4.—
Northern analysis of Pi-starved wild-type cells and pho88 deletion strains. (A) Samples (10 μg) of small RNAs from BY4741 (wild-type) cells grown in SC (column 1) or in SC–Pi for the amount of time indicated (columns 2–5) at 23° from pho88Δ MATa cells (column 6) and from YD05055 (los1Δ) cells (column 7) were separated by electrophoresis and then transferred to a membrane. The membrane was probed with 32P-labeled oligonucleotides complementary to tRNAIle and detected by autoradiography. Arrows indicate intron-containing pre-tRNAs. Dashes indicate mature tRNAs. (B, top) Samples (10 μg) of small RNAs from BY4742 (wild-type) cells, from YD05055 (los1Δ) cells, and from cells of three independently derived pho88Δ strains (BY88ΔD1, BY88ΔF1, BY88ΔG4) grown in SC at 23° were separated by electrophoresis and then transferred to a membrane. The membrane was probed with 32P-labeled oligonucleotides complementary to tRNATyr and detected by autoradiography. Arrow indicates intron-containing pre-tRNAs. Dashes indicate mature tRNAs. (Bottom) Photo of the ethidium–bromide-stained polyacrylamide gel, prior to transfer, illuminated by ultraviolet light.
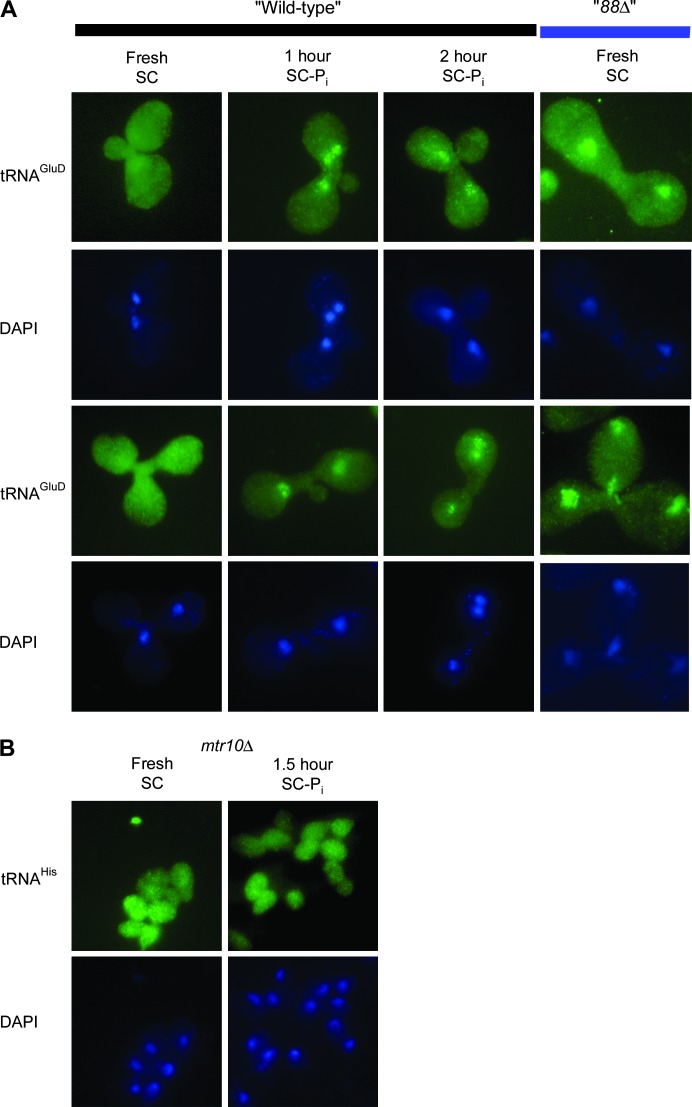
Since Northern analysis indicated that the tRNA nuclear accumulation observed in response to Pi deprivation was not due to impaired export of newly synthesized tRNA, tRNA nuclear accumulation was likely to have occurred through the tRNA retrograde pathway, similar to amino-acid-starvation-induced tRNA redistribution (Shaheen and Hopper 2005). To verify that tRNA retrograde nuclear accumulation occurs during Pi deprivation and when Pho88 is absent, the distribution of tRNAGlu-D was determined in “wild-type” heterokaryons grown in SC–Pi and in heterokaryons without Pho88. Heterokaryons were generated by mating yeast cells, in which one of the mating types carries a plasmid-expressing tRNAGlu-D from Dictyostelium discoideum and a kar1-1 mutation (strains MS739 + tRNAGlu-D or 8MS88ΔH2D + tRNAGlu-D), with KAR1 yeast cells (strain BY4741 or BY88ΔD1). The kar1-1 mutation blocked nuclear fusion upon mating and resulted in cells with a shared cytosol and two separate nuclei, where only one nucleus carried the plasmid. If mature tRNA, in response to Pi deprivation, had entered nuclei through retrograde tRNA transport, then all nuclei in a heterokaryon would have accumulated tRNAGlu-D when grown in SC–Pi. If it is independent of retrograde tRNA transport, then only nuclei that carried the plasmid-expressing tRNAGlu-D should have accumulated tRNAGlu-D when grown in SC–Pi.
Heterokaryons grown at 23° in SC–Pi for 1 or 2 hr, or in fresh SC for 2 hr, were subjected to FISH analysis. To quantify the data, heterokaryons were scored by determining if all, some, or none of the nuclei accumulate tRNAGlu-D. Nearly every heterokaryon grown in SC had an even distribution of tRNAGlu-D throughout the cell (Figure 5A, column 1; Table 4). Of the heterokaryons grown in SC–Pi for 1 hr, 83.5% accumulated tRNAGlu-D in all nuclei (Figure 5A, column 2; Table 4), 14.4% accumulated tRNAGlu-D in some nuclei, and 2.1% had no nuclear accumulation of tRNAGlu-D. The majority of heterokaryons grown in SC–Pi for 2 hr still accumulated tRNAGlu-D in all nuclei (72.7%; Figure 5A, column 3); however, as previously observed in haploid cells (Figure 3), the accumulation was not as prominent as the 1-hr time point and there was an increase in cells that have no nuclear accumulation (17.2 vs. 2.1%). The reduction of the total number of cells exhibiting tRNA nuclear accumulation after 2 hr of growth in SC–Pi was expected because the tRNA nucleus–cytosol distribution of wild-type cells returned to an even distribution between 2 and 3.5 hr of growth in SC–Pi. Since tRNAGlu-D accumulates in all of the nuclei of the majority of Pi-deprived “wild-type” heterokaryons, similar to amino-acid-deprived “wild-type” heterokaryons (Shaheen and Hopper 2005), the tRNA that accumulated in response to Pi deprivation entered the nucleus via retrograde tRNA transport of cytosolic tRNA.
Figure 5.—
Retrograde transport in pho88Δ and Pi-deprived wild-type cells. (A) “Wild-type” heterokaryon cells grown in fresh SC (column 1), SC–Pi for 1 hr (column 2) or SC–Pi for 2 hr (column 3), and “88Δ” heterokaryons (column 4) were analyzed by FISH. (Rows 1 and 2) tRNAGlu-D. (Rows 2 and 4) DAPI stain. (B) mtr10Δ MATa cells grown in fresh SC or SC–Pi for 1.5 hr at 23° were analyzed by FISH. (Row 1) tRNAHis. (Row 2) DAPI stain.
TABLE 4.
Heterokaryon assay of tRNA retrograde movement
| Cross | Media | PMI | All nuclei accumulate tRNAGlu-D (%) | Some nuclei accumulate tRNAGlu-D (%) | No nuclei accumulate tRNAGlu-D (%) |
|---|---|---|---|---|---|
| Wild type | SC | 2 | 0 (0) | 1 (1.2) | 83 (98.8) |
| Wild type | SC–Pi | 1 | 81 (83.5) | 14 (14.4) | 2 (2.1) |
| Wild type | SC–Pi | 2 | 64 (72.7) | 9 (10.2) | 15 (17.0) |
| 88Δ | SC | 2 | 79 (95.2) | 4 (4.8) | 0 (0) |
| 88Δ | YEPD | 2 | 36 (97.3) | 2 (2.7) | 0 (0) |
PMI, postmating incubation time.
The anticipated cause of tRNA nuclear accumulation within pho88Δ cells was low intracellular levels of Pi caused by defective Pi uptake. Therefore, the tRNA accumulated within the nuclei of pho88Δ cells was also expected to have entered the nucleus through retrograde transport. Heterokaryons homozygous for pho88Δ (“88Δ”) were subjected to FISH analysis. Consistent with our prediction, 95.2% of “88Δ” heterokaryons accumulated tRNAGlu-D in all nuclei (Figure 5A, column 5; Table 4). The data indicate that the tRNA accumulated within the nucleus of pho88Δ cells entered via retrograde tRNA transport.
Amino-acid-starvation-induced tRNA nuclear accumulation requires the β-importin, Mtr10, as mtr10Δ cells exhibit an even distribution of tRNA when grown in SC lacking amino acids (Shaheen and Hopper 2005). If Mtr10 is necessary for Pi-deprivation-induced tRNA nuclear accumulation, then Pi-deprived mtr10Δ cells will exhibit an even distribution of tRNA. When grown in SC–Pi at 23° for 1.5 hr, mtr10Δ cells showed the same tRNA nucleus–cytosol distribution as mtr10Δ cells grown in SC (Figure 5B, columns 2 and 1, respectively). The data indicated that Pi-deprivation-induced tRNA nuclear accumulation was Mtr10 dependent and therefore occurred by a mechanism shared with amino-acid-starvation-induced tRNA nuclear accumulation.
DISCUSSION
We undertook these studies to systematically query nonessential yeast genes for synthetic interactions with los1Δ to learn more about tRNA subcellular dynamics and we discovered a role for the availability of Pi in this process. SGA technology uncovered 131 candidate interactions with los1Δ. While 6 candidates were identified in both the 22° and the 37° assays, only 3 (lrp1Δ, rpl13bΔ, arc1Δ) of these had bona fide interactions as determined by tetrad analysis and growth assays. Of the 97 candidates evaluated by tetrad dissection, eight novel interactions were uncovered (Table 2). The SGA false-positive rate for los1Δ is 90.7% compared to the 25–50% success rate reported for other baits (Tong et al. 2004). The false-positive rate of the candidates that appeared in both screens (3 of 6) is 50%. This indicates that many false positives could have been eliminated by repeating the assays at both 22° and 37°. However, we note that PHO88 would not have been analyzed if SGA had been performed only at 37°. Since we previously noted that amino acid starvation causes tRNA nuclear accumulation (Shaheen and Hopper 2005), one may have expected to uncover SHR3, a likely equivalent to Pho88 for amino acid permeases, as a candidate. However, deletion of SHR3 was inviable by large-scale deletion (Winzeler et al. 1999) and was not included in the assay.
The eight novel synthetic interactions uncovered in this study affect amino acid synthesis, translation, mRNA and tRNA processing, and Pi uptake. The variety of cellular processes affected by the identified deletions and the data indicating that some of the identified deletions do not affect tRNA subcellular dynamics, raises the question as to why these synthetic interactions with los1Δ occur. RPL12A and RPL13B encode ribosomal proteins. The synthetic interactions between los1Δ and mutations of protein synthesis machinery, including tef2Δ and gcd11 (Hellmuth et al. 1998; Grosshans et al. 2000), are likely the result of the cumulative impairment of translation by reduced availability of cytoplasmic tRNA (los1Δ) and inefficient ribosomes. Aro7 is required for synthesis of tyrosine and phenylalanine. Deletion of ARO7 affects amino acid synthesis, and reduced amino acid availability may also affect the rate of protein synthesis. The genetic interaction between lrp1Δ and los1Δ may be caused by multiple defects in cellular RNA levels because previous studies uncovered synthetic lethal interactions between lrp1Δ and a variety of genes encoding defective tRNA, mRNA, and rRNA processing activities (Hieronymus et al. 2004; Davierwala et al. 2005). Our studies uncovered a single synthetic-enhanced growth interaction between snt309Δ and los1Δ. Since Snt309 is a component of spliceosomes (Chen et al. 1999), it is unclear why snt309Δ and los1Δ would have enhanced genetic interactions.
Different synthetic interactions between los1Δ and pho88Δ were observed when SGA or direct mutagenesis was employed to generate double mutants. The cold-sensitive nature of the synthetic slow-growth interaction observed in los1Δ pho88Δ cells may explain why PHO88 was found as a SGA candidate when the array was performed at 22° but not at 37°. Tetrad dissection was performed at 23° on YEPD medium, which has low levels of Pi (Yoshida et al. 1989b). This may have contributed to the absence of los1Δ pho88Δ progeny. If low temperature and/or medium prevented the appearance of los1Δ pho88Δ colonies, then tetrad dissection of pho88Δ by los1Δ crosses on high-Pi medium at 30° may produce los1Δ pho88Δ colonies. Tetrad dissection also involves a starvation period to induce meiosis, whereas direct mutagenesis occurs on rich media. Thus, it is also possible that los1Δ pho88Δ spores are unable to germinate after this starvation.
Deletion of PHO88, GTR1, or GTR2 caused nuclear accumulation of mature tRNA. Simulating the Pi-uptake defect observed in pho88Δ cells by growing wild-type cells in SC–Pi caused transient, temperature-dependent tRNA nuclear accumulation without causing accumulation of intron-containing tRNAs. The data indicate that tRNA nuclear accumulation was not due to impaired export of newly synthesized tRNAs and that Pi-deprived wild-type and pho88Δ cells accumulated mature tRNA only in nuclei that entered through tRNA retrograde transport. Consistent with amino-acid-starvation-induced tRNA redistribution, the data also demonstrated that the tRNA nuclear accumulation observed in Pi-deprived cells was Mtr10 dependent.
Although we have demonstrated that the mature tRNA accumulating within nuclei of pho88Δ and Pi-deprived wild-type cells entered the nuclei via the retrograde transport pathway, the mechanism for the accumulation remains unclear. Accumulation could have occurred due to increased retrograde transport, blocked re-export, or simultaneous occurrence of both increased retrograde transport and blocked re-export. Mtr10 may function in a signal transduction pathway regulating retrograde transport and/or re-export or as part of a tRNA importing complex.
Nuclear accumulation of tRNA that occurred due to PHO88 deletion largely paralleled the accumulation that occurred in Pi-deprived wild-type cells. However, pho88Δ cells constitutively accumulated tRNA within nuclei, whereas the accumulation observed during Pi deprivation was transient in nature. This difference may be explained by the differences in how the cells were impaired for Pi uptake. In the absence of Pho88, cells were able to constitutively acquire very low levels of Pi, sufficient for slow growth (Yompakdee et al. 1996). In contrast, Pi deprivation caused wild-type cells to undergo a two-phase response. During the first phase, when tRNA nuclear accumulation occurred, polyphosphate is mobilized (Mouillon and Persson 2006), Pho84 is highly expressed, and Pho5 is slightly expressed. The second phase, when tRNA returned to normal distribution, was marked by increased expression of Pho5. Similarly, recent data demonstrated that acute glucose starvation causes tRNA nuclear accumulation, whereas an even distribution of tRNA occurred during prolonged starvation (Whitney et al. 2007). This indicates that cells may have acclimatized to prolonged starvation and redistributed previously accumulated tRNA via re-export. Cells with PHO88 deletions may never reach the extremely low levels of intracellular Pi required for this transition. As Pi-deprivation-induced tRNA nuclear accumulation was independent of Pho4 and Pho81, the signaling pathway(s) controlling tRNA distribution during the Pi deprivation time course have yet to be determined.
Although a goal of this work was to uncover tRNA export pathways that function in parallel to Los1 to export newly synthesized tRNA, such a pathway was not identified. This unidentified exporter(s) may have been missed by SGA or not included in the deletion collection, or it may be encoded by an essential gene that has yet to be recognized as a tRNA transporter. Instead, we successfully uncovered novel connections between los1Δ and mRNA processing (lrp1Δ) and stable RNA processing (snt309Δ) and additional connections to the ribosome (rpl13bΔ, rpl12aΔ) and amino acid synthesis (aro7Δ). The novel synthetic interactions between los1Δ and gtr1Δ, gtr2Δ, or pho88Δ identified in this work provided a clear connection between tRNA subcellular dynamics and phosphate availability. Further studies are required to understand the signal transduction and the mechanism by which Pi levels regulate tRNA nucleus–cytosol distribution.
Acknowledgments
We thank H. Shaheen, M. Whitney, A. Murthi, and K. Stauffer for valuable scientific interactions. This work was supported by a fellowship to R.L.H. from the American Heart Association, by a grant to A.K.H. from the National Institutes of Health, and by grants to C.B. from the Canadian Institute of Health Research, Genome Canada, and Genome Ontario.
References
- Arts, G. J., M. Fornerod and I. W. Mattaj, 1998. a Identification of a nuclear export receptor for tRNA. Curr. Biol. 8 305–314. [DOI] [PubMed] [Google Scholar]
- Arts, G. J., S. Kuersten, P. Romby, B. Ehresmann and I. W. Mattaj, 1998. b The role of exportin-t in selective nuclear export of mature tRNAs. EMBO J. 17 7430–7441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bun-Ya, M., S. Harashima and Y. Oshima, 1992. Putative GTP-binding protein, Gtr1, associated with the function of the Pho84 inorganic phosphate transporter in Saccharomyces cerevisiae. Mol. Cell. Biol. 12 2958–2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne, M., N. Miller, M. Springer and E. K. O'Shea, 2004. A distal, high-affinity binding site on the cyclin-CDK substrate Pho4 is important for its phosphorylation and regulation. J. Mol. Biol. 335 57–70. [DOI] [PubMed] [Google Scholar]
- Carroll, A. S., and E. K. O'Shea, 2002. Pho85 and signaling environmental conditions. Trends Biochem. Sci. 27 87–93. [DOI] [PubMed] [Google Scholar]
- Chen, H. R., T. Y. Tsao, C. H. Chen, W. Y. Tsai, L. S. Her et al., 1999. Snt309p modulates interactions of Prp19p with its associated components to stabilize the Prp19p-associated complex essential for pre-mRNA splicing. Proc. Natl. Acad. Sci. USA 96 5406–5411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett, A. H., D. M. Koepp, G. Schlenstedt, M. S. Lee, A. K. Hopper et al., 1995. Rna1p, a Ran/TC4 GTPase activating protein, is required for nuclear import. J. Cell Biol. 130 1017–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davierwala, A. P., J. Haynes, Z. Li, R. L. Brost, M. D. Robinson et al., 2005. The synthetic genetic interaction spectrum of essential genes. Nat. Genet. 37 1147–1152. [DOI] [PubMed] [Google Scholar]
- Feng, W., and A. K. Hopper, 2002. A Los1p-independent pathway for nuclear export of intronless tRNAs in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 99 5412–5417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein, A. L., and J. H. McCusker, 1999. Three new dominant drug resistance cassettes for gene disruption in Saccharomyces cerevisiae. Yeast 15 1541–1553. [DOI] [PubMed] [Google Scholar]
- Gorlich, D., and U. Kutay, 1999. Transport between the cell nucleus and the cytoplasm. Annu. Rev. Cell Dev. Biol. 15 607–660. [DOI] [PubMed] [Google Scholar]
- Grosshans, H., E. Hurt and G. Simos, 2000. An aminoacylation-dependent nuclear tRNA export pathway in yeast. Genes Dev. 14 830–840. [PMC free article] [PubMed] [Google Scholar]
- Hellmuth, K., D. M. Lau, F. R. Bischoff, M. Kunzler, E. Hurt et al., 1998. Yeast Los1p has properties of an exportin-like nucleocytoplasmic transport factor for tRNA. Mol. Cell. Biol. 18 6374–6386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hieronymus, H., M. C. Yu and P. A. Silver, 2004. Genome-wide mRNA surveillance is coupled to mRNA export. Genes Dev. 18 2652–2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopper, A. K., and E. M. Phizicky, 2003. tRNA transfers to the limelight. Genes Dev. 17 162–180. [DOI] [PubMed] [Google Scholar]
- Hopper, A. K., F. Banks and V. Evangelidis, 1978. A yeast mutant which accumulates precursor tRNAs. Cell 14 211–219. [DOI] [PubMed] [Google Scholar]
- Hopper, A. K., L. D. Schultz and R. A. Shapiro, 1980. Processing of intervening sequences: a new yeast mutant which fails to excise intervening sequences from precursor tRNAs. Cell 19 741–751. [DOI] [PubMed] [Google Scholar]
- Huh, W. K., J. V. Falvo, L. C. Gerke, A. S. Carroll, R. W. Howson et al., 2003. Global analysis of protein localization in budding yeast. Nature 425 686–691. [DOI] [PubMed] [Google Scholar]
- Hurt, D. J., S. S. Wang, Y. H. Lin and A. K. Hopper, 1987. Cloning and characterization of LOS1, a Saccharomyces cerevisiae gene that affects tRNA splicing. Mol. Cell. Biol. 7 1208–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadowaki, T., D. Goldfarb, L. M. Spitz, A. M. Tartakoff and M. Ohno, 1993. Regulation of RNA processing and transport by a nuclear guanine nucleotide release protein and members of the Ras superfamily. EMBO J. 12 2929–2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaffman, A., N. M. Rank, E. M. O'Neill, L. S. Huang and E. K. O'Shea, 1998. The receptor Msn5 exports the phosphorylated transcription factor Pho4 out of the nucleus. Nature 396 482–486. [DOI] [PubMed] [Google Scholar]
- Kutay, U., G. Lipowsky, E. Izaurralde, F. R. Bischoff, P. Schwarzmaier et al., 1998. Identification of a tRNA-specific nuclear export receptor. Mol. Cell 1 359–369. [DOI] [PubMed] [Google Scholar]
- Lagerstedt, J. O., I. Reeve, J. C. Voss and B. L. Persson, 2005. Structure and function of the GTP binding protein Gtr1 and its role in phosphate transport in Saccharomyces cerevisiae. Biochemistry 44 511–517. [DOI] [PubMed] [Google Scholar]
- Martzen, M. R., S. M. McCraith, S. L. Spinelli, F. M. Torres, S. Fields et al., 1999. A biochemical genomics approach for identifying genes by the activity of their products. Science 286 1153–1155. [DOI] [PubMed] [Google Scholar]
- Mouillon, J. M., and B. L. Persson, 2006. New aspects on phosphate sensing and signalling in Saccharomyces cerevisiae. FEMS Yeast Res. 6 171–176. [DOI] [PubMed] [Google Scholar]
- Nakashima, N., N. Hayashi, E. Noguchi and T. Nishimoto, 1996. Putative GTPase Gtr1p genetically interacts with the RanGTPase cycle in Saccharomyces cerevisiae. J. Cell Sci. 109(Pt. 9): 2311–2318. [DOI] [PubMed] [Google Scholar]
- Nakashima, N., E. Noguchi and T. Nishimoto, 1999. Saccharomyces cerevisiae putative G protein, Gtr1p, which forms complexes with itself and a novel protein designated as Gtr2p, negatively regulates the Ran/Gsp1p G protein cycle through Gtr2p. Genetics 152 853–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar, S., and A. K. Hopper, 1998. tRNA nuclear export in Saccharomyces cerevisiae: in situ hybridization analysis. Mol. Biol. Cell 9 3041–3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar, S., A. K. Azad and A. K. Hopper, 1999. Nuclear tRNA aminoacylation and its role in nuclear export of endogenous tRNAs in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 96 14366–14371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiestl, R. H., and R. D. Gietz, 1989. High efficiency transformation of intact yeast cells using single stranded nucleic acids as a carrier. Curr. Genet. 16 339–346. [DOI] [PubMed] [Google Scholar]
- Shaheen, H. H., and A. K. Hopper, 2005. Retrograde movement of tRNAs from the cytoplasm to the nucleus in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 102 11290–11295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma, K., E. Fabre, H. Tekotte, E. C. Hurt and D. Tollervey, 1996. Yeast nucleoporin mutants are defective in pre-tRNA splicing. Mol. Cell. Biol. 16 294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simos, G., A. Segref, F. Fasiolo, K. Hellmuth, A. Shevchenko et al., 1996. a The yeast protein Arc1p binds to tRNA and functions as a cofactor for the methionyl- and glutamyl-tRNA synthetases. EMBO J. 15 5437–5448. [PMC free article] [PubMed] [Google Scholar]
- Simos, G., H. Tekotte, H. Grosjean, A. Segref, K. Sharma et al., 1996. b Nuclear pore proteins are involved in the biogenesis of functional tRNA. EMBO J. 15 2270–2284. [PMC free article] [PubMed] [Google Scholar]
- Springer, M., D. D. Wykoff, N. Miller and E. K. O'Shea, 2003. Partially phosphorylated Pho4 activates transcription of a subset of phosphate-responsive genes. PLoS Biol. 1 E28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford, D. R., M. L. Whitney, R. L. Hurto, D. M. Eisaman, W. C. Shen et al., 2004. Division of labor among the yeast Sol proteins implicated in tRNA nuclear export and carbohydrate metabolism. Genetics 168 117–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano, A., T. Endo and T. Yoshihisa, 2005. tRNA actively shuttles between the nucleus and cytosol in yeast. Science 309 140–142. [DOI] [PubMed] [Google Scholar]
- Toh-e, E. A., Y. Ueda, S. I. Kakimoto and Y. Oshima, 1973. Isolation and characterization of acid phosphatase mutants in Saccharomyces cerevisiae. J. Bacteriol. 113 727–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong, A. H., M. Evangelista, A. B. Parsons, H. Xu, G. D. Bader et al., 2001. Systematic genetic analysis with ordered arrays of yeast deletion mutants. Science 294 2364–2368. [DOI] [PubMed] [Google Scholar]
- Tong, A. H., G. Lesage, G. D. Bader, H. Ding, H. Xu et al., 2004. Global mapping of the yeast genetic interaction network. Science 303 808–813. [DOI] [PubMed] [Google Scholar]
- Weis, K., 2003. Regulating access to the genome: nucleocytoplasmic transport throughout the cell cycle. Cell 112 441–451. [DOI] [PubMed] [Google Scholar]
- Whitney, M. L., R. L. Hurto, H. H. Shaheen and A. K. Hopper, 2007. Rapid reversible nuclear accumulation of cytoplasmic tRNA in response to nutrient availability. Mol. Biol. Cell (in press). [DOI] [PMC free article] [PubMed]
- Winzeler, E. A., D. D. Shoemaker, A. Astromoff, H. Liang, K. Anderson et al., 1999. Functional characterization of the S. cerevisiae genome by gene deletion and parallel analysis. Science 285 901–906. [DOI] [PubMed] [Google Scholar]
- Yompakdee, C., N. Ogawa, S. Harashima and Y. Oshima, 1996. A putative membrane protein, Pho88p, involved in inorganic phosphate transport in Saccharomyces cerevisiae. Mol. Gen. Genet. 251 580–590. [DOI] [PubMed] [Google Scholar]
- Yoshida, K., Z. Kuromitsu, N. Ogawa and Y. Oshima, 1989. a Mode of expression of the positive regulatory genes PHO2 and PHO4 of the phosphatase regulon in Saccharomyces cerevisiae. Mol. Gen. Genet. 217 31–39. [DOI] [PubMed] [Google Scholar]
- Yoshida, K., N. Ogawa and Y. Oshima, 1989. b Function of the PHO regulatory genes for repressible acid phosphatase synthesis in Saccharomyces cerevisiae. Mol. Gen. Genet. 217 40–46. [DOI] [PubMed] [Google Scholar]
- Yoshihisa, T., K. Yunoki-Esaki, C. Ohshima, N. Tanaka and T. Endo, 2003. Possibility of cytoplasmic pre-tRNA splicing: the yeast tRNA splicing endonuclease mainly localizes on the mitochondria. Mol. Biol. Cell 14 3266–3279. [DOI] [PMC free article] [PubMed] [Google Scholar]