Abstract
In the unicellular algae Chlamydomonas reinhardtii, the plus and minus mating types are controlled by a complex locus, MT, where the dominant MID gene in the MT− locus has been shown to be necessary for expression of minus-specific gamete-specific genes in response to nitrogen depletion. We report studies on MID expression patterns during gametogenesis and on a second gene unique to the MT− locus, MTD1. Vegetative cells express basal levels of MID. An early activation of MID transcription after nitrogen removal, and its sequence similarity to plant RWP-RK proteins involved in nitrogen-responsive processes, suggest that Mid conformation/activity may be nitrogen sensitive. A second stage of MID upregulation correlates with the acquisition of mating ability in minus gametes. Knockdown of MTD1 by RNAi in minus strains results in a failure to differentiate into gametes of either mating type after nitrogen deprivation. We propose that intermediate Mid levels are sufficient to activate MTD1 transcription and to repress plus gamete-specific genes and that MTD1 expression in turn allows the threshold-level MID expression needed to turn on minus gamete-specific genes. We further propose that an MTD1-equivalent system, utilizing at least one gene product encoded in the MT+ locus, is operant during plus gametogenesis.
CHLAMYDOMONAS reinhardtii is a flagellated unicellular green alga that has two mating types, plus and minus, determined by the mating type (MT) loci (MT+ and MT−). The center of this ∼1-Mb locus of recombinational suppression carries translocations and inversions and is called the rearranged (R) domain (Ferris and Goodenough 1994). Both housekeeping and sex-limited genes are found in this region (Ferris et al. 2002), similar to mating-type loci and sex chromosomes in other organisms (Graves 2006). Six unique regions (a–f) are found within the R domain, three (a–c) specific to MT+ and three (d–f) specific to MT−. Four genes have been identified in these regions: MTA1 (MT locus, region a) in a, FUS1 (fusion) in c, MTD1 (MT locus, region d) in d, and MID (minus dominance) in f (Ferris et al. 2002). The two MT−-specific genes are the focus of this study.
In response to nitrogen starvation, haploid vegetative Chlamydomonas cells differentiate into gametes. Gametes of opposite mating type are able to agglutinate and fuse to form zygotes (Harris 1989). Occasionally, heterozygous mt+/mt− diploids form after mating, resume vegetative growth, and differentiate as gametes with N-starvation. The fact that these diploids always mate as minus indicates that minus is dominant to plus (Harris 1989), a phenomenon found to be controlled by the MID gene (Galloway and Goodenough 1985). MID encodes a transcription factor in the RWP-RK family that also includes several proteins in higher plants that are suggested to exert their function during nitrogen limitation (Schauser et al. 1999, 2005; Borisov et al. 2003).
Previous studies revealed that MID is necessary and sufficient to convert wild-type plus gametes to mate as minus: mt+ cells transformed with the MID gene differentiate as minus gametes (Ferris and Goodenough 1997), and mt− cells carrying loss-of-function MID mutations (mid-1 or mid-2) differentiate as plus gametes (Ferris and Goodenough 1997; Ferris et al. 2002). In fact, although the mid mutants express plus flagellar agglutinins (Ferris and Goodenough 1997; Ferris et al. 2002) and plus mating structures (Ferris and Goodenough 1997), they are unable to fuse with minus gametes due to the lack of FUS1, a gene restricted to the MT+ locus and encoding a glycoprotein required for fusion (Ferris et al. 1996; Misamore et al. 2003); hence the phenotype of mid mutants is designated pseudo-plus. The pseudo-plus phenotype can be rescued by transforming mid mutants with FUS1 (Ferris et al. 1996).
MID has been shown to be involved in the activation/repression of the following genes:
SAD1 (sexual adhesion), located within the MT locus but just outside the R domain, encodes the minus agglutinin. Expression of SAD1 is inhibited in mid mutants (Ferris et al. 2005) and restored by transformation with MID (data not shown).
SAG1 (sexual agglutination), unlinked to MT, encodes the plus agglutinin. It is expressed in mid mutants and wild-type plus gametes but not in wild-type minus gametes (Ferris et al. 2005).
GSP1 [gamete-specific plus (mating type) molecule 1], unlinked to MT, encodes a plus gamete-specific homeodomain protein that functions in the zygote. Expression of GSP1 occurs in mid-1 and wild-type plus gametes but not in wild-type minus gametes nor in mt+/mt− diploids (Kurvari et al. 1998; Wilson et al. 1999).
GSM1 [gamete-specific minus (mating type) molecule 1], unlinked to MT, encodes a homeodomain partner of Gsp1 in minus gametes and shows MID-dependent activation in wild-type minus cells (J.-H. Lee, H. Lin and U. W. Goodenough, unpublished results).
Previous studies of the MTD1 gene showed that it encodes a protein with five predicted NXT/S glycosylation sites, three predicted transmembrane regions, and no homologs in the current database (Ferris et al. 2002). This protein is not essential to Chlamydomonas: MID-transformed mt+ gametes are able to form viable zygotes with wild-type plus gametes where MTD1 is not present in either cell line (Ferris and Goodenough 1997). Both MID and MTD1 are MT− localized and only ∼20 kb apart (Ferris et al. 2002), and both are gamete specific by Northern blotting (Ferris and Goodenough 1997; Ferris et al. 2002), suggesting that MTD1 might be involved in minus gametogenesis.
We report here studies on the expression of MID and MTD1 upon nitrogen removal using synchronous cell culture. The results reveal an early (∼30 min) upregulation of MID expression in response to nitrogen starvation. A second stage of MID expression is induced when cells display the gametic phenotype. We propose that this second activation is dependent on Mtd1 function. We also show that knockdown of MTD1 by RNA interference (RNAi) compromises or prevents minus gametogenesis, indicating an essential role for MTD1 in this process.
MATERIALS AND METHODS
Cells and cell culture:
C. reinhardtii strains (available from the Chlamydomonas Genetics Center, Duke University, Chapel Hill, NC) were maintained on Tris–acetate–phosphate (TAP) plates (Harris 1989). Vegetative cells were cultured in flasks of TAP medium with gentle shaking for 3 days. Gametes were prepared by resuspending at-least-5-day-old cells from TAP plates in nitrogen-free high salt minimal (NFHSM) medium (Martin and Goodenough 1975) for 2–3 hr. Synchronous cells were cultured with aeration in liquid high-salt minimal medium on a 12-hr light/12-hr dark cycle for 3 days (Harris 1989). A portion of cells was saved as the vegetative cell sample while the rest were harvested and resuspended in NFHSM immediately. At the time points indicated, cells were collected by centrifugation and prepared for RNA isolation or SDS–PAGE.
Transformation of Chlamydomonas:
Nine copies of FLAG (Castrucci et al. 1992) were inserted into the MID gene just before the stop codon. FLAG-tagged and nontagged MID were transformed into mid-2 cells by glass-bead transformation (Kindle 1990), using pSI103 (Sizova et al. 2001) as a selection marker. Transformants were selected on paromomycin plates and screened by PCR for the MID gene. Transformants were further screened for their ability to mate with wild-type plus gametes. The MTD1 RNAi construct was transferred into wild-type minus cells using pSI103 as a cotransformant by electroporation (Shimogawara et al. 1998).
BLAST and phylogenetic analysis:
The C-terminal sequence (aa 96–147) of C. reinhardtii Mid, which includes the conserved RWP-RK motif, was used in a protein BLAST against translated nucleotides in the Chlamydomonas JGI (Doe Joint Genome Institute) genome database version 3.0 (http://genome.jgi-psf.org/cgi-bin/runAlignment?db=Chlre3&advanced=1) with expected values ≤1e–3. Among the 14 proteins obtained from BLAST, 1 of them contained RWP but no RK in the conserved region and was omitted from alignment and phylogenetic analysis. In scaffold 27, 2 RWP-RK proteins were 5 kb away from each other although BLAST recognized only 1 of them (RWP11); the second protein (RWP4) was added to this study. Sequences containing the conserved motif from different proteins were aligned using Clustal X 1.83 (Thompson et al. 1997) and the alignment output was prepared using BOXSHADE. The aligned sequences were used to draw a neighbor-joining tree with bootstrap repeats of 1000 using MEGA 3.1 (Kumar et al. 2004).
SDS–PAGE and immunoblotting:
For antibody preparation, full-size MID or MTD1 cDNA was cloned into pET21a vectors (Novagen) and transformed into bacteria. Recombinant His-tagged proteins were induced by IPTG and purified using a His-affinity purification kit (Novagen) according to the manufacturer's protocol. The purified proteins were used to generate anti-Mid and anti-Mtd1 antibodies in rabbits (Cocalico Biological). Both antibodies were subjected to affinity purification using recombinant a protein-conjugated Sephorase 4B (Amersham Biosciences) column.
For detection of Mid, freshly harvested cells were resuspended in 1× SDS gel-loading buffer (50 mm Tris–HCl, pH 6.8, 100 mm DTT, 2% SDS, 0.1% bromophenol blue, 10% glycerol) and boiled for 5 min (Sambrook and Russell 2001). Typically, proteins from 1 × 107 cells were separated by 15% acrylamide SDS–PAGE (Laemmli 1970) at room temperature (RT), 85 V for stacking gel and 120 V for resolving gel. After electrophoresis, proteins were transferred to Immobilon-P membranes (Millipore, Bedford, MA) at 12 V for 1 hr using the semidry method at 4°. Membranes were blocked in 5% milk in TBST (137 mm NaCl, 20 mm Tris–HCl, pH 7.6, 0.05% Tween-20) for 1 hr at RT. Blocked membranes were washed for 5 min with TBST and inoculated with anti-Mid antibody (1:5000 dilution) or anti-FLAG antibody M2 (1:10,000 dilution, Sigma, St. Louis) in TBST containing 3% milk for 1 hr at RT or overnight at 4°. Membranes were rinsed once and washed with TBST three times, 5 min each. Peroxidase-conjugated goat-anti-rabbit or goat-anti-mouse antibodies (1:10,000 dilution each, Bio-Rad, Hercules, CA) were used as secondary antibodies for 1 hr at RT. Membranes were washed as above (Harlow and Lane 1988). Signals were detected using homemade enhanced chemiluminescence reagent (Yang and Widmann 2001).
For detection of Mtd1, the anti-Mtd1 antibody was further purified by preabsorption with acetone-precipitated proteins from wild-type plus gametes (Harlow and Lane 1988). Freshly harvested cells were resuspended in buffer (10 mm Tris, pH 7.0, 1 mm NaCl) containing proteinase inhibitors (Sigma) and flash frozen in liquid nitrogen for >1 hr (Wilson et al. 1999). An equal amount of boiling 2× SDS gel-loading buffer was added to the frozen samples and the samples were boiled immediately for 5 min. Proteins were separated by 10% acrylamide SDS–PAGE and transferred to Immobilon-P membranes as above. Membrane was blocked and inoculated with anti-Mtd1 antibody (1:1000 dilution) and peroxidase-conjugated goat-anti-rabbit secondary antibody (1:10,000 dilution, Bio-Rad) sequentially.
RNA preparation and Northern blotting:
For RNA isolation, 108–109 cells were collected and resuspended in RNA lysis buffer (20 mm Tris, pH 8.0, 20 mm EDTA, pH 8.0, 5% SDS, and 50 μg/ml proteinase K). The cell mixture was incubated at RT without stirring for 4–24 hr. Sodium acetate (3 m, pH 5.2) was added to the cell mixture to a final concentration of 0.3 m and vortexed. RNA was extracted by an equal volume of phenol/chloroform (1:1) and precipitated by an equal volume of isopropanol. Precipitated RNA was washed and dissolved in DEPC water. RNA was further purified by precipitation using an equal volume of lithium chloride overnight at 4°, followed sequential precipitation using 2.5 vol of ethanol. RNA from ethanol precipitation was vacuum dried and resuspended in DEPC water. RNA concentration was determined by spectrophotometry at 260 nm. For Northern blotting, RNA was loaded to 1% agarose formaldehyde gels and the gels were run at 35 V overnight at RT. After electrophoresis, RNA was transferred to nylon membranes (Hybond-XL, Amersham, Piscataway, NJ) by dry blotting overnight and crosslinked at 1200 μJ × 100 (UV Stratalinker 1800, Stratagene, La Jolla, CA). For hybridization, cDNA probes were randomly radiolabeled. Hybridization and washes were done following Church and Gilbert (1984).
cDNA synthesis and RT–PCR:
Poly(A) RNA was isolated from 5 μg of total RNA using Dynabeads oligo(dT)25 (Invitrogen, San Diego), according to the manufacturer's protocol. Beads with bound mRNA were inoculated with RQ1 RNase-free DNase (Promega, Madison, WI) in a 10-μl reaction at 37° for 30 min. SuperScript II reverse transcriptase (Invitrogen) was used for cDNA synthesis using random primers with reaction conditions of 25° for 10 min, 42° for 1 hr, 50° for 30 min, and 65° for 15 min. After these reactions, RNA was digested by the addition of RNase H (Invitrogen) at 37° for 30 min. One microliter from the reaction was used in a 20-μl PCR using Taq polyermase (Promega). PCR cycle numbers were determined experimentally to ensure that the products were within a linear range. In the study of MID expression, the intensity of individual RT–PCR MID and CRY1 signals was measured by Quantity One software (Bio-Rad). The relative amount of the MID was standardized by the intensity of corresponding CRY1 and further standardized by the relative amount of MID in vegetative cells.
Primers used in this study are the following: MID (5′-ATGGCCTGTTTCTTAGCC-3′; 5′-CTACATGTGTTTCTTGACG-3′); MTD1 (5′-GCTACCGGAGGCTCCTAC-3′; 5′-GACACGTTGCAAGACAGA-3′); CRY1 (5′-TTCGGCGTTGCTCACATCTT-3′; 5′-TCGATGCGGCCAATCTTCAT-3′); GSM1 (5′-CAGTGGACACGGCGACTG-3′; 5′-CCGAAGAAACTCAGAGTACG-3′); SAD1 (5′-TTCAGAGCGCTGGATCTG-3′; 5′-GCCATGCTGGTGTACCTG-3′); NSG6 (5′-TGAGCGGCAGTTTGCTGA-3′; 5′-ACCATGGCGCCCATCAAT-3′); and NSG17 (5′-TGCAGGCCATGCAAATGA-3′; 5′-ACAACCGCGTGCGAAACT-3′).
Ribonuclease protection assay:
RNA probes for ribonuclease protein assay (RPA) were synthesized by in vitro transcription using linearized plasmids containing full-length MID or partial MAT3 cDNA sequences as templates. T7 or T3 RNA polymerase (Ambion) and radiolabeled UTP were used. The transcribed probes were gel purified using 5% acrylamide/8 m urea gel. RPA was performed using an RPAIII kit (Ambion) according to the manufacturer's instructions.
RESULTS
The RWP-RK protein family in Chlamydomonas:
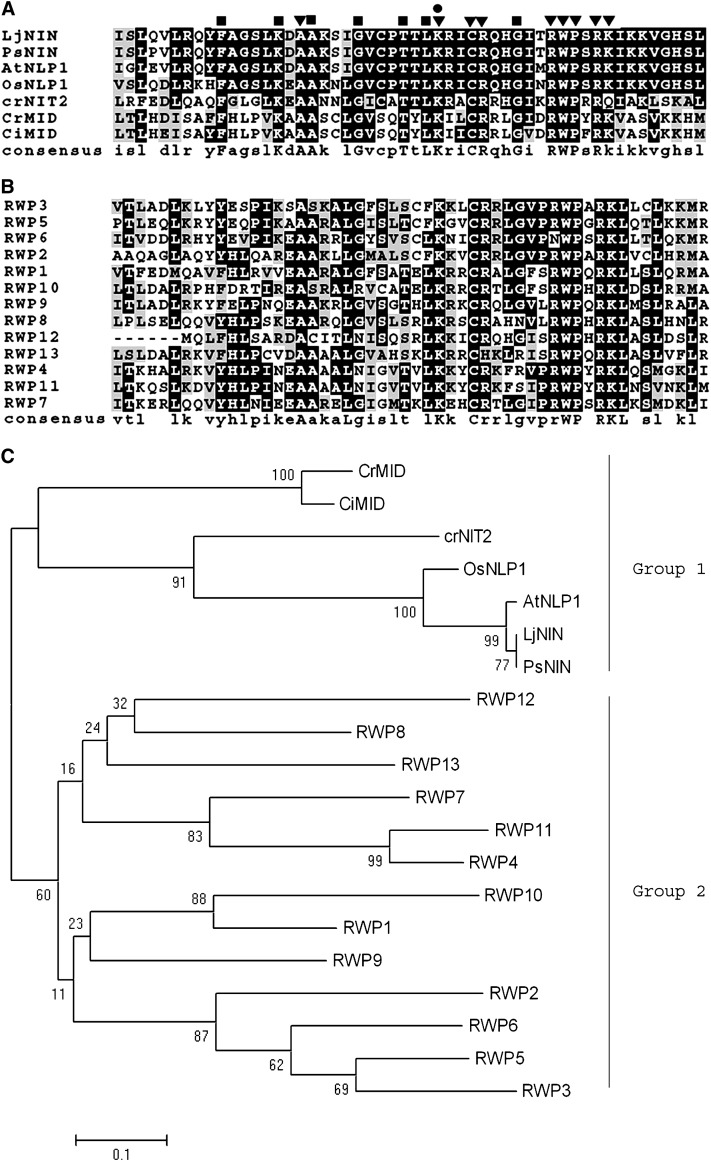
Two years after Mid was identified (Ferris and Goodenough 1997), a protein named NIN (nodule inception) was identified in lotus (Lotus japonicus) (Schauser et al. 1999). Sequence comparison between NIN and Mid revealed a conserved RWP-RK motif, which contains an invariant RWP×RK sequence (Schauser et al. 1999). More NIN-like proteins were lately identified in pea (Borisov et al. 2003), rice, and Arabidopsis (Schauser et al. 2005). In total, 14 proteins in Arabidopsis and 16 in rice contain this conserved motif.
Fourteen RWP-RK proteins in addition to Mid were identified in the current Chlamydomonas genome using the conserved domain (aa 96–147) of Mid (materials and methods). One protein is the gene product of the nitrate assimilation regulatory gene NIT2 (Schnell and Lefebvre 1993; Galvan and Fernandez 2001); the rest are unknown proteins, named RWP1–13 (Table 1). Sequence comparisons (Figure 1A and 1B) and phylogenetic analysis (Figure 1C) reveal that Mid is phylogenetically closer to Nit2 in Chlamydomonas and to NIN and NIN-like proteins in lotus, pea, rice, and Arabidopsis (group 1) than to other RWP proteins in Chlamydomonas (group 2). Some amino acids other than RWP×PK are conserved in all proteins (Figure 1A, inverted triangles), including the lysine that is mutated in the mid-1 mutant (Figure 1A, circle) (Ferris and Goodenough 1997). Other sites are conserved among group 1 but not group 2 proteins (Figure 1A, squares). Given that group 1 proteins are all involved in processes induced by nitrogen limitation, these sites may play a role in the nitrogen response.
TABLE 1.
RWP-RK proteins in Chlamydomonas
| RWP-RK proteins | Localization in JGI Genome Project |
|---|---|
| NIT2 | Chlre3/scaffold_9:322348-327145 |
| RWP1 | Chlre3/scaffold_34:980727-982909 |
| RWP2 | Chlre3/scaffold_3:54841-64925 |
| RWP3 | Chlre3/scaffold_43:243606-250716 |
| RWP4 | Chlre3/scaffold_27:360353-364613 |
| RWP5 | Chlre3/scaffold_26:825065-832955 |
| RWP6 | Chlre3/scaffold_26:1532616-1536494 |
| RWP7 | Chlre3/scaffold_14:53086-56143 |
| RWP8 | Chlre3/scaffold_15:44952-49225 |
| RWP9 | Chlre3/scaffold_12:1929625-1934390 |
| RWP10 | Chlre3/scaffold_17:985300-986840 |
| RWP11 | Chlre3/scaffold_27:345625-354103 |
| RWP12 | Chlre3/scaffold_72:313007-321422 |
| RWP13 | Chlre3/scaffold_22:137799-143173 |
Figure 1.—
RWP-RK proteins. (A) Alignment of RWP-RK domains from C. reinhardtii Mid (CrMid, AAC49753), C. incerta Mid (CiMid, AAB60944), C. reinhardtii Nit2 (CrNit2, ABC42493), and several Nin-like plant proteins: Lj, L. japonicus (CAB61243); Ps, Pisum sativum (CAD37949); At, Arabidopsis thaliana (F84548); Os, Oryza sativa (AAM22710.1). ▾, conserved amino acids within all listed proteins (except in CrNit2, in which lysine in RWP-RK is replaced by glutamine); •, mutation of this amino acid from lysine to isoleucine in mid-1 mutant leads to pseudo-plus gametes; ▪, conserved amino acids within all proteins listed in A but not in B. (B) Alignment of Chlamydomonas RWP proteins. (C) Neighbor-joining tree showing the relationship of all listed RWP-RK proteins. Numbers at nodes represent bootstrap percentages of 1000 repeated runs. Proteins in group 1 all respond to nitrogen limitation in different organisms; the function of proteins in group 2 is currently unknown.
Patterns of MID expression:
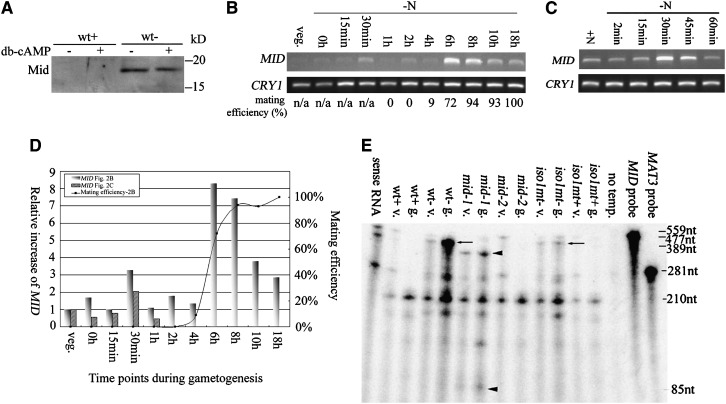
During Chlamydomonas mating, cell adhesion triggers elevation of intracellular cyclic AMP (cAMP), which in turn activates a series of mating responses; addition of exogenous, membrane-permeant dibutyryl cyclic AMP (db-cAMP) is able to mimic these responses (Pasquale and Goodenough 1987; Goodenough 1989). To ask whether MID expression is affected by cAMP, db-cAMP was added to wild-type plus and minus gametes. Western blotting using anti-Mid antibody showed that addition of db-cAMP in minus cells made no difference in Mid protein level (Figure 2A). Western blotting also confirmed that Mid protein is present in minus but not plus gametes, with the observed molecular weight (∼17 kDa) close to that calculated (16,390 Da) (Ferris and Goodenough 1997).
Figure 2.—
Patterns of MID expression. (A) Western blot of Mid in wild-type plus (wt+) and minus (wt−) gametes with or without the addition of db-cAMP. (B) RT–PCR of MID during gametogenesis. Synchronous wild-type minus cells were transferred to nitrogen-free (−N) media and samples were collected at various time points as indicated. RT–PCR products of poly(A) selected RNA were detected by ethidium bromide staining. CRY1, encoding ribosomal protein S14, is used as an internal control. Mating efficiencies of cells when samples were collected are standardized using mating efficiencies of wild-type tester cells. (C) RT–PCR of MID during early gametogenesis. Wild-type minus cells from 3-day-old TAP plates were transferred to nitrogen-free media and collected at various time points as indicated. Mating efficiencies of cells were not determined since vegetative cells do not differentiate into gametes within 1 hr. (D) Relative increases of MID during gametogenesis. The expression levels of MID in B and C were obtained by quantitation of the MID RT–PCR signals with the internal loading control, CRY1, and standardized by the relative amount of MID in vegetative cells. The relative increases of MID were plotted against time points when samples were removed during gametogenesis. The mating efficiency of individual samples from B is also plotted. (E) Expression of MID in vegetative cells. Total RNA isolated from wild-type and various mutant cells was hybridized with both a MID antisense RNA probe and a MAT3 antisense RNA probe and subjected to the ribonuclease protection assay. Arrows and arrowheads indicate the protected fragment of MID. mid-1, MID mutant with point mutations; mid-2, MID deletion mutant; iso1 mt−, an isoagglutination mutant; and iso1 mt+, mutant that carries the same mutation as in iso1 mt− but has a normal plus phenotype. The MID probe, 559 nucleotides (nt); the protected MID messages in wild-type minus and iso1 mt− cells, 477 nt (arrows); and the protected MID fragments in mid-1, 389 and 85 nt, respectively (arrowheads). MAT3, encoding a retinoblastoma homolog (Umen and Goodenough 2001), was used as an internal loading control. The MAT3 probe, 281 nt; and the protected MAT3 message, 210 nt. The third-to-last lane, no template, serves as a negative control with no RNA template.
When MID was first identified, Northern blotting showed a very weak and hence ambiguous MID signal in minus vegetative cells and a strong signal in mature gametes (Ferris and Goodenough 1997). To ask whether or not MID is indeed expressed in vegetative cells, and to understand when and under what conditions expression is upregulated, vegetative cells were transferred into nitrogen-free medium and the level of MID was studied at different time points during gametogenesis using RT–PCR. Cells were prepared for these studies in two different ways.
In the first, wild-type minus cells were synchronized by light/dark cycles. Cells remain in a prolonged G1 during the light phase, undergo alternative rounds of S and mitosis in the dark, and re-enter G1 when reilluminated (Umen and Goodenough 2001). Cells were collected at this G1 and immediately transferred into nitrogen-free medium (set as time 0) (Abe et al. 2004). As shown in Figure 2B, some cells started to become gametic at 4 hr, capable of agglutinating and fusing with wild-type plus testers with a relative mating efficiency of 9%, and the mating ability of the culture increased dramatically to 72% within the next 2 hr. Sensitive RT–PCR was able to detect MID transcript in vegetative cells at what will be referred to as basal levels. Its expression increased approximately threefold (level 1) at 30 min (Figure 2D, shadowed bars), returned to basal levels at 1 hr, and was then strongly upregulated to approximately eightfold (level 2) at 6 hr (Figure 2D) in concert with the augmentation of mating ability (Figure 2D, curve).
A second approach was taken to study in detail the upregulation of MID within the first 30 min. It has been shown that cells growing on TAP plates are uniformly vegetative after 3 days in culture (Martin and Goodenough 1975). Such 3-day vegetative cells were washed into N-free medium, collected at different time points, and subjected to RT–PCR. Consistent with the results from Figure 2B, basal levels of MID message were detected in the vegetative cells; levels increased at 30 min to approximately twofold those of the basal levels, and abated at 60 min (Figure 2, C and D, diagonal bars).
An RPA was employed to detect the MID transcripts in various strains in both vegetative and gametic cells. As shown in Figure 2E, and consistent with results summarized in Figure 2D, a low level of MID mRNA was found in wild-type minus vegetative cells, with strong expression (approximately fourfold) in mature wild-type minus gametes.
Basal levels of MID transcripts were also observed in mid-1 vegetative cells, with a slightly upregulated message (approximately twofold) in mature mid-1 gametic cells. Ferris and Goodenough (1997) found that the mid-1 mutant carries two very close nucleotide changes within the coding region of MID: one leads to a synonymous change while the other leads to the change of a single amino acid, conserved within all the RWP-RK proteins reported here, from lysine to isoleucine. These nucleotide changes caused mismatches between the in vitro-transcribed antisense MID probe and the endogenous MID message in mid-1; therefore two shorter MID fragments were observed. Observation of the MID messages at the basal levels in vegetative cells suggests that the mutations within the coding region do not affect its initial transcription. However, upregulation of the MID message from its basal levels in mid-1 gametes (approximately twofold) is not as robust as that in wild-type minus gametes (approximately fourfold), suggesting that the mutations may affect some feature of its stability.
Also shown in Figure 2E are results with the iso1 mt− strain. This mutant, described in Campbell et al. (1995), displays an isoagglutinating gametic phenotype because cells in a clonal population differentiate as either minus or pseudo-plus. Since MID is not expressed in the pseudo-plus cells, the mixed cell population fails to display evidence of MID upregulation from basal levels.
Expression levels of Mid correlate with mating efficiency:
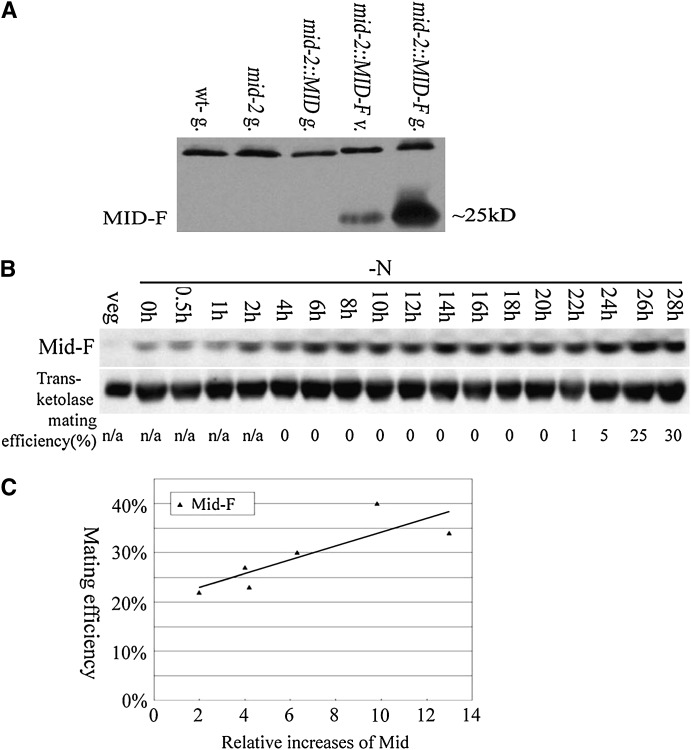
Experiments were next performed to evaluate the relationship between Mid protein levels and gametogenesis. Although anti-Mid antibody is able to detect Mid (Figure 2A), it also recognizes many other bands in immunoblots even after affinity purification and/or preabsorption with plus gametic proteins. Therefore, nine copies of an epitope tag, FLAG, were introduced to the C terminus of Mid right before the stop codon. Under the regulation of its own promoter, either MID or MID-F was cotransformed into the MID-deletion mutant mid-2 with pSI103, which provides paromomycin resistance. In previous studies with the missense mid-1 mutant (Ferris and Goodenough 1997), MID transformation was found to yield only partial rescue: some cells differentiated as minus while the rest continued to differentiate as pseudo-plus, the result being clonal isoagglutination. The same outcome was obtained with mid-2 transformants: 1 of 88 paromomycin-resistant colonies from the MID transformation displayed an isoagglutination phenotype, and 1 of 183 paromomycin-resistant colonies from the MID-F transformation displayed isoagglutination. Such partial rescue presumably reflects the sensitivity of minus differentiation to MID expression levels (Figure 2, B–D) and the failure to achieve full expression in exogenous integration sites.
An ∼25-kDa protein (size change due to nine copies of FLAG) was recognized by anti-FLAG antibody in MID-F transformants at both vegetative and gametic stages (Figure 3A, mid-2∷MID-F v. and g.) with the level of Mid-F strongly upregulated in fully differentiated gametes. This protein was absent in wild-type minus, mid-2, or mid-2 gametes transformed with non-epitope-tagged MID (Figure 3A).
Figure 3.—
Mid protein levels related to minus mating efficiency. (A) An anti-FLAG antibody was used to detected FLAG-tagged Mid in mid-2∷MID-F cells. Gametes from wild-type minus, mid-2, and mid-2∷MID (a mid-2 transformant carrying MID without FLAG tag) were used as negative controls. The top ∼35-kDa band in all lanes was due to cross-hybridization between the anti-FLAG antibody and an unknown Chlamydomonas protein and served as a loading control. (B) mid-2∷MID-F expression in synchronous culture. The anti-FLAG antibody was used to detect Mid-FLAG; an anti-transketolase antibody was used to detect transketolase as an internal control. Mating efficiencies of cells when samples were collected were standardized using mating efficiencies of wild-type tester cells. (C) Relationship between the amount of Mid-F and the mating efficiency of mature mid-2∷MID-F cells. Six individual mid-2∷MID-F subclones were grown synchronously and the mating abilities of individual subclones were tested at 28 hr after nitrogen removal. The increases of Mid-F levels at 28 hr were obtained by quantitation of the Mid-F protein signal with the transketolase signals and standardized by the relative amount of Mid in corresponding vegetative cells. The relative increases of Mid-F were plotted against the mating efficiency of individual mid-2∷MID-F subclones.
This mid-2∷MID-F transformant was used to analyze the relationship between the level of Mid expression and mating ability. The strain proved to undergo gametogenesis slowly in synchronous culture and to mate with low efficiency even after long periods (>20 hr) in nitrogen-free medium due to its mixed minus/pseudo-plus phenotype. In addition, individual subclones isolated from the same strain displayed different mating abilities at a given time point after nitrogen removal. Six individual subclones were grown synchronously and cell samples were taken at different time points after nitrogen deprivation. As detected by immunoblots, Mid-F protein increased gradually even though mating capacity was not observed until >20 hr later. Figure 3B showed the expression pattern of Mid-F in one of the subclones. At 28 hr after nitrogen removal, when cells were considered to have reached maximum mating efficiency, the relative amounts of Mid-F in each subclone were quantitated against an internal transketolase control and further standardized by the relative amounts of Mid-F in vegetative samples, yielding an estimate of the relative increase of Mid in each subclone. These values were plotted against the mating efficiencies of individual subclones at 28 hr after nitrogen deprivation (Figure 3C). Although the values reflect population levels and give no indication of the Mid levels attained in individual cells, the experiment demonstrates that MID protein levels indeed correlate with the ability to differentiate as minus gametes.
Expression of MTD1 during gametogenesis:
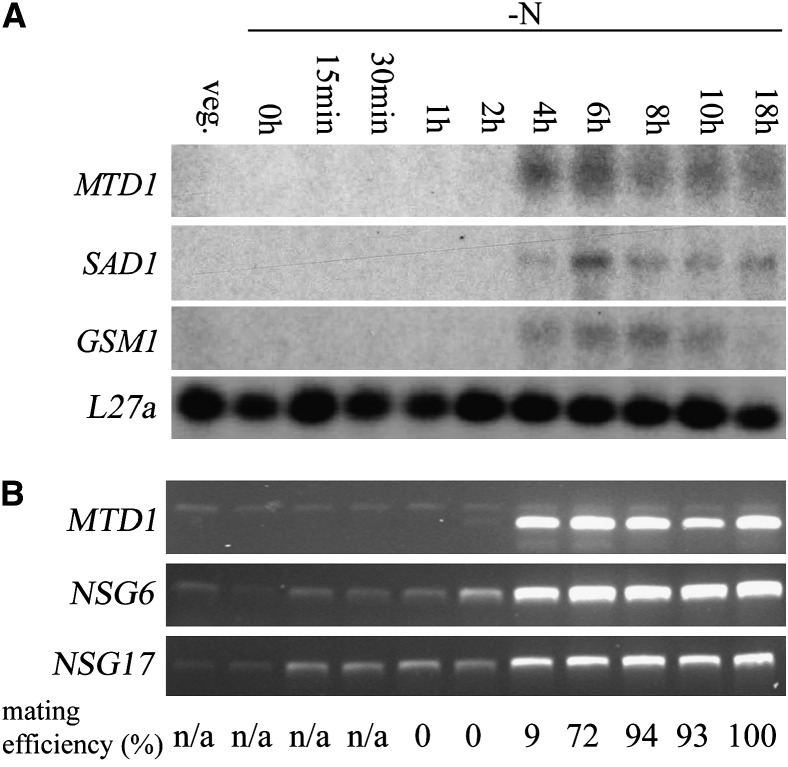
We next turned to the MTD1 gene. Previous studies using Northern blotting showed that expression of MTD1 was restricted to minus gametes (Ferris et al. 2002). To follow expression during synchronous gametogenesis, we used the same RNA samples as in Figure 2B but employed Northern blotting. MTD1 expression was activated 4 hr after cells were transferred to nitrogen-free medium, coincident with the onset of mating ability (Figure 4A). It was also coincident with the onset of the expression of two other minus gamete-specific genes, SAD1 and GSM1 (Figure 4A), but whereas MTD1 expression reaches a sustained plateau level at 4 hr, SAD1 and GSM1 expression do not reach their maximum until 2 hr later, and expression abates with time.
Figure 4.—
Transcription of MTD1 during gametogenesis. (A) Total RNA samples were prepared as in Figure 2B. Ten micrograms of total RNA were loaded in each lane. A membrane was probed with MTD1, stripped, and reprobed with SAD1, GSM1, and L27a, sequentially. SAD1 encodes minus agglutinin; GSM1 encodes a homeodomain protein expressed in minus gametes. L27a, encoding a 60S ribosomal protein, serves as a loading control. (B) RT–PCR results of MTD1 and two nitrogen-starved gametogenesis (NSG) genes, NSG6 and NSG17. mRNA samples were prepared as in Figure 2B. The top band in the MTD1 lanes derives from contaminating genomic DNA. Mating efficiencies of cells when samples were collected are standardized using mating efficiencies of wild-type tester cells.
Recent studies on synchronous gametogenesis in plus C. reinhardtii strains (Abe et al. 2004, 2005) identified 18 novel nitrogen-starved gametogenesis (NSG) genes that were assigned to three temporal classes: early, middle, and late. Among them, NSG17 was fully induced within 1 hr after cells were transferred to nitrogen-free medium and therefore classified as an early gene, while NSG6 was induced between 3 and 4 hr and placed in the middle class (Abe et al. 2004). To determine in which class MTD1 belongs, expression patterns of MTD1, NSG17, and NSG6 were evaluated using RT–PCR (Figure 4B). Consistent with our Northern blotting results (Figure 4A), activation of MTD1 expression starts at 4 hr, placing it in the middle class. The NSG6 pattern agreed with the published data for plus cells (Abe et al. 2004): very weak expression up to 1 hr (barely detectable by Northern blotting); a relatively weak upregulation (∼10-fold) at 2 hr; and an ∼30-fold upregulation compared to vegetative cells at 4 hr and after. By contrast, NSG17 expression differed from the data published for plus cells, showing a pattern of relative weak upregulation (∼10-fold) between 15 min and 2 hr and strong upregulation (∼40-fold) at 4 hr and after. It is not yet known whether this reflects a plus/minus difference or results from different experimental conditions in the two laboratories.
RNAi demonstrates an essential role for MTD1 in minus gametogenesis:
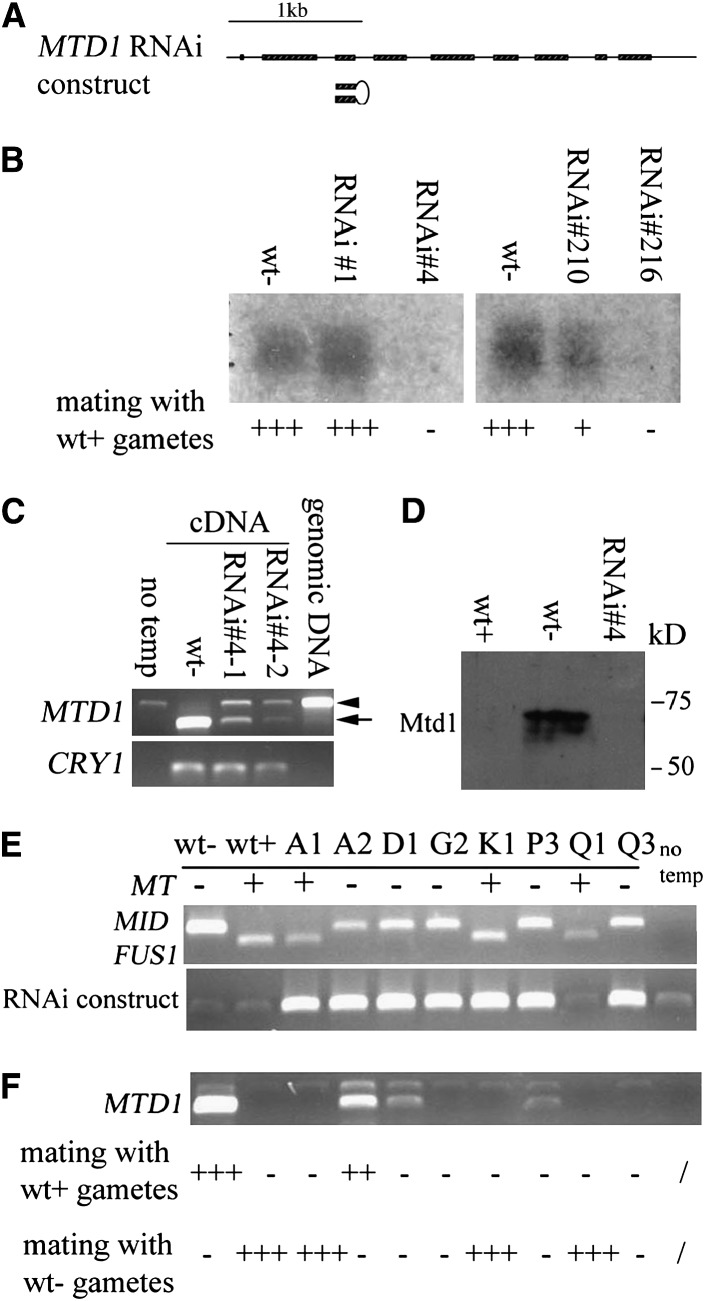
MTD1 function was evaluated using RNAi (Sineshchekov et al. 2002; Koblenz et al. 2003). A hairpin RNAi plasmid containing inverted pairs of the third exon of MTD1, with the third intron serving as the middle loop, was driven by the constitutive HSP70A/rbcS2 promoter (Figure 5A) (Schroda et al. 2000; Koblenz et al. 2003). This RNAi construct was cotransformed into wild-type mt− cells with pSI103. From two individual transformation experiments, 195 and 168 paromomycin-resistant colonies were isolated. Crude DNA was extracted from each colony and subjected to a PCR screen targeting the HSP70A/rbcS2 promoter. Four individual clones were identified: #1 and #4 from the first transformation and #210 and #216 from the second transformation. Among them, #4 and #216 showed no agglutination or fusion with either wild-type plus or wild-type minus gametes. Addition of db-cAMP did not change the nonmating phenotype of these transformants, indicating that, like MID, MTD1 expression is cAMP independent. Prolonged incubation with plus gametes overnight did not lead to any zygote formation. Gametes of a third strain (#210) showed weak agglutination and fusion with plus gametes and ∼1/3 of number of wild-type zygotes with overnight incubation, while a fourth (#1) mated normally, and produced normal zygote levels. As shown in Figure 5B, levels of MTD1 message in the various strains were strongly correlated with mating ability: strong MTD1 transcripts were found in both wild-type minus and MTD1 RNAi #1 gametes; reduced level of MTD1 in #210; and no MTD1 message found in #4 and #216, which had no mating ability.
Figure 5.—
RNAi of MTD1 in minus cells. (A) Structure of the MTD1 gene and RNAi construct. Boxes, exons; lines, 5′-UTR, introns, and 3′-UTR. RNAi hairpin structure is presented by inverted pairs of exon 3 with intron 3 serving as the loop. The construct was driven by a constitutive HSP70A/rbcS2 promoter. (B) Northern blotting of MTD1 levels in different RNAi lines. The mating abilities of individual lines with wild-type plus gametes are indicated: +++, strong mating efficiency (80–100%); +, weak mating efficiency (20–50%); −, no mating. (C) RT–PCR of MTD1 in strain 4. Poly(A) RNA was isolated from two different cultures (#4-1 and #4-2). The arrow indicates the amplified cDNA fragment. The arrow head represents a weak contaminating genomic DNA fragment amplified in PCR. CRY1 is used as an internal control. The “no temp” control served as a negative control for PCR with no addition of DNA template. “Genomic DNA” served as a positive control for PCR by using genomic DNA as template. (D) Western blotting of Mtd1 in wild type and in the Mtd knockdown strain 4. (E) PCR of genomic DNA from progeny obtained from a cross between wt+ and strain 4. (Top) PCR of MID and FUS1 to determine mating types of individual progeny. (Bottom) PCR to detect the existence of RNAi construct. “No temp,” no DNA template control. (F) RT–PCR to detect the expression level of MTD1 in these progeny. The mating efficiency of individual progeny with either wild-type plus or wild-type minus gametes is indicated: +++, strong mating efficiency (80–100%); ++, moderate mating efficiency (50–80%); +, weak mating efficiency (20–50%); −, no mating.
Strain #4 was selected for further study. RT–PCR was performed to detect any weak level of MTD1 missed by Northern blotting. RNA was isolated from two different cultures of strain #4 at different times. Both samples show strongly reduced levels of MTD1 (Figure 5C, arrow)—∼1/10 of that in wild-type minus gametes—while the control message, CRY1, displayed a similar expression level in all three samples (Figure 5C). [An additional band is observed in the RNAi samples (Figure 5C, arrowhead) with the same size as genomic DNA amplified by the same set of primers. Since this band is also present in the negative PCR control (no template), it suggests that some genomic DNA contaminated the PCR reaction. This contaminating genomic DNA is not observed in the wild-type minus sample, presumably either because it is masked by the intensive signal from the amplified cDNA copy of MTD1 or because the abundant cDNA template overwhelmed the low-abundance genomic DNA contamination for binding and amplification by PCR primers.]
Consistent with the RNA results, immunoblotting using anti-Mtd1 antibody showed an ∼73-kDa protein in wild-type minus gametes but not in plus or strain #4 gametes (Figure 5D). The size of the detected protein was somewhat larger than the calculated molecular weight of 64.7 kDa, presumably due to glycosylation on putative N-glycosylation sites (Ferris et al. 2002).
Two months after isolation, the original RNAi lines had gradually recovered, with increasing levels of MTD1 correlating with increased mating ability. Such RNAi instability has been observed in mice (Bartlett and Davis 2006) and in Chlamydomonas (Koblenz et al. 2003). Strain #4 was therefore backcrossed to wild-type plus, and progeny were screened by PCR to amplify the HSP70A/rbcs2 promoter in the RNAi construct. The mating types of these progeny were determined by amplification of both MID and FUS1 from crude genomic DNA extract: all the plus progeny contained the plus-specific FUS1 gene only, while all the minus progeny carried MID only. Analyses of several progeny are shown in Figure 5, E and F: five minus progeny strains carrying the construct (A2, D1, G2, P3, Q3) displayed various levels of mating ability: one (A2) agglutinated and fused with wild-type plus but not with wild-type minus gametes, while the rest displayed little or no agglutination and fusion ability with either plus or minus testers (Figure 5F, bottom). RT–PCR revealed that transcription of MTD1 was greatly reduced or absent in those four progeny but only slightly reduced in A2 (Figure 5F). Two plus progeny carrying the construct (A1, K1) agglutinated and fused normally with wild-type minus gametes but not with wild-type plus gametes (Figure 5F, bottom), indicating that the presence of the construct per se is not toxic to gametic differentiation. The G2, P3, and K1 strains were used in subsequent studies.
MID deletion and MTD1 knockdown affect minus gamete-specific gene expression:
The inability of MTD1 knockdowns to agglutinate as minus indicates a block in Sad1 agglutinin synthesis. This mimics the phenotype of mid mutants with one important difference: in mid mutants, SAG1 expression occurs instead, and the gametes agglutinate as plus, whereas in the MTD1 knockdowns, no agglutinin of either type is made. It therefore became important to understand the relationship between MID and MTD1.
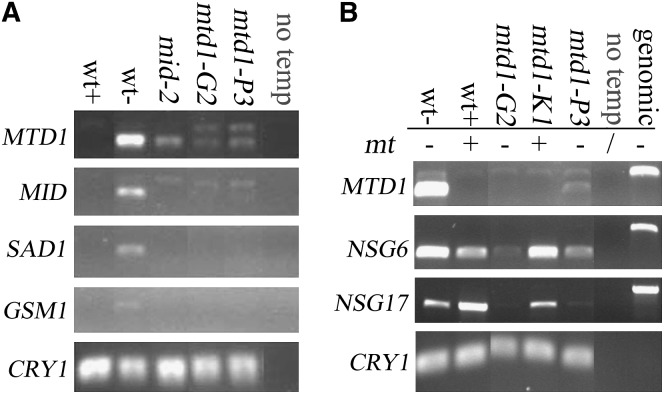
RT–PCR was performed to detect MID, MTD1, SAD1, and GSM1 expression in wild type, in mid mutants, and in MTD1 knockdowns. As shown in Figure 6A, expression of MTD1 is greatly reduced, but not eliminated, in mid-2 cells, suggesting that Mid strongly influences MTD1 expression but that a weak Mid-independent MTD-1 expression pathway exists as well. Expression of MID was also greatly reduced in MTD1 knockdowns, suggesting that Mtd1 is a determinant of strong (level 2) MID expression. Expression of SAD1 and GSM1 was inhibited in both mid-2 and MTD1 knockdowns, indicating that their expression is dependent on both Mid and Mtd1. Given that MID has two activation stages during gametogenesis (Figure 2, B–D), these observations generate the proposal (see discussion) that the very early activation of MID by nitrogen starvation may act as a positive regulator of MTD1 and that MTD1 expression in turn leads to a second increase of MID to its threshold level, which is necessary to activate SAD1 and GSM1.
Figure 6.—
Effects of MID mutation and MTD1 knockdown on expression of some gamete-specific genes. (A) RT–PCR of various minus-specific genes in wild type, mid-2, and MTD1 RNAi minus progeny G2 and P3. CRY1 is used as an internal control. “No temp,” negative control in PCR with no addition of DNA template. (B) RT–PCR to detect expression levels of NSG6 and NSG17 in different MTD1 RNAi progeny. “No temp,” negative control in PCR with no addition of DNA template. “Genomic,” positive control in PCR using genomic DNA as templates.
We also tested the effect of MTD1 knockdown on the expression of NSG6 and NSG17. RT–PCR (Figure 6B) revealed that NSG6 and NGS17 were affected in minus knockdown strains G2 and P3, but not in the plus (K1) strain carrying the RNAi construct, as expected. Thus expression of these two NSG genes during minus gametogenesis is also influenced by Mtd1 levels.
DISCUSSION
Responses to ammonium depletion in Chlamydomonas:
Adverse environmental conditions such as nitrogen deprivation commonly trigger gametogenesis in algae (Sager and Granick 1954; Harris 1989) and in fungi such as Schizosaccharomyces pombe (Mochizuki and Yamamoto 1992; Breeding et al. 1998; Davey 1998) and Candida lusitaniae (Young et al. 2000). In fission yeast, nitrogen starvation stimulates activation of a heterotrimeric G protein followed by activation of adenylate cyclase, with cAMP activating the signal transduction pathway leading to sexual development (Davey 1998). By contrast, in C. reinhardtii, nitrogen starvation activates the expression of gamete-specific genes, while sexual agglutination activates a cAMP-dependent signal transduction pathway that triggers subsequent events in the mating reaction (Pasquale and Goodenough 1987; Goodenough 1989; Saito et al. 1993).
Ammonium depletion has been shown to trigger three changes in vegetative C. reinhardtii cells:
A rapid (within 1 hr) transcriptional activation of genes involved in nitrate utilization that are repressed by ammonium [NIA1-encoding nitrate reductase (Loppes et al. 1999), NRT2;1-encoding nitrate transporters (Quesada et al. 1994), and NIT2, a positive regulator of nitrate-assimilation genes (Schnell and Lefebvre 1993)].
An onset of massive protein and nucleic acid catabolism (Beck and Haring 1996) and ribosome degradation (Martin et al. 1976), accompanied by an early (within 1–3 hr) expression of genes encoding proteasome subunits (Abe et al. 2004), amino acid oxidase (Vallon et al. 1993), urate oxidase (Merchan et al. 2001), and other proteins presumably involved in this catabolic response (Abe et al. 2004). The outcome of this is that cells enter a stable G0 stage in which they can survive for weeks without an exogenous nitrogen source. Following the terminology of Abe et al. (2005), we refer to this as the N-adaptation program.
A later (3–4 hr) expression of genes encoding proteins necessary for mating and early zygote development, hereafter called the gamete program (Abe et al. 2004). These changes are fully reversible: gametes provided ammonium will redifferentiate into cycling vegetative cells within 18 hr (Sager and Granick 1954; Goodenough 1991).
The fact that many of the genes involved in the N-adaptation program are expressed prior to most of the genes involved in the gamete program suggests that the former are responsive to transcription factors that are directly activated by ammonium depletion and that the latter are responsive to transcription factors that are expressed or activated as a consequence of some feature(s) of the N-adaptation program. Such a two-stage model helps to clarify an otherwise puzzling facet of the process: once catabolism is underway, intracellular ammonium levels presumably rise, dampening the N-starvation signal, but gametogenesis is able to proceed because it is regulated by a system acting downstream of N-starvation.
Distinctive features of the MID gene and Mid protein:
The MID gene, resident in the MT− locus and required for expression of minus-specific mating- and zygote-related genes, is shown here to have a unique expression pattern:
Unlike most gamete-specific genes, including MTD1, MID is expressed at low (basal) levels in minus vegetative cells.
MID undergoes a small transient rise in expression (to level 1) within 30 min of ammonium depletion, in concert with the ammonium-repressed and N-adaptation-program genes.
Several hours later, MID undergoes a second, sustained increase in expression (to level 2) in concert with the onset of mating-related minus gene expression. The upregulation to level 2, which we propose serves as a threshold level, is required to drive the expression of minus-specific genes necessary for agglutination and fusion, possibly because their cis-regulatory elements have relatively low Mid affinity.
These observations indicate that MID expression is under complex regulation: basal expression in vegetative cells is possibly constitutive; level 1 expression appears to be a direct response to ammonium depletion; and level 2 expression is presumably dependent on features instantiated by a pathway downstream of the initial ammonium-withdrawal response.
A second feature of the Mid protein is also documented in this report: Of the 15 genes (13 of unknown function) in the C. reinhardtii genome that encode proteins with an RWP-RK motif, the Mid sequence alone carries a contiguous additional set of seven conserved amino acids found in the N-sensitive Nit2 transcription factor of C. reinhardtii (Schnell and Lefebvre 1993; Galvan and Fernandez 2001) and in transcription factors involved in nitrogen-deprivation-induced nodulation events in lotus (Schauser et al. 1999) and pea (Borisov et al. 2003). Hence, MID possesses two properties of a protein influenced by nitrogen limitation: upregulated expression in response to ammonium depletion and a protein motif that possibly adopts distinctive configurations in response to ammonium levels. The fact that vegetative cells express basal levels of Mid is consistent with the possibility that Mid may play some role in the ability of minus vegetative cells to sense the occurrence of ammonium depletion.
Our study also clarifies a third feature of the MID system. Previous reports showed that whereas deletion (mid-2) or loss of function (mid-1) of Mid disallows minus-specific gene expression, plus-specific gene expression is not affected, and the cells differentiate as pseudo-plus gametes, lacking only those plus functions that are encoded in the absent MT+ locus (Ferris et al. 1996; Ferris and Goodenough 1997). This observation has been open to two interpretations: (1) Mid acts both as a transcriptional activator of minus genes and as a transcriptional repressor of plus genes or (2) the plus program is the “default” program expressed when the minus program fails without positing any direct repressor activity for Mid. We show here that in an MTD1-knockdown background, wherein functional Mid protein is expressed at low (but not high) levels, plus genes fail to be expressed, in contrast to their full expression when Mid is absent or nonfunctional. This observation strongly suggests that low levels of Mid are adequate for preventing the expression of plus genes.
The role of Mtd1 in minus gametogenesis:
Sex-determination systems are typically complex, an example being the elaborate interplay between SRY and Sox-9 in mammalian testis determination (Koopman 1999; Kanai et al. 2005). This study indicates that sex determination in Chlamydomonas entails a similarly complex pattern of gene regulation.
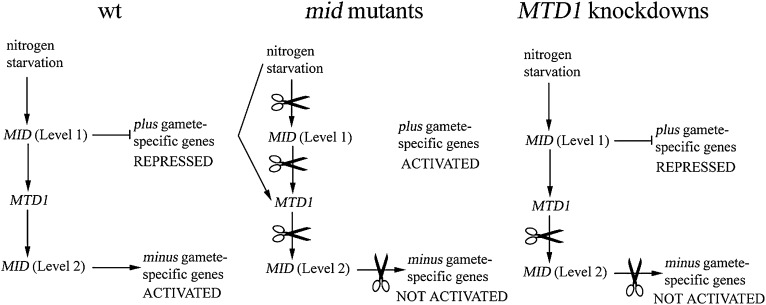
Our working model is shown in Figure 7. Upregulation of MID to level 1 immediately follows nitrogen depletion and leads to activation of MTD1 expression, which in turn leads to the second stage of MID activation, level 2. Level 1 is sufficient for preventing expression of plus gamete-specific genes while level 2 represents the threshold necessary to activate minus gamete-specific genes. When MID undergoes loss-of-function mutations, expression of plus gamete-specific genes is not prevented, nor does activation of minus-specific genes occur. As a result, cells differentiate as pseudo-plus gametes. Expression of MTD1 is strongly reduced but not eliminated in the mid mutants, indicating that nitrogen deprivation controls the expression of MTD1 via both MID-dependent and -independent pathways. On the other hand, when MTD1 is knocked down, cells fail to differentiate as either pseudo-plus or minus gametes: plus gamete-specific genes are not expressed due to the level 1 presence of MID, and minus gamete-specific genes are not induced since level 2 cannot be reached. That is, a threshold level of MTD1 is apparently required for the critical second stage of MID activation in minus cells.
Figure 7.—
Proposed model of gametogenesis in minus cells. In wild-type (wt) cells, plus gamete-specific genes are repressed and minus gamete-specific genes are activated by MID. In mid mutants, loss of function of MID fails to repress plus gamete-specific genes and activate minus genes; therefore cells differentiate into pseudo-plus cells. Nitrogen starvation is able to activate MTD1 through a MID-independent pathway. In MTD1 knockdowns, low levels (level 1) of MID are able to repress plus gamete-specific genes. However, MID cannot reach its threshold level (level 2) to activate minus gamete-specific genes due to the defect in MTD1. Therefore, cells fail to display any gametic phenotypes.
This model does not account for two published observations: An mt+ strain carrying the MID gene transposed to an autosome differentiates as minus, as do mt+ cells transformed with the MID gene, even though neither possesses a copy of the MTD1 gene (Ferris and Goodenough 1997). To reconcile these observations with the results reported here, we are led to propose that plus gametes express a system, the “MTD1-equivalent system,” that is functionally equivalent to the “MTD1 system” but achieves this outcome without requiring the Mtd1 protein itself. When MID is introduced into a plus background, the MTD1-equivalent system enables sufficiently high MID expression to allow transformants to undergo minus differentiation, albeit success is usually incomplete (see results and Ferris and Goodenough 1997), meaning that the MTD1-equivalent system is not repressible by Mid. Importantly, at least one essential gene in the posited plus MTD-equivalent system must be resident in the MT+ locus. If the system were fully encoded elsewhere in the genome and Mid repressible, then the mt+ cells carrying a MID gene would fail to differentiate. If it were fully encoded elsewhere in the genome and not Mid repressible, then MTD1 knockdowns would presumably be complemented by this second system and would not have a mating-null phenotype.
That plus cells possess an MTD-equivalent system is also indicated by the phenotypes of two mutant strains, the conditional dif2 and the nonconditional dif3, both studied in a plus background (Abe et al. 2005). In the dif2 mutant, expression of all known N-adaptation genes and of one plus-specific gamete-program gene (FUS1) is blocked at restrictive temperature and the cells are unable to mate; gene expression and mating ability occur normally with temperature downshift. In dif3, three N-adaptation genes (NSG3, NSG6, and NSG7) and FUS1 are not expressed, and gametogenesis also fails. When dif2 is crossed into an mt− background, the nondifferentiation phenotype is also observed (Saito and Matsuda 1991); therefore, dif2 does not correspond to the posited gene linked to mt+. The dif3 mutant is sterile and cannot be tested for mt+ linkage.
The MT+ locus contains no ORF with any similarity to MTD1, and indeed MTD1 lacks homology with any known gene. Hence the posited plus MTD-equivalent system must be specified in a fashion different from the minus system. The one gene currently known to be unique to the MT+ locus—expressed exclusively in gametes, not repressible by Mid (Ferris et al. 2002), and not yet assigned a function—is MTA1, which encodes a small protein with a predicted coiled-coil domain and repeated motifs predicted to form a leucine histidine zipper (Ferris et al. 2002). This is a totally different species from Mtd1, which is predicted to be a transmembrane protein (Ferris et al. 2002). Experiments to test the phenotype of MTA1 knockdowns are clearly of high priority. Alternatively, the posited MT+-linked gene may await identification.
Of high priority as well is the ascertainment of the cellular location of Mtd1 since, despite considerable effort, our antibodies have failed to generate definitive immunolocalization. Given its predicted three-spanner transmembrane configuration with N-glycosylation sites, however, one can reasonably speculate that Mtd1 functions to monitor or respond to features of the external environment, the obvious feature being nitrogen status.
This study provides an answer to a puzzle pertaining to the sex-determination system of C. reinhardtii. When it was assumed that MID was the sole determinant of mating type, it was not obvious why C. reinhardtii possesses complex MT loci under recombinational repression. Would it not be sufficient that cells carrying the MID gene differentiate as minus, and cells not carrying MID differentiate as plus? Our finding that MID and MTD1 are mutually dependent on one another for bringing about minus gametogenesis, and that at least one component of the posited complementary system in plus is encoded in the MT+ locus, indicates that it may be essential that MID and MTD1 remain in genetic linkage. If so, the puzzle shifts to the question of how such a system evolved in the first place.
Acknowledgments
We thank Christoph Beck for providing the mid-2 deletion strain and Yoshihiro Matsuda for providing antibodies against Chlamydomonas transketolase. We also thank Susan Dutcher, Patrick Ferris, Sabine Waffenschmidt, James Umen, Takeaki Kubo, and Jae-Hyeok Lee for their helpful advice. This study was supported by a grant (MCB-0326829) from the National Science Foundation to U.W.G. and in part from a Monsanto Fellowship to H.L.
References
- Abe, J., T. Kubo, Y. Takagi, T. Saito, K. Miura et al., 2004. The transcriptional program of synchronous gametogenesis in Chlamydomonas reinhardtii. Curr. Genet. 46 304–315. [DOI] [PubMed] [Google Scholar]
- Abe, J., T. Kubo, T. Saito and Y. Matsuda, 2005. The regulatory networks of gene expression during the sexual differentiation of Chlamydomonas reinhardtii, as analyzed by mutants for gametogenesis. Plant Cell Physiol. 46 312–316. [DOI] [PubMed] [Google Scholar]
- Bartlett, D. W., and M. E. Davis, 2006. Insights into the kinetics of siRNA-mediated gene silencing from live-cell and live-animal bioluminescent imaging. Nucleic Acids Res. 34 322–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck, C. F., and M. A. Haring, 1996. Gametic differentiation of Chlamydomonas. Int. Rev. Cytol. 168 259–302. [Google Scholar]
- Borisov, A. Y., L. H. Madsen, V. E. Tsyganov, Y. Umehara, V. A. Voroshilova et al., 2003. The Sym35 gene required for root nodule development in pea is an ortholog of Nin from Lotus japonicus. Plant Physiol. 131 1009–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breeding, C. S., J. Hudson, M. K. Balasubramanian, S. M. Hemmingsen, P. G. Young et al., 1998. The cdr2(+) gene encodes a regulator of G2/M progression and cytokinesis in Schizosaccharomyces pombe. Mol. Biol. Cell 9 3399–3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell, A. M., H. J. Rayala and U. W. Goodenough, 1995. The iso1 gene of Chlamydomonas is involved in sex determination. Mol. Biol. Cell 6 87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castrucci, M. R., P. Bilsel and Y. Kawaoka, 1992. Attenuation of influenza A virus by insertion of a foreign epitope into the neuraminidase. J. Virol. 66 4647–4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church, G. M., and W. Gilbert, 1984. Genomic sequencing. Proc. Natl. Acad. Sci. USA 81 1991–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey, J., 1998. Fusion of a fission yeast. Yeast 14 1529–1566. [DOI] [PubMed] [Google Scholar]
- Ferris, P. J., and U. W. Goodenough, 1994. The mating-type locus of Chlamydomonas reinhardtii contains highly rearranged DNA sequences. Cell 76 1135–1145. [DOI] [PubMed] [Google Scholar]
- Ferris, P. J., and U. W. Goodenough, 1997. Mating type in Chlamydomonas is specified by mid, the minus-dominance gene. Genetics 146 859–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris, P. J., J. P. Woessner and U. W. Goodenough, 1996. A sex recognition glycoprotein is encoded by the plus mating-type gene fus1 of Chlamydomonas reinhardtii. Mol. Biol. Cell 7 1235–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris, P. J., E. V. Armbrust and U. W. Goodenough, 2002. Genetic structure of the mating-type locus of Chlamydomonas reinhardtii. Genetics 160 181–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris, P. J., S. Waffenschmidt, J. G. Umen, H. Lin, J.-H. Lee et al., 2005. Plus and minus sexual agglutinins from Chlamydomonas reinhardtii. Plant Cell 17 597–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galloway, R. E., and U. W. Goodenough, 1985. Genetic analysis of mating locus linked mutations in Chlamydomonas reinhardtii. Genetics 111 447–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan, A., and E. Fernandez, 2001. Eukaryotic nitrate and nitrite transporters. Cell. Mol. Life Sci. 58 225–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodenough, U. W., 1989. Cyclic AMP enhances the sexual agglutinability of Chlamydomonas flagella. J. Cell Biol. 109 247–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodenough, U. W., 1991. Chlamydomonas mating interactions, pp. 71–112 in Microbial Cell-Cell Interactions, edited by M. Dworkin American Society for Microbiology, Washington, DC.
- Graves, J. A., 2006. Sex chromosome specialization and degeneration in mammals. Cell 124 901–914. [DOI] [PubMed] [Google Scholar]
- Harlow, E., and D. Lane, 1988. Antibodies: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Harris, E. H., 1989. The Chlamydomonas Sourcebook: A Comprehensive Guide to Biology and Laboratory Use. Academic Press, San Diego. [DOI] [PubMed]
- Kanai, Y., R. Hiramatsu, S. Matoba and T. Kidokoro, 2005. From SRY to SOX9: mammalian testis differentiation. J. Biochem. 138 13–19. [DOI] [PubMed] [Google Scholar]
- Kindle, K. L., 1990. High-frequency nuclear transformation of Chlamydomonas reinhardtii. Proc. Natl. Acad. Sci. USA 87 1228–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koblenz, B., J. Schoppmeier, A. Grunow and K.-F. Lechtreck, 2003. Centrin deficiency in Chlamydomonas causes defects in basal body replication, segregation and maturation. J. Cell Sci. 116 2635–2646. [DOI] [PubMed] [Google Scholar]
- Koopman, P., 1999. Sry and Sox9: mammalian testis-determining genes. Cell. Mol. Life Sci. 55 839–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar, S., K. Tamura and M. Nei, 2004. MEGA3: integrated software for molecular evolutionary genetics analysis and sequence alignment. Brief. Bioinformatics 5 150–163. [DOI] [PubMed] [Google Scholar]
- Kurvari, V., N. V. Grishin and W. J. Snell, 1998. A gamete-specific, sex-limited homeodomain protein in Chlamydomonas. J. Cell Biol. 143 1971–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli, U. K., 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227 680–685. [DOI] [PubMed] [Google Scholar]
- Loppes, R., M. Radoux, M. C. Ohresser and R. F. Matagne, 1999. Transcriptional regulation of the Nia1 gene encoding nitrate reductase in Chlamydomonas reinhardtii: effects of various environmental factors on the expression of a reporter gene under the control of the Nia1 promoter. Plant Mol. Biol. 41 701–711. [DOI] [PubMed] [Google Scholar]
- Martin, N., and U. Goodenough, 1975. Gametic differentiation in Chlamydomonas reinhardtii. I. Production of gametes and their fine structure. J. Cell Biol. 67 587–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, N. C., K. S. Chiang and U. W. Goodenough, 1976. Turnover of chloroplast and cytoplasmic ribosomes during gametogenesis in Chlamydomonas reinhardtii. Dev. Biol. 51 190–201. [DOI] [PubMed] [Google Scholar]
- Merchan, F., H. van den Ende, E. Fernandez and C. F. Beck, 2001. Low-expression genes induced by nitrogen starvation and subsequent sexual differentiation in Chlamydomonas reinhardtii, isolated by the differential display technique. Planta 213 309–317. [DOI] [PubMed] [Google Scholar]
- Misamore, M. J., S. Gupta and W. J. Snell, 2003. The Chlamydomonas Fus1 protein is present on the mating type plus fusion organelle and required for a critical membrane adhesion event during fusion with minus gametes. Mol. Biol. Cell 14 2530–2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki, N., and M. Yamamoto, 1992. Reduction in the intracellular cAMP level triggers initiation of sexual development in fission yeast. Mol. Gen. Genet. 233 17–24. [DOI] [PubMed] [Google Scholar]
- Pasquale, S. M., and U. W. Goodenough, 1987. Cyclic AMP functions as a primary sexual signal in gametes of Chlamydomonas reinhardtii. J. Cell Biol. 105 2279–2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quesada, A., A. Galvan and E. Fernandez, 1994. Identification of nitrate transporter genes in Chlamydomonas reinhardtii. Plant J. 5 407–419. [DOI] [PubMed] [Google Scholar]
- Sager, R., and S. Granick, 1954. Nutritional control of sexuality in Chlamydomonas reinhardtii. J. Gen. Physiol. 37 729–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito, T., and Y. Matsuda, 1991. Isolation and characterization of Chlamydomonas temperature-sensitive mutants affecting gametic differentiation under nitrogen-starved conditions. Curr. Genet. 19 65–71. [DOI] [PubMed] [Google Scholar]
- Saito, T., L. Small and U. W. Goodenough, 1993. Activation of adenylyl cyclase in Chlamydomonas reinhardtii by adhesion and by heat. J. Cell Biol. 122 137–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook, J., and D. Russell, 2001. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Schauser, L., A. Roussis, J. Stiller and J. Stougaard, 1999. A plant regulator controlling development of symbiotic root nodules. Nature 402 191–195. [DOI] [PubMed] [Google Scholar]
- Schauser, L., W. Wieloch and J. Stougaard, 2005. Evolution of NIN-like proteins in Arabidopsis, rice, and Lotus japonicus. J. Mol. Evol. 60 229–237. [DOI] [PubMed] [Google Scholar]
- Schnell, R. A., and P. A. Lefebvre, 1993. Isolation of the Chlamydomonas regulatory gene NIT2 by transposon tagging. Genetics 134 737–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroda, M., D. Blöcker and C. F. Beck, 2000. The HSP70A promoter as a tool for the improved expression of transgenes in Chlamydomonas. Plant J. 21 121–131. [DOI] [PubMed] [Google Scholar]
- Shimogawara, K., S. Fujiwara, A. Grossman and H. Usuda, 1998. High-efficiency transformation of Chlamydomonas reinhardtii by electroporation. Genetics 148 1821–1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sineshchekov, O. A., K.-H. Jung and J. L. Spudich, 2002. Two rhodopsins mediate phototaxis to low- and high-intensity light in Chlamydomonas reinhardtii. Proc. Natl. Acad. Sci. USA 99 8689–8694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sizova, I., M. Fuhrmann and P. Hegemann, 2001. A Streptomyces rimosus aphVIII gene coding for a new type phosphotransferase provides stable antibiotic resistance to Chlamydomonas reinhardtii. Gene 277 221–229. [DOI] [PubMed] [Google Scholar]
- Thompson, J. D., T. J. Gibson, F. Plewniak, F. Jeanmougin and D. G. Higgins, 1997. The ClustalX windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25 4876–4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umen, J. G., and U. W. Goodenough, 2001. Control of cell division by a retinoblastoma protein homolog in Chlamydomonas. Genes Dev. 15 1652–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallon, O., L. Bulte, R. Kuras, J. Olive and F. A. Wollman, 1993. Extensive accumulation of an extracellular L-amino-acid oxidase during gametogenesis of Chlamydomonas reinhardtii. Eur. J. Biochem. 215 351–360. [DOI] [PubMed] [Google Scholar]
- Wilson, N. F., J. S. O'Connell, M. Lu and W. J. Snell, 1999. Flagellar adhesion between mt(+) and mt(−) Chlamydomonas gametes regulates phosphorylation of the mt(+)-specific homeodomain protein GSP1. J. Biol. Chem. 274 34383–34388. [DOI] [PubMed] [Google Scholar]
- Yang, J.-Y., and C. Widmann, 2001. Antiapoptotic signaling generated by caspase-induced cleavage of RasGAP. Mol. Cell. Biol. 21 5346–5358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young, L. Y., M. C. Lorenz and J. Heitman, 2000. A STE12 homolog is required for mating but dispensable for filamentation in Candida lusitaniae. Genetics 155 17–29. [DOI] [PMC free article] [PubMed] [Google Scholar]