Abstract
Monounsaturated fatty acids are essential components of membrane and storage lipids. Their synthesis depends on the conversion of saturated fatty acids to unsaturated fatty acids by Δ9 desaturases. Caenorhabditis elegans has three Δ9 desaturases encoded by the genes fat-5, fat-6, and fat-7. We generated nematodes that display a range of altered fatty acid compositions by constructing double-mutant strains that combine mutations in fat-5, fat-6, and fat-7. All three double-mutant combinations have reduced survival at low temperatures. The fat-5;fat-6 double mutants display relatively subtle fatty acid composition alterations under standard conditions, but extreme fatty acid composition changes and reduced survival in the absence of food. The strain with the most severe defect in the production of unsaturated fatty acids, fat-6;fat-7, exhibits slow growth and reduced fertility. Strikingly, the fat-6;fat-7 double-mutant animals have decreased fat stores and increased expression of genes involved in fatty acid oxidation. We conclude that the Δ9 desaturases, in addition to synthesizing unsaturated fatty acids for properly functioning membranes, play key roles in lipid partitioning and in the regulation of fat storage.
Δ9 desaturases, also known as stearoyl-CoA desaturases (SCDs), are key lipogenic enzymes that catalyze the biosynthesis of monounsaturated fatty acids (MUFAs) from saturated fatty acids. These monounsaturated products are the most abundant fatty acids found in phospholipids, triglycerides, and cholesterol esters (Enoch et al. 1976). As components of phospholipids, MUFAs are key in maintaining optimal membrane fluidity and also serve as mediators of signal transduction (Ntambi 1999). In humans, alterations in ratios of saturated to unsaturated fatty acids are associated with various diseases including diabetes, atherosclerosis, cancer, and obesity (Wang et al. 2003a,b; Warensjo et al. 2005; Bougnoux et al. 2006). The mechanisms in which Δ9 desaturase activity affects these disease conditions are not well understood.
The Δ9 desaturases are essential and are ubiquitous among eukaryotes. Previous work has shown that Δ9 desaturases are regulated to respond to changing environmental conditions. In poikilotherms, organisms that are physiologically unable to regulate body temperature, expression of desaturases is induced upon exposure to low temperatures to maintain fluid and functioning cell membranes (Tiku et al. 1996; Gracey et al. 2004; Los and Murata 2004). The Δ9 desaturases are also highly regulated in response to diet. In yeast, expression of the OLE1 desaturase is repressed by exposure to exogenous unsaturated fatty acids in the growth media (Choi et al. 1996). Mice display a similar reduction in stearoyl-CoA desaturase 1 (SCD1) expression when unsaturated fatty acids are provided in the diet, and this isoform is also regulated by various dietary carbohydrates and by hormones such as insulin and leptin (Ntambi and Miyazaki 2003, 2004). Mouse mutants lacking SCD1 activity are lean and resistant to diet-induced obesity and insulin resistance (Dobrzyn and Ntambi 2005a).
In Caenorhabditis elegans, the fat-6 and fat-7 genes encode SCDs and a similar gene, fat-5, encodes a palmitoyl-CoA desaturase (Watts and Browse 2000). The pathway for unsaturated fatty acid synthesis in C. elegans begins with palmitic acid (16:0), obtained from the Escherichia coli diet or synthesized de novo, which is converted to palmitoleic acid (16:1) by FAT-5 (Figure 1A). This fatty acid is then elongated to cis-vaccenic acid (18:1Δ11), which is the most abundant fatty acid in phospholipids and triglycerides (Tanaka et al. 1996). Palmitic acid (16:0) can also be elongated to stearic acid (18:0), the substrate for FAT-6 and FAT-7 desaturation to oleic acid (18:1Δ9), which is further desaturated and elongated to form all of the polyunsaturated fatty acids (PUFAs), including arachidonic acid (20:4n-6) and eicosapentaenoic acid (20:5n-3) (Watts and Browse 2002) The desaturases involved in PUFA production downstream of the Δ9 desaturases include FAT-2 (Δ12 desaturase), FAT-3 (Δ6 desaturase), FAT-4 (Δ5 desaturase), and FAT-1 (omega-3 desaturase). Long-chain PUFAs are components of membrane phospholipids where they play important roles in membrane function and lipid signaling (Kahn-Kirby et al. 2004; Kubagawa et al. 2006). We recently characterized mutant strains that lack each Δ9 desaturase activity and these studies revealed only slight effects on fatty acid composition due to compensation by the remaining isoforms. Although these genes display functional overlap, the Δ9 desaturation is essential because fat-5;fat-6;fat-7 triple mutants that lack all activity are unable to survive unless they are supplemented with dietary oleic acid (Brock et al. 2006).
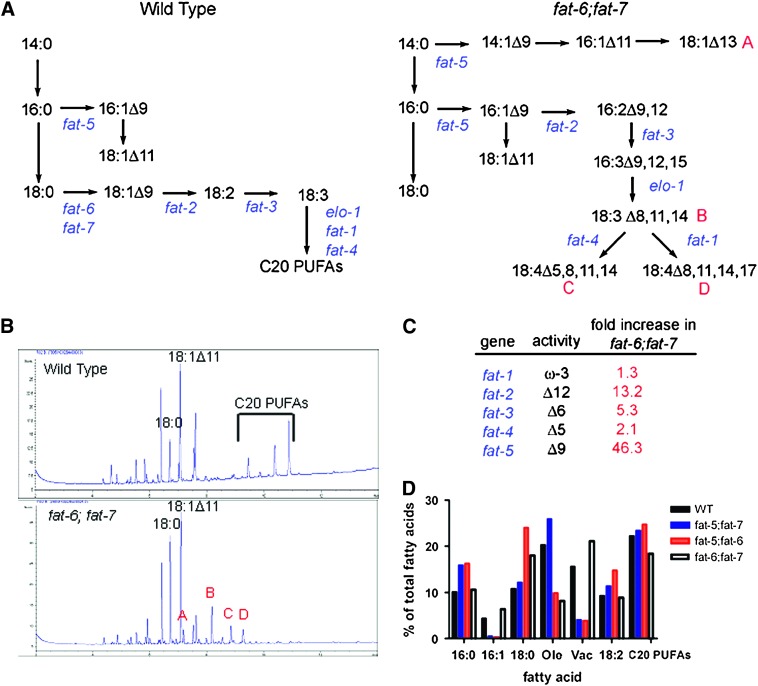
Figure 1.—
Fatty acid composition is altered in Δ9 desaturase double mutants. (A) Simplified scheme of fatty acid desaturation in wild-type C. elegans highlighting the roles of the fatty acid desaturases fat-1–fat-7 (left). (Right) The proposed pathway for generation of the unusual fatty acids produced by the fat-6;fat-7 double mutant. Fatty acid nomenclature: X:YΔZ, as in 18:1Δ11, fatty acid chain of X carbon atoms and Y methylene-interrupted cis double bonds; Z indicates the position of a double bond relative to the carboxyl end of the molecule. (B) Gas chromatography traces showing the fatty acid profiles of wild-type and fat-6;fat-7 double mutants. The fat-6;fat-7 double mutants lack 18:1Δ9 and the 20-carbon PUFAs. In addition, they accumulate higher levels of 18:0 as well as unusual fatty acids, labeled A–D in red. The identities of these fatty acids are A-18:1Δ13, B-18:3(Δ8,11,14), C-18:4(Δ5,8,11,14), and D-18:4(Δ8,11,14,17). (C) Changes in desaturase gene expression in fat-6;fat-7 double mutants compared to wild type. Gene expression was measured by QRT–PCR. (D) Simplified fatty acid composition of Δ9 desaturase double mutants grown in axenic culture. Ole, oleic acid (18:1Δ9); Vac, vaccenic acid (18:1Δ11).
In this study, we generated Δ9 desaturase double-mutant strains to examine intermediate, nonlethal effects that arise from reduced Δ9 desaturase activity. Our characterization of the double mutants reveals striking roles for Δ9 desaturases in maintaining energy homeostasis as well as for growth and development. All three double-mutant combinations affect growth and viability at low temperatures. The fat-5;fat-6 double mutants display relatively subtle fatty acid composition alterations under standard conditions, but extreme fatty acid composition changes and reduced survival in the absence of food. The fat-6;fat-7 double mutant shows the greatest fatty acid composition alterations under standard conditions and exhibits the broadest range of defects, including slow growth and reduced fertility. A key finding of these studies is that the fat-6;fat-7 double mutants have reduced fat stores and induction of genes encoding components of peroxisomal and mitochondrial β-oxidation. Other mutant strains with reduced PUFAs (fat-2 and fat-3 mutants) show similar defects in growth and fertility, yet do not display fat storage defects, indicating that ability to convert stearic acid (18:0) to oleic acid (18:1) is vital for proper energy partitioning and fat synthesis.
MATERIALS AND METHODS
Culture of nematodes:
Unless otherwise noted, animals were grown on nematode growth media plates (NGM) at 20° with the E. coli (OP50) strain as a food source (Wood 1988). The wild-type strain used was N2. The axenic culture media consisted of 3% soy peptone, 3% yeast extract, 0.5 mg/ml hemoglobin in 1 m KOH, and 20% ultra-high-temperature pasteurized skim milk (Houthoofd et al. 2002). The liquid axenic cultures were grown at room temperature (22°–23°) with constant shaking. Plates containing dietary fatty acid supplementation were prepared fresh for each supplementation experiment as described (Watts et al. 2003).
Generation of double mutants:
Single-worm PCR (Wicks et al. 2001) was used to determine the genotype of worms during crossing to generate fat-5(tm420);fat-6(tm331), fat-5(tm420);fat-7(wa36), and fat-6(tm331);fat-7(wa36) double-mutant lines. For fat-5(tm420) and fat-6(tm331) identification, standard PCR was used (for primer sequences see Brock et al. 2006). For fat-7(wa36) identification, real-time quantitative PCR was used with two primer sets. One primer set was designed to preferentially amplify the wild-type allele and the other primer set to preferentially amplify the fat-7(wa36) mutation (Drenkard et al. 2000).
Fatty acid and lipid analysis:
Fatty acid composition of adult nematodes was determined as previously described (Watts and Browse 2002; Brock et al. 2006). Thin-layer chromatography and lipid analysis was performed as described in Ashrafi et al. (2003) and Watts and Browse (2006). Nile Red staining was performed as described in Ashrafi et al. (2003). Images were captured at ×40 magnification using identical settings and exposure time for each image. To identify the unusual fatty acids in the fat-6;fat-7 double mutants, the fatty acid 4,4-dimethyloxazoline (DMOX) derivatives were prepared from fatty acid methyl esters to stabilize them for analysis by gas chromatography (GC)/mass spectroscopy (MS). For the DMOX reaction, the fatty acid methyl esters were evaporated using argon (Ar). A solution of 9:1 ethanol:benzene was added and after evaporation warmed 2-amino-2-methylpropanol was added. The reaction was capped and incubated 6 hr at 190°. After cooling, the DMOX derivatives were dissolved in hexane and washed twice with water. The hexane layer was then passed through a drying column of glass wool and Na2SO4. After evaporation of the solvent with Ar, 9:1 ethanol:benzene was added and then evaporated. The DMOX derivatives were then dissolved in hexane and separated on a 30 m × 0.25 mm AT-WAXms column (Alltech) with an HP6890 series GC system (Hewlett Packard) and the mass spectra were determined on the HP 5973 Mass Selective Detector (Hewlett Packard) (Watts and Browse 1999). The mass spectra of the peaks identified as DMOX-13-octadecenoate, DMOX-6,9,12-hexadecatrienoate, and DMOX-8,11,14,17-octadecatetraenoate matched the spectra presented by W. W. Christie on the lipid library website (http://www.lipidlibrary.co.uk/index.html). The mass spectrum of the peak identified as DMOX-5,8,11,14-octadecatetraenoate contained a prominent peak at m/z 153, which is diagnostic of a double bond at the Δ5 position, in addition to the characteristic gaps of 12 atomic mass units at m/z 182 and 194, m/z 222 and 234, and m/z 262 and 274, which correspond to double bonds at the 8, 11, and 14th carbon of DMOX-derived 18-carbon fatty acids.
Quantitative RT–PCR analysis:
Adult nematodes were harvested and RNA was prepared using TRIzol Reagent (Invitrogen, San Diego). A DNA-FREE RNA kit (Zymo Research) was used for Dnase treatment and purification. After quantification, 1 μg of RNA was used in a reverse-transcription reaction with SuperScriptIII (Invitrogen) to generate cDNA. Primer sequences for the metabolism genes were obtained from Marc Van Gilst (Van Gilst et al. 2005a). The PCR mixture consisted of 0.3 μm primers, cDNA, ROX, and 1× SYBR green mix (Invitrogen Platinum SYBR green qPCR Supermix UDG). The quantitative RT–PCR (QRT–PCR) was run and monitored on a MX3000P machine (Stratagene, La Jolla, CA). Relative abundance was determined using the ΔΔCt method and the reference genes tbb-2 and ubc-2 to control for template levels (Wong and Medrano 2005).
Growth and development phenotype analysis:
Life-span analysis:
Aging experiments were performed on adult nematodes grown at 20°. Worms were moved to plates containing 5-fluoro-2′-deoxyuridine (Sigma, St. Louis) at the fourth larval stage of development (L4). Live animals were assayed for movement in response to touch every 1–2 days (Apfeld and Kenyon 1998). Movement assays were performed with 1-day-old adults as described in Miller et al. (1996) and Watts et al. (2003).
Growth rate analysis:
Eggs were isolated from gravid adults using hypochlorite treatment and plated onto NGM plates. Twice a day the number of worms at each life stage was counted.
Fertility analysis:
For analysis of total progeny produced per worm, L4 (nonreproductive) worms were isolated and moved to fresh plates. After reaching reproductive viability, adults were moved to fresh plates twice daily, as needed. Two days after removal of the adult, the live progeny were counted. For analysis of biochemical complementation of fertility, worms were grown from hatching on supplemented plates. They were moved as young adults to supplemented plates of the same type and allowed to lay eggs for 2 days after which the adult was removed. The number of live progeny was counted on the following day.
Cold temperature growth:
Equal numbers of synchronous L1 worms were placed on plates at 20°, 15°, and 10°. The number of live nonarrested worms was counted on each plate when the wild-type population reached adulthood. These values are expressed relative to the number of live nonarrested worms counted at 20°.
L1 starvation survival:
Embryos were collected from adult worms by hypochlorite treatment and hatched on unseeded NGM plates without peptone. This produced a population of C. elegans arrested in the first larval stage. These larvae were washed from the plate and incubated at room temperature in M9 buffer with cholesterol (10 μg/ml). Every 48 hr, aliquots were transferred to standard NGM plates seeded with E. coli (OP50) bacteria. After 3 days of growth at 20°, viable adult nematodes were counted (Derry et al. 2001).
RESULTS
Fatty acid composition is altered in Δ9 desaturase double mutants:
To examine the roles of the Δ9 desaturases and the effects of altered saturated and monounsaturated fatty acid compositions, we generated double-mutant strains for all combinations of the three C. elegans Δ9 desaturase genes. The fat-5;fat-7, fat-5;fat-6, and fat-6;fat-7 double-mutant strains are all capable of reaching adulthood and reproducing under standard growth conditions even though they rely on only one of the three Δ9 desaturase isoforms. GC was used to measure the fatty acid composition of worms grown under standard conditions feeding on E. coli bacteria (Table 1). The fat-5;fat-7 and fat-5;fat-6 double mutants displayed subtle alterations compared to wild type, with increased saturated fatty acid content (19.3 and 17.7% saturated fatty acids compared to 15.6% in wild type) and slightly decreased MUFA and PUFA content.
TABLE 1.
Fatty acid composition of wild-type and Δ9 desaturase double mutants
| Fatty acid | Wild type | fat-5;fat-7 | fat-5;fat-6 | fat-6;fat-7 |
|---|---|---|---|---|
| 14:0 | 1.6 ± 0.1 | 2.3 ± 0.1*** | 1.9 ± 0.3 | 1.4 ± 0.3 |
| 16:0 | 5.5 ± 0.5 | 7.1 ± 0.6*** | 6.4 ± 0.6* | 1.6 ± 0.3*** |
| 18:0 | 8.5 ± 1.0 | 9.9 ± 1.2* | 9.4 ± 0.5 | 22.2 ± 1.3*** |
| Total saturated | 15.6 | 19.3 | 17.7 | 25.2 |
| 16:1 | 1.4 ± 0.1 | 1.2 ± 0.1 | 1.1 ± 0.1** | 3.4 ± 0.8*** |
| 18:1Δ9 | 3.2 ± 0.2 | 4.2 ± 0.4** | 3.2 ± 0.2 | —*** |
| 18:1Δ11 | 14.3 ± 1.3 | 13.5 ± 0.6 | 12.6 ± 1.3* | 24.0 ± 1.6*** |
| Total MUFA | 18.9 | 18.9 | 16.9 | 27.4 |
| 18:2 | 3.1 ± 0.1 | 3.5 ± 0.3* | 3.0 ± 0.3 | —*** |
| 20:3 | 4.4 ± 0.4 | 4.1 ± 0.3 | 3.9 ± 0.5 | —*** |
| 20:4n-6 | 2.0 ± 0.4 | 1.7 ± 0.1 | 1.5 ± 0.2 | —*** |
| 20:4n-3 | 5.0 ± 0.6 | 4.5 ± 0.6 | 4.4 ± 0.6 | —*** |
| 20:5 | 11.7 ± 1.7 | 11.0 ± 1.4 | 10.2 ± 1.2 | —*** |
| Total PUFA | 26.2 | 24.8 | 23.0 | — |
| 18:1 Δ13 | — | — | — | 2.0 ± 0.3*** |
| 18:3 Δ8,11,14 | — | — | — | 8.0 ± 2.0*** |
| 18:4 Δ5,8,11,14 | — | — | — | 4.1 ± 0.3*** |
| 18:4 Δ8,11,14,17 | — | — | — | 3.7 ± 0.3*** |
| Total unusual | — | — | — | 17.8 |
| C15iso | 3.9 ± 0.3 | 3.6 ± 0.2 | 4.3 ± 0.4 | 0.7 ± 0.3*** |
| C17iso | 3.1 ± 0.3 | 2.4 ± 0.2* | 3.5 ± 0.2* | 1.5 ± 0.6*** |
| Total branched | 7.0 | 6.0 | 7.4 | 2.2 |
| 17Δ | 19.9 ± 0.9 | 19.1 ± 1.0 | 20.9 ± 1.8 | 18.8 ± 2.1 |
| 19Δ | 13.2 ± 0.9 | 11.7 ± 0.7* | 13.8 ± 0.9 | 8.8 ± 1.5* |
| Total cyclopropane | 33.1 | 30.8 | 34.7 | 27.6 |
Data are weight percentages (mean ± SD) of four to six independent determinations of total worm fatty acids measured by gas chromatography. 17Δ, 9,19-methylenehexadecanoic acid; 19Δ, 11,12-methyleneoctadecanoic acid. Dash indicates fatty acids <0.5%. Values determined to be significantly different from wild-type worms using an unpaired t-test are *P < 0.05, **P < 0.001, and ***P < 0.0001.
In contrast, the fatty acid composition of fat-6;fat-7 double mutants was dramatically altered from wild type. The fat-6;fat-7 double mutants accumulated very high levels of 18:0 (22.2% of total fatty acids compared to 8.5% in wild type) and completely lack oleic acid (18:1Δ9) and PUFAs derived from this fatty acid, such as linolenic acid (18:2) and eicosapentaenoic acid (20:5n-3). In addition, the mono-methyl branched-chain fatty acids were reduced approximately threefold below wild-type levels. GC traces for fat-6;fat-7 contained novel peaks that were not present in wild type. To identify the novel peaks, fatty acid methyl esters were converted to DMOX derivatives to stabilize them for analysis of double-bond position by GC/mass spectroscopy (see materials and methods). We found that these peaks correspond to four unusual isomers of C18 unsaturated fatty acids that make up nearly 18% of total fatty acids (Figure 1B, Table 1).
Quantitative real time PCR (QRT–PCR) was used to monitor the expression of fatty acid desaturase genes in the fat-6;fat-7 double mutants (Figure 1C). We found that, compared to wild-type nematodes grown under the same conditions, adult fat-6;fat-7 double mutants showed induction of four of five fatty acid desaturases. The biggest change in expression was seen for the fat-5 gene, which encodes the only remaining Δ9 desaturase. This gene showed 46-fold greater expression in the fat-6;fat-7 double mutants than in wild type. The increased fat-5 expression likely leads to the increased vaccenic acid (18:1Δ11) content of the fat-6;fat-7 double mutants, since this fatty acid is elongated from 16:1 and cannot be further modified into PUFAs. In addition, the fat-2 Δ12 desaturase showed 13-fold induction and the fat-3 Δ6 desaturase showed 5-fold induction. Increased expression of desaturases, together with the absence of their normal substrates, likely leads to the formation of unsaturated fatty acids that are not normally produced. Presumably these unusual unsaturated fatty acids compensate for some of the functions that are ordinarily provided by C20 PUFAs. A proposed pathway for the formation of the unusual C18 unsaturated fatty acids in fat-6;fat-7 double mutants is shown in Figure 1A.
To determine if altered growth conditions affected fatty acid composition of the double mutants, we grew the worms in liquid axenic media. While the E. coli diet contains saturated fatty acids [myristic and palmitic acids (14:0 and 16:0)] as well as the MUFAs palmitoleic and cis-vaccenic acids (16:1 and 18:1Δ11) (Tanaka et al. 1996), the axenic media consists of yeast extract, peptone, and skim milk, which provides small amounts of palmitic acid (16:0), palmitoleic acid (16:1), oleic acid (18:1Δ9), and linoleic acid (18:2) (Brock et al. 2006). We found that both the fat-5;fat-7 and the fat-5;fat-6 double mutant showed amplified fatty acid composition changes when grown in axenic culture, with palmitoleic acid (16:1) and vaccenic acid (18:1Δ11) decreasing more than threefold compared to wild type in both double mutants and with 18:0 increasing twofold in the fat-5;fat-6 double (Figure 1D). This indicates that both fat-5 and fat-6 are particularly important for maintaining fatty acid composition during axenic growth. In contrast, the fat-6;fat-7 double mutant grown in axenic media had a less severe fatty acid composition change than when grown on E. coli plates. In axenic media, the fat-6;fat-7 double mutant accumulated 18:2 and C20 PUFAs, due to the presence of oleic and linoleic acids in the media. This, together with the 18:1 and 20:5 supplementation studies (see below), illustrates the importance of dietary contribution to fatty acid composition.
Δ9 desaturase activity is necessary for survival at low temperatures:
As a poikilothermic animal, C. elegans is sensitive to environmental temperatures. To determine if the Δ9 desaturases and the MUFAs that they produce play a role in survival at low temperatures, we plated fat-5;fat-6, fat-5;fat-7, and fat-6;fat-7 double mutants and wild-type L1 larvae at 20°, 15°, and 10°. When the wild-type larvae had reached adulthood, the nonarrested living worms on the plates were counted. With survival at 20° set at 100%, wild-type animals had a 96 and 85% survival rate at 15° and 10°, respectively. All three of the Δ9 desaturase double mutants exhibited reduced survival at 10° (Figure 2A). The fat-5;fat-7 double mutant displayed only 60% survival at 10° while the fat-5;fat-6 double mutant was more strongly affected with only 33% of the population surviving. The fat-6;fat-7 double mutant had the most extreme cold-sensitive phenotype. Only 1% of the worms survived at 10° and only 28% survived when grown at 15°. Thus, higher saturated fatty acid and lower unsaturated fatty acid composition is detrimental to nematodes at low temperatures.
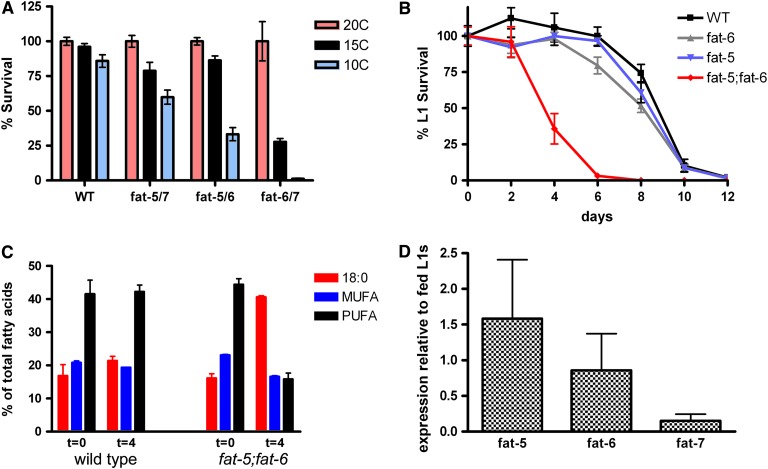
Figure 2.—
Δ9 desaturase double mutants have reduced survival at low temperature and during L1 starvation. (A) Survival at 15° and 10° relative to that at 20° for fat-5;fat-6, fat-5;fat-7 and fat-6;fat-7 double mutants and wild type. Synchronized L1 larvae of each genotype were plated at various temperatures and live animals were counted several days later when wild-type nematodes reached adulthood. (B) Survival as L1's for fat-5, fat-6, fat-5;fat-6, and wild type in the absence of food. Synchronized L1 larvae were incubated in M9 buffer + 0.01% cholesterol. Larvae were plated every 48 hr and allowed to develop on plates for 3 days, after which viable animals were counted. Error bars represent the standard error. (C) Simplified fatty acid composition of newly hatched L1 larvae (t = 0) and starved L1 larvae that have been incubated in M9 buffer for 4 days (t = 4). (D) Change of expression of the fat-5, fat-6, and fat-7 genes in 4-day-starved wild-type L1 larvae relative to expression in newly hatched L1 larvae. Data shown are the average of three determinations, each from four biological replicates; error bars represent the standard deviation.
fat-5;fat-6 double mutants reveal a role for Δ9 desaturation in survival of L1 arrest:
In the course of maintaining our stocks, we noted that the fat-5;fat-6 nematodes were difficult to revive from old plates containing starved worms. Several days after C. elegans worms had depleted their E. coli food, adult animals died and the surviving nematode population typically consisted of numerous L1 larvae that arrested development until food is reintroduced (Johnsen et al. 1984). We examined the ability of wild-type and mutant L1 larvae to survive in the absence of food by counting viable animals that had been incubated as L1 larvae for increasing periods of time in the absence of food. We found a shorter survival time for the fat-5;fat-6 L1 larvae compared to wild type. The time for half of the L1 larva population to die in the absence of food for wild type was ∼9 days, while the time for half of the fat-5;fat-6 L1 larva population to die in the absence of food was only 3.5 days (Figure 2B). The single fat-5 and fat-6 mutants, as well as other Δ9 desaturases double mutants, fat-5;fat-7 and fat-6;fat-7, displayed a similar ability to survive L1 arrest as wild type (Figure 2B and data not shown). Thus, the fat-5;fat-6 double mutant displays a specific defect in surviving starvation.
In an attempt to explain the reduced survival time of starved fat-5;fat-6 larvae, we characterized the fatty acid composition of fed and starved L1 animals. We found that starved wild-type L1 larvae showed very small changes in fatty acid composition compared to fed L1 larvae, with only a 4% increase in 18:0 and little change in MUFAs and PUFAs (Figure 2C). In contrast, the fat-5;fat-6 larvae showed greatly increased 18:0 accumulation (41% in starved larvae compared to 16% in fed L1 larvae) and decreased MUFAs and PUFAs (16% each of MUFAs and PUFAs in starved animals compared to 23% MUFAs and 44% PUFAs in fed L1 larvae). A recent study showed that the fat-7 gene is repressed 10-fold in wild-type adults after removal from food for 12 hr (Van Gilst et al. 2005b). We performed QRT–PCR on the three Δ9 desaturase genes using wild-type RNA isolated from fed and 4-day-old starved L1 larvae and found that the fat-7 gene was repressed >6-fold in the starved larvae while the fat-5 and fat-6 genes were unchanged or slightly induced (Figure 2D). While FAT-7 compensates for the lack of FAT-5 and FAT-6 under standard conditions, it appears that FAT-7 alone cannot provide the level of desaturation needed for survival in the absence of food.
MUFAs or PUFAs are required for proper movement, growth, and fertility:
Compared to the fat-5;fat-7 and fat-5;fat-6 strains, both phenotypes of the fat-6;fat-7 double mutants are more varied and more severe, which correlates with the more severe alterations in fatty acid composition. Relative to wild type, the fat-6;fat-7 worms grow slowly and produce fewer progeny, a phenotype similar to that of the other PUFA-deficient C. elegans strains fat-2 and fat-3 (Watts and Browse 2002; Watts et al. 2003). While the fat-6;fat-7 double mutants do not display the dumpy body shape observed in fat-2 and fat-3 mutants, the fat-6;fat-7 double mutants appear to move more sluggishly than wild type, a phenotype shared with fat-3 and fat-2 mutants. Indeed, when we quantified movement by assaying thrashing in liquid, we found that the fat-6;fat-7 double mutants generated less than half the number of thrashes per minute observed for wild type (Figure 3A). This rate is comparable to that of fat-3 mutants, but substantially higher than that of fat-2.
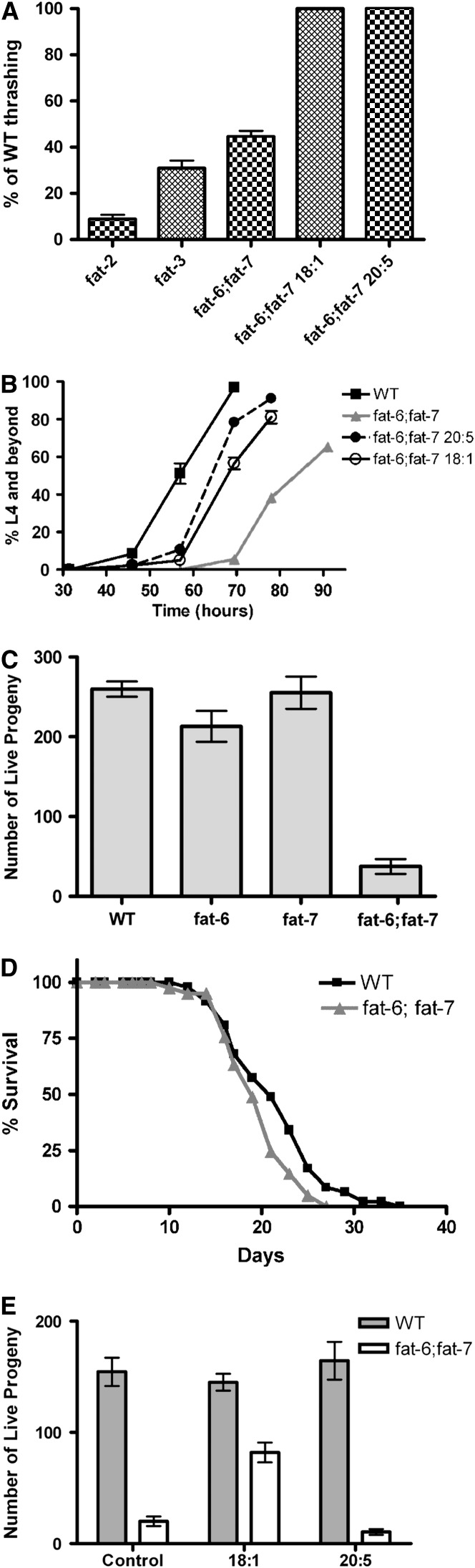
Figure 3.—
Movement, growth, and fertility defects in the fat-6;fat-7 double mutants. (A) Movement assayed by thrashing in liquid. (B) Time in hours for animals to reach the L4 stage. Nonsupplemented wild type are compared to fat-6;fat-7 double mutants grown without supplementation or supplementation with 18:1Δ9 or 20:5. (C) Average number of progeny produced per adult for wild type, fat-6, fat-7, and fat-6;fat-7 individuals. (D) Life span of wild type and fat-6;fat-7 at 20°. (E) Average progeny produced by individual adults over 2 days for wild-type and fat-6;fat-7 double mutants grown with control and 18:1Δ9 or 20:5 supplementation. All error bars represent the standard error.
We determined the growth rate of the fat-6;fat-7 double mutants by measuring the time that it takes isolated early stage embryos to develop and reach the fourth larval stage of development (L4). The fat-6;fat-7 double mutants reached the L4 stage well after wild-type animals (Figure 3B). The time for half of the population to reach L4 (L450) was 57 hr for wild type and 77 hr for fat-6;fat-7 double mutants. In comparison, fat-2 mutants display an even greater growth retardation than fat-6;fat-7, not reaching the L450 until 110 hr at 20°. The fat-3 worms grew considerably faster than the fat-6;fat-7 animals, reaching L450 at 64 hr.
We observed reduced brood size in the fat-6;fat-7 double mutants. By counting the progeny of single animals, we found that the fat-6;fat-7 double mutants produced an average of only 37 ± 9 live progeny/adult worm compared with 260 ± 10 live progeny for wild type (Figure 3C). The number of eggs laid by fat-6;fat-7 double mutants was only 36% of the number of eggs laid by wild type and only 34% of the eggs laid by fat-6;fat-7 double mutants that hatched into viable larvae. Microscopic examination revealed abnormalities in eggshells or absent eggshells for 58% of the unhatched eggs. Similarly, fat-2 (wa17) mutants layed an average of 53 eggs, and only 29% of these hatched. An abnormal eggshell was observed in ∼10% of the unhatched eggs produced by fat-2 mutants. In comparison, fat-3 mutants have greater reproductive success, as they lay an average of 160 eggs and 94% of them hatched (Watts et al. 2003). Interestingly, in spite of the movement, growth, and fertility defects observed in the fat-6;fat-7 strain, we found that the double mutants have a life span only slightly shorter than that of wild type (Figure 3D).
Fatty acid supplementation with 18:1Δ9 or 20:5 resulted in the amelioration of some, but not all, of the defects observed in fat-6;fat-7 animals. Supplementation with 0.1 mm 18:1 or 0.1 mm 20:5 fully restored movement (Figure 3A). The slow growth rate of fat-6;fat-7 could be partially complemented by dietary supplementation of 18:1 or 20:5 (Figure 3B). Fertility requirements were somewhat more specific, as the number of progeny produced by the fat-6;fat-7 double mutants was partially rescued by dietary oleic acid (18:1Δ9) but eicosapentaenoic acid (20:5n-3) did not improve either the number of eggs laid or their hatch rate (Figure 3E).
fat-6;fat-7 double mutants display reduced fat stores and induced expression of mitochondrial and peroxisomal β-oxidation genes:
In addition to the decreased growth rate, reduced fertility, and altered fatty acid profile, we noted that the fat-6;fat-7 double mutants grown on E. coli have a clear intestine, a phenotype often associated with reduced fat stores (McKay et al. 2003). We used the lipophilic dye Nile Red to stain the lipid droplets in the intestine of adult worms. The staining in fat-6;fat-7 mutants was qualitatively different from wild type: it was fainter and more diffuse, and the Nile Red-staining granules common in wild type were not distinct (Figure 4). To quantify this decrease in fat storage, we used thin-layer chromatography and gas chromatography to characterize the lipid profile of the fat-6;fat-7 double mutants and found a decrease in triglyceride stores. The average percentage of lipids as triglycerides in three independently grown samples was 51 ± 1% in wild-type animals and 41 ± 2% in fat-6;fat-7 double mutants. Analysis of the fatty acid composition of the various lipid classes revealed that the unusual 18-carbon PUFAs found in the fat-6;fat-7 double mutants were mainly partitioned to phospholipids and may replace the C20 PUFAs that are a major component of phospholipids (Table 2). The triglyceride fraction showed a dramatic increase in saturated fatty acids, containing 24.8% saturated fatty acids compared to only 9.9% in wild type.
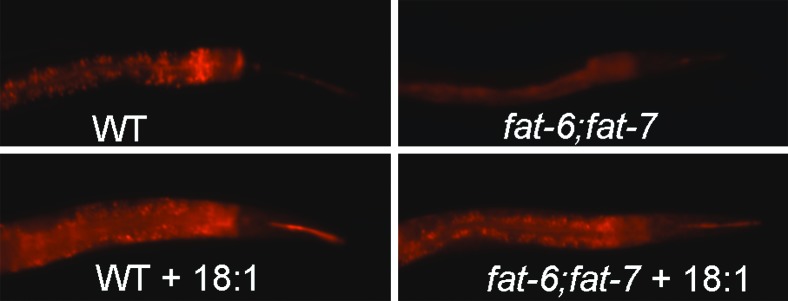
Figure 4.—
Reduced adiposity in fat-6;fat-7 double mutants. Wild-type and fat-6;fat-7 1-day-old adults stained with Nile Red. Animals were grown on unsupplemented plates (top) or on plates containing 0.1 mm 18:1 (bottom).
TABLE 2.
Fatty acid composition of lipid fractions from wild-type and fat-6;fat-7 double mutants
| Fatty acid | Wild-type TAG | fat-6;fat-7 TAG | Wild type PL | fat-6;fat-7 PL |
|---|---|---|---|---|
| 14:0 | 2.3 ± 0.3 | 2.4 ± 0.6 | 0.7 ± 0.1 | 0.5 ± 0.1 |
| 16:0 | 4.0 ± 2.5 | 2.2 ± 1.9 | 4.1 ± 0.5 | 1.8 ± 1.3* |
| 18:0 | 3.6 ± 0.1 | 20.2 ± 3.1** | 9.3 ± 1.8 | 23.5 ± 4.0* |
| Total saturated | 9.9 | 24.8 | 14.1 | 25.8 |
| 16:1 | 2.3 ± 0.2 | 3.2 | 0.9 ± 0.1 | 3.0 ± 0.1*** |
| 18:1Δ9 | 6.0 ± 0.1 | —*** | 2.2 ± 0.2 | —*** |
| 18:1Δ11 | 14.6 ± 2.6 | 16.8 ± 0.9 | 15.0 ± 1.7 | 26.0 ± 3.9* |
| Total MUFA | 21.8 | 20.0 | 16.8 | 29.0 |
| 18:2 | 3.0 ± 0.6 | —* | 4.6 ± 0.5 | —*** |
| 20:3 | 2.5 ± 0.3 | —** | 6.8 ± 0.5 | —*** |
| 20:4n-6 | 0.8 ± 0.4 | — | 2.5 ± 0.4 | —* |
| 20:4n-3 | 1.5 ± 0.3 | —* | 9.4 ± 0.1 | —*** |
| 20:5 | 3.1 ± 0.5 | —** | 22.0 ± 1.1 | —*** |
| Total PUFA | 11.5 | — | 46.1 | — |
| 18:1 Δ13 | — | 2.2 ± 0.2** | — | 1.7 ± 0.1*** |
| 18:3 Δ8,11,14 | — | 0.8 ± 0.7 | — | 8.3 ± 0.9*** |
| 18:4 Δ5,8,11,14 | — | 0.5 ± 0.5 | — | 5.6 ± 0.8** |
| 18:4 Δ8,11,14,17 | — | 0.5 ± 0.5 | — | 6.3 ± 1.3*** |
| Total unusual | — | 4.0 | — | 21.9 |
| C15iso | 5.7 ± 0.2 | 2.0 ± 0.5** | 1.8 ± 0.1 | —*** |
| C17iso | 3.2 ± 0.2 | 2.1 ± 0.4* | 2.6 ± 0.1 | 0.9 ± 0.3** |
| Total branched | 8.9 | 4.1 | 4.5 | 0.9 |
| 17Δ | 28.8 ± 0.6 | 31.1 ± 2.4 | 6.7 ± 0.7 | 10.6 ± 1.7* |
| 19Δ | 16.8 ± 1.2 | 12.2 ± 1.5* | 6.8 ± 0.5 | 7.7 ± 2.9 |
| Total cyclopropane | 45.6 | 43.3 | 13.5 | 18.3 |
Data are weight percentages of total fatty acids (mean ± SD) of lipid extractions from three independently grown nematode populations measured by gas chromatography. 17Δ, 9,19-methylenehexadecanoic acid; 19Δ, 11,12-methyleneoctadecanoic acid; TAG, triacylglyceride fraction; PL, phospholipid fraction. Dashes indicate fatty acids <0.5%. Values determined to be significantly different from wild-type lipid fractions using an unpaired t-test are *P < 0.05, **P < 0.001, and ***P < 0.0001.
The fat-2 and fat-3 mutants, which have normal Δ9 desaturase activity and are capable of synthesizing MUFAs, but are deficient in the synthesis of downstream PUFAs, do not show reduced Nile Red staining (Ashrafi et al. 2003). Therefore, it appears that the reduced synthesis of monounsaturated fatty acids or the reduction of Δ9 desaturase activity specifically, rather than the reduction in PUFAs, leads to lower adiposity in nematodes. Dietary supplementation with oleic acid (18:1) restored normal Nile Red staining to the fat-6;fat-7 double mutants (Figure 4). Thus, dietary fats are able to compensate for the fatty acid desaturation defect and restore the normal fat accumulation.
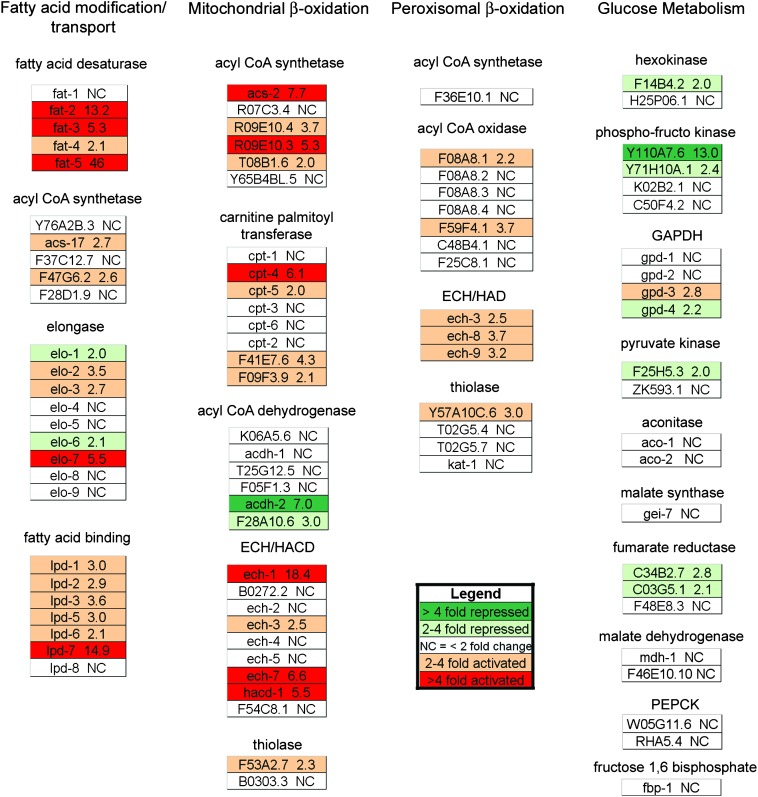
We used QRT–PCR to characterize the expression of a panel of genes involved in lipid and glucose metabolism in the fat-6;fat-7 double mutant compared to wild type (Van Gilst et al. 2005a). In addition to the increased expression of desaturase genes mentioned earlier, this analysis revealed a dramatic increase in expression of genes predicted to be involved in the mitochondrial and peroxisomal β-oxidation pathways (Figure 5). Notably, genes predicted to encode an acyl-CoA synthetase (acs-2) and a trifunctional β-oxidation enzyme (ech-1) showed increased expression (7.7-fold and 18.4-fold, respectively) in the fat-6;fat-7 double mutants. Previous studies have shown that the expression of these two genes is downregulated in high-fat nhr-49 mutants and that reducing their activity by RNA interference leads to increased fat stores (Van Gilst et al. 2005a). Another group of genes that are induced in the fat-6;fat-7 double mutants are the fatty-acid-binding proteins lpd-1–lpd-7. These proteins are predicted to encode cytosolic fatty-acid-binding proteins but their precise function is unknown. Genes involved in glucose metabolism were largely unaffected, with the exception of one isoform of phosphofructo kinase that was repressed 13-fold in the fat-6;fat-7 double mutants. The induction of enzymes involved in fatty acid oxidation indicates that, in addition to maintaining optimal unsaturation in membranes, Δ9 desaturase activity plays an important role in fat storage and energy homeostasis.
Figure 5.—
Changes in energy metabolism gene expression in fat-6;fat-7 double mutants compared to wild type. Gene expression was measured by QRT–PCR. The genes shown are predicted by homology to participate in C. elegans fatty acid and glucose metabolism pathways (Van Gilst et al. 2005a). Genes expressed at lower levels in fat-6;fat-7 are shown in green and genes with higher expression in fat-6;fat-7 are shown in orange (two to fourfold higher) and red (more than fourfold higher).
DISCUSSION
The generation of three Δ9 desaturase double-mutant strains enabled us to examine the consequences of altering the content of saturated and monounsaturated fatty acids in an animal. Our previous studies determined that the deletion of each of the single Δ9 desaturase genes had only subtle effects on fatty acid composition and no physiological consequences for the mutant animals, while the deletion of all three Δ9 desaturases led to lethality (Brock et al. 2006). The double-mutant strains created for this study showed a range of fatty acid compositions as well as a range of physical defects that provide clues to the biological processes that depend most on the synthesis of MUFAs.
To maintain membrane fluidity at low temperatures, plants and poikilothermic animals alter their fatty acid composition by increasing the level of unsaturation of their membrane phospholipids (Los and Murata 2004). We found that the fat-6;fat-7 double mutant, which lacks all 20-carbon PUFAs, is not able to survive at 10° and shows reduced survival at 15°. Other PUFA-deficient mutants, fat-2 and fat-3, also show decreased survival at low temperatures (Watts et al. 2003 and our unpublished observations). Thus, growth at low temperature is very sensitive to changes in fatty acid composition. The only apparent defect in the fat-5;fat-7 double mutants is a small reduction in survival at 10°. The lack of phenotypic consequences in the fat-5;fat-7 double mutants indicates that the FAT-6 Δ9 desaturase isoform, the most highly expressed of the three C. elegans Δ9 desaturases (Brock et al. 2006), provides sufficient production of MUFAs and PUFAs under standard growth conditions.
Characterization of the fat-5;fat-6 double mutants revealed roles for FAT-5 and FAT-6 in maintaining fatty acid composition during axenic growth conditions and in surviving L1 starvation arrest. While the FAT-7 Δ9 desaturase compensates for the loss of the other Δ9 desaturases when grown on E. coli-seeded plates, more extreme fatty acid composition changes occur in fat-5;fat-6 mutants when the bacterial food source is removed or when worms are grown in axenic media. In addition, the fat-5;fat-6 L1 larvae are severely compromised in their ability to survive periods of starvation. Under starvation conditions, the expression of the fat-7 gene in wild-type adults and L1-arrested animals falls to very low levels (Figure 2D and Van Gilst et al. 2005b). Thus, when food is absent, the fat-5;fat-6 double mutants experience a lethal deficiency in Δ9 desaturation activity and a rapid accumulation of stearic acid. This lethality is consistent with the inability of the fat-5;fat-6;fat-7 triple mutants to survive unless they are supplemented with dietary unsaturated fatty acids . Presumably, signals from food sources positively regulate fat-7 expression. This regulation is apparently complex, as previous studies have shown that FAT-7 expression depends on at least three transcription factors—NHR-49, NHR-80, and SREBP—as well as the transcriptional mediator MDT-15 (Van Gilst et al. 2005a; Brock et al. 2006; Taubert et al. 2006; Yang et al. 2006).
The fat-6;fat-7 double mutant displays the most extensive physiological defects of the Δ9 desaturase double mutants. The fatty acid composition of the fat-6;fat-7 double mutant confirms that FAT-5 is a true palmitoyl-CoA desaturase and cannot use stearic acid (18:0) as a substrate for desaturation. Like fat-2 and fat-3, C. elegans mutants with severely reduced PUFA production, the fat-6;fat-7 double mutants grow slowly and show reduced movement and low fertility (Figure 3). Slow growth is a symptom of essential fatty acid deficiency in humans (Heird 2005; Heird and Lapillonne 2005). Growth defects in humans with essential fatty acid deficiency as well as the C. elegans mutants can be rescued by dietary supplementation with PUFAs. The mechanisms linking PUFAs and growth in humans are not yet known (Smit et al. 2004). The reduced movement in all three strains is likely due to lack of C20 PUFAs that are required for efficient neurotransmission (Lesa et al. 2003). A reduction in fertility in the fat-6;fat-7 double mutants also occurs in fat-3 mutants but the fat-6;fat-7 double-mutant phenotype is more severe. The fat-3 mutants produce oleic (18:1Δ9) and linoleic acids (18:2) that the fat-6;fat-7 mutants cannot produce (Figure 1A), indicating that these may be crucial fatty acids for the maintenance of optimal fertility. In spite of the greatly reduced growth rate and fertility of the fat-6;fat-7 double mutants, their life span is nearly as long as that of wild type, indicating that a high proportion of saturated fatty acids (>23% compared to 8% in wild type) does not significantly reduce life span as has been suggested previously (Van Gilst et al. 2005a).
A key finding of these studies is the low-fat phenotype of the fat-6;fat-7 double mutant (Figure 4). The fat-2 and fat-3 mutants generate normal fat stores, indicating that the low adiposity is caused by the inability to synthesize oleic acid (18:1Δ9), rather than by a lack of PUFAs (see Figure 1A). Consistent with this, wild-type triacylglyceride stores contain <12% PUFAs, with the remaining fatty acids consisting of saturated, monounsaturated, branched, and cyclopropane fatty acids (Table 2). While fat-6;fat-7 triacylglyceride stores contain a similar proportion of monounsaturated fatty acids as wild type, they contain a more than fourfold greater proportion of stearic acid (18:0). Previous studies show that stearic acid is a poor substrate for triglyceride synthesis in cultured hepatocytes (Pai and Yeh 1996); thus it is possible that the kinetics of triglyceride synthesis are affected by the altered substrate availability. If the rate of incorporation of stearic acid is reduced, this may trigger increased catabolism of the accumulating substrates. Indeed, we find an increase in the expression of genes that encode the mitochondrial β-oxidation machinery, leaving open the possibility that the low-adiposity phenotype of the fat-6;fat-7 double mutant may be a consequence of increased energy expenditure as well as a consequence of reduced triglyceride synthesis.
The reduced-adiposity phenotype is similar to the mouse SCD1 targeted knockout, in which the SCD1-deficient mice have low fat stores, increased insulin sensitivity, and resistance to diet-induced obesity (Dobrzyn and Ntambi 2005a). Mice lacking SCD1 consume 25% more food than wild-type mice even though they accumulated less fat than their wild-type counterparts (Cohen et al. 2002). Indirect calorimetry studies indicate that the SCD1-deficient mice consume higher rates of oxygen and have increased rates of β-oxidation in the liver and in brown adipose tissue (Ntambi et al. 2002; Lee et al. 2004). Like the fat-6;fat-7 double mutants, SCD1-deficient mice display increased expression of mitochondrial β-oxidation genes (Ntambi et al. 2002).
Thus, in both vertebrates and invertebrates, Δ9 desaturases play a key role in cellular lipid partitioning and homeostasis. It has been suggested that Δ9 desaturases may be a potential therapeutic target in the treatment of obesity and metabolic syndrome (Dobrzyn and Ntambi 2005b). In support of this suggestion, studies in humans demonstrate that elevated Δ9 desaturase activity in skeletal muscle contributes to excess lipid accumulation and is associated with obesity, insulin resistance, and diabetes (Hulver et al. 2005). Targeting Δ9 desaturases directly may lead to unwanted side effects due to their essential function in maintaining pro-per membrane fluidity; however, the C. elegans model should allow rapid identification of factors that modify Δ9 desaturase gene expression and enzyme activity. Lowering the Δ9 desaturase activity in C. elegans mutants with increased fat stores in combination with genomewide reverse genetics screens promises to clarify the contributions of metabolic and neuroendocrine signaling pathways in the regulation of fat storage.
Acknowledgments
We thank Eric Phillips and Kyle Ann Brooks for technical assistance, Marc Van Gilst for providing primer sequences, and Monika Tzoneva and Bin Liang for helpful comments on the manuscript. The fat-5(tm420) and fat-6(tm331) mutants were provided by Shohei Mitani and the National BioResource Project for the Nematode (Tokyo). This work was supported by the National Institutes of Health R01 grant DK074114 and the Agricultural Research Center, Washington State University (WSU). Additional funding for T.J.B. was provided by the National Institutes of Health Biotechnology Training Program at WSU.
References
- Apfeld, J., and C. Kenyon, 1998. Cell nonautonomy of C. elegans daf-2 function in the regulation of diapause and life span. Cell 95 199–210. [DOI] [PubMed] [Google Scholar]
- Ashrafi, K., F. Y. Chang, J. L. Watts, A. G. Fraser, R. S. Kamath et al., 2003. Genome-wide RNAi analysis of Caenorhabditis elegans fat regulatory genes. Nature 421 268–272. [DOI] [PubMed] [Google Scholar]
- Bougnoux, P., B. Giraudeau and C. Couet, 2006. Diet, cancer, and the lipidome. Cancer Epidemiol. Biomarkers Prev. 15 416–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock, T. J., J. Browse and J. L. Watts, 2006. Genetic regulation of unsaturated fatty acid composition in C. elegans. PloS Genet. 2 e108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi, J., J. Stukey, S. Hwang and C. Martin, 1996. Regulatory elements that control transcription activation and unsaturated fatty acid-mediated repression of the Saccharomyces cerevisiae OLE1 gene. J. Biol. Chem. 271 3581–3589. [DOI] [PubMed] [Google Scholar]
- Cohen, P., M. Miyazaki, N. D. Socci, A. Hagge-Greenberg, W. Liedtke et al., 2002. Role for stearoyl-CoA desaturase-1 in leptin-mediated weight loss. Science 297 240–243. [DOI] [PubMed] [Google Scholar]
- Derry, W. B., A. P. Putzke and J. H. Rothman, 2001. Caenorhabditis elegans p53: role in apoptosis, meiosis, and stress resistance. Science 294 591–595. [DOI] [PubMed] [Google Scholar]
- Dobrzyn, A., and J. M. Ntambi, 2005. a The role of stearoyl-CoA desaturase in the control of metabolism. Prostaglandins Leukot. Essent. Fatty Acids 73 35–41. [DOI] [PubMed] [Google Scholar]
- Dobrzyn, A., and J. M. Ntambi, 2005. b Stearoyl-CoA desaturase as a new drug target for obesity treatment. Obes. Rev. 6 169–174. [DOI] [PubMed] [Google Scholar]
- Drenkard, E., B. G. Richter, S. Rozen, L. M. Stutius, N. A. Angell et al., 2000. A simple procedure for the analysis of single nucleotide polymorphisms facilitates map-based cloning in Arabidopsis. Plant Physiol. 124 1483–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch, H. G., A. Catala and P. Strittmatter, 1976. Mechanism of rat liver microsomal stearyl-CoA desaturase. Studies of the substrate specificity, enzyme-substrate interactions, and the function of lipid. J. Biol. Chem. 251 5095–5103. [PubMed] [Google Scholar]
- Gracey, A. Y., E. J. Fraser, W. Li, Y. Fang, R. R. Taylor et al., 2004. Coping with cold: an integrative, multitissue analysis of the transcriptome of a poikilothermic vertebrate. Proc. Natl. Acad. Sci. USA 101 16970–16975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heird, W. C., 2005. Biochemical homeostasis and body growth are reliable end points in clinical nutrition trials. Proc. Nutr. Soc. 64 297–303. [DOI] [PubMed] [Google Scholar]
- Heird, W. C., and A. Lapillonne, 2005. The role of essential fatty acids in development. Annu. Rev. Nutr. 25 549–571. [DOI] [PubMed] [Google Scholar]
- Houthoofd, K., B. P. Braeckman, I. Lenaerts, K. Brys, A. De Vreese et al., 2002. Axenic growth up-regulates mass-specific metabolic rate, stress resistance, and extends life span in Caenorhabditis elegans. Exp. Gerontol. 37 1371–1378. [DOI] [PubMed] [Google Scholar]
- Hulver, M. W., J. R. Berggren, M. J. Carper, M. Miyazaki, J. M. Ntambi et al., 2005. Elevated stearoyl-CoA desaturase-1 expression in skeletal muscle contributes to abnormal fatty acid partitioning in obese humans. Cell Metab. 2 251–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnsen, T. E., D. H. Mitchell, S. Kline, R. Kemal and J. Foy, 1984. Arresting development arrests aging in the nematode Caenorhabditis elegans. Mech. Ageing Dev. 28 23–40. [DOI] [PubMed] [Google Scholar]
- Kahn-Kirby, A. H., J. L. Dantzker, A. J. Apicella, W. R. Schafer, J. Browse et al., 2004. Specific polyunsaturated fatty acids drive TRPV-dependent sensory signaling in vivo. Cell 119 889–900. [DOI] [PubMed] [Google Scholar]
- Kubagawa, H. M., J. L. Watts, C. Corrigan, J. W. Edmonds, E. Sztul et al., 2006. Oocyte signals derived from polyunsaturated fatty acids control sperm recruitment in vivo. Nat. Cell Biol. 8 1143–1148. [DOI] [PubMed] [Google Scholar]
- Lee, S. H., A. Dobrzyn, P. Dobrzyn, S. M. Rahman, M. Miyazaki et al., 2004. Lack of stearoyl-CoA desaturase 1 upregulates basal thermogenesis, but causes hypothermia in cold environment. J. Lipid Res. 45 1674–1682. [DOI] [PubMed] [Google Scholar]
- Lesa, G. M., M. Palfreyman, D. H. Hall, M. T. Clandinin, C. Rudolph et al., 2003. Long chain polyunsaturated fatty acids are required for efficient neurotransmission in C. elegans. J. Cell Sci. 116 4965–4975. [DOI] [PubMed] [Google Scholar]
- Los, D. A., and N. Murata, 2004. Membrane fluidity and its roles in the perception of environmental signals. Biochim. Biophys. Acta 1666 142–157. [DOI] [PubMed] [Google Scholar]
- McKay, R. M., J. P. McKay, L. Avery and J. M. Graff, 2003. C. elegans: a model for exploring the genetics of fat storage. Dev. Cell 4 131–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, K., A. Alfonso, M. Nguyen, J. Crowell, C. Johnson et al., 1996. A genetic selection for Caenorhabditis elegans synaptic transmission mutants. Proc. Natl. Acad. Sci. USA 93 12593–12598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ntambi, J., 1999. Regulation of stearoyl-CoA desaturase by polyunsaturated fatty acids and cholesterol. J. Lipid Res. 40 1549–1558. [PubMed] [Google Scholar]
- Ntambi, J. M., and M. Miyazaki, 2003. Recent insights into stearoyl-CoA desaturase-1. Curr. Opin. Lipidol. 14 255–261. [DOI] [PubMed] [Google Scholar]
- Ntambi, J. M., and M. Miyazaki, 2004. Regulation of stearoyl-CoA desaturases and role in metabolism. Prog. Lipid Res. 43 91–104. [DOI] [PubMed] [Google Scholar]
- Ntambi, J. M., M. Miyazaki, J. P. Stoehr, H. Lan, C. M. Kendziorski et al., 2002. Loss of stearoyl-CoA desaturase-1 function protects mice against adiposity. Proc. Natl. Acad. Sci. USA 99 11482–11486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pai, T., and Y. Y. Yeh, 1996. Stearic acid unlike shorter-chain saturated fatty acids is poorly utilized for triacylglycerol synthesis and beta-oxidation in cultured rat hepatocytes. Lipids 31 159–164. [DOI] [PubMed] [Google Scholar]
- Smit, E. N., F. A. Muskiet and E. R. Boersma, 2004. The possible role of essential fatty acids in the pathophysiology of malnutrition: a review. Prostaglandins Leukot. Essent. Fatty Acids 71 241–250. [DOI] [PubMed] [Google Scholar]
- Tanaka, T., K. Ikita, T. Ashida, Y. Motoyama, Y. Yamaguchi et al., 1996. Effects of growth temperature on the fatty acid composition of the free-living nematode Caenorhabditis elegans. Lipids 31 1173–1178. [DOI] [PubMed] [Google Scholar]
- Taubert, S., M. R. Van Gilst, M. Hansen and K. R. Yamamoto, 2006. A Mediator subunit, MDT-15, integrates regulation of fatty acid metabolism by NHR-49-dependent and -independent pathways in C. elegans. Genes Dev. 20 1137–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiku, P. E., A. Y. Gracey, A. I. Macartney, R. J. Beynon and A. R. Cossins, 1996. Cold-induced expression of delta 9-desaturase in carp by transcriptional and posttranslational mechanisms. Science 271 815–818. [DOI] [PubMed] [Google Scholar]
- Van Gilst, M. R., H. Hadjivassiliou, A. Jolly and K. R. Yamamoto, 2005. a Nuclear hormone receptor NHR-49 controls fat consumption and fatty acid composition in C. elegans. PloS Biol. 3 e53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Gilst, M. R., H. Hadjivassiliou and K. R. Yamamoto, 2005. b A Caenorhabditis elegans nutrient response system partially dependent on nuclear receptor NHR-49. Proc. Natl. Acad. Sci. USA 102 13496–13501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, L., A. R. Folsom and J. H. Eckfeldt, 2003. a Plasma fatty acid composition and incidence of coronary heart disease in middle aged adults: the Atherosclerosis Risk in Communities (ARIC) Study. Nutr. Metab. Cardiovasc. Dis. 13 256–266. [DOI] [PubMed] [Google Scholar]
- Wang, L., A. R. Folsom, Z. J. Zheng, J. S. Pankow and J. H. Eckfeldt, 2003. b Plasma fatty acid composition and incidence of diabetes in middle-aged adults: the Atherosclerosis Risk in Communities (ARIC) Study. Am. J. Clin. Nutr. 78 91–98. [DOI] [PubMed] [Google Scholar]
- Warensjo, E., U. Riserus and B. Vessby, 2005. Fatty acid composition of serum lipids predicts the development of the metabolic syndrome in men. Diabetologia 48 1999–2005. [DOI] [PubMed] [Google Scholar]
- Watts, J. L., and J. Browse, 1999. Isolation and characterization of a delta 5-fatty acid desaturase from Caenorhabditis elegans. Arch. Biochem. Biophys. 362 175–182. [DOI] [PubMed] [Google Scholar]
- Watts, J. L., and J. Browse, 2000. A palmitoyl-CoA-specific delta9 fatty acid desaturase from Caenorhabditis elegans. Biochem. Biophys. Res. Commun. 272 263–269. [DOI] [PubMed] [Google Scholar]
- Watts, J. L., and J. Browse, 2002. Genetic dissection of polyunsaturated fatty acid synthesis in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 99 5854–5859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts, J. L., and J. Browse, 2006. Dietary manipulation implicates lipid signaling in the regulation of germ cell maintenance in C. elegans. Dev. Biol. 292 381–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts, J. L., E. Phillips, K. R. Griffing and J. Browse, 2003. Deficiencies in C20 polyunsaturated fatty acids cause behavioral and developmental defects in Caenorhabditis elegans fat-3 mutants. Genetics 163 581–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicks, S. R., R. T. Yeh, W. R. Gish, R. H. Waterston and R. H. Plasterk, 2001. Rapid gene mapping in Caenorhabditis elegans using a high density polymorphism map. Nat. Genet. 28 160–164. [DOI] [PubMed] [Google Scholar]
- Wong, M. L., and J. F. Medrano, 2005. Real-time PCR for mRNA quantitation. BioTechniques 39 75–85. [DOI] [PubMed] [Google Scholar]
- Wood, W. B. (Editor), 1988. The Nematode Caenorhabditis elegans. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Yang, F., B. W. Vought, J. S. Satterlee, A. K. Walker, Z. Y. Jim Sun et al., 2006. An ARC/Mediator subunit required for SREBP control of cholesterol and lipid homeostasis. Nature 442 700–704. [DOI] [PubMed] [Google Scholar]