Abstract
Mitochondrial dysfunction is involved in many neurodegenerative disorders in humans. Here we report mutations in a gene (designated levy) that codes for subunit VIa of cytochrome c oxidase (COX). The mutations were identified by the phenotype of temperature-induced paralysis and showed the additional phenotypes of decreased COX activity, age-dependent bang-induced paralysis, progressive neurodegeneration, and reduced life span. Germ-line transformation using the levy+ gene rescued the mutant flies from all phenotypes including neurodegeneration. The data from levy mutants reveal a COX-mediated pathway in Drosophila, disruption of which leads to mitochondrial encephalomyopathic effects including neurodegeneration, motor dysfunction, and premature death. The data present the first case of a mutation in a nuclear-encoded structural subunit of COX that causes mitochondrial encephalomyopathy rather than lethality, whereas several previous attempts to identify such mutations have not been successful. The levy mutants provide a genetic model to understand the mechanisms underlying COX-mediated mitochondrial encephalomyopathies and to explore possible therapeutic interventions.
MITOCHONDRIA are involved in several neurodegenerative diseases such as Parkinson's disease (Abou-Sleiman et al. 2006; Keeney et al. 2006), Alzheimer's disease (Atamna and Boyle 2006; Esposito et al. 2006), and Huntington's disease (Choo et al. 2004; Benchoua et al. 2006). In addition, several disorders arise directly from mutations that disrupt mitochondrial function. These include Leber hereditary optic neuropathy (LHON), mitochondrial encephalopathy with lactic acidosis and stroke-like episodes (MELAS), myoclonic epilepsy with ragged red fibers (MERRF), chronic progressive external ophthalmoplegia and Kearns Sayre syndrome (CPEO/KSS), myoneurogastrointestinal encephalopathy syndrome (MNGIE), mitochondrial DNA depletion syndrome (MDS), Barth syndrome, and Leigh syndrome (LS) (McFarland et al. 2002; DiMauro and Schon 2003).
Complex I (NADH dehydrogenase) genes are responsible for the majority of LHON cases. Reduced activities of complexes I and IV (cytochrome c oxidase) are commonly associated with MELAS, and mutations in the thymidine phosphorylase gene on chromosome 22 and multiple mtDNA mutations are commonly associated with MERRF (DiMauro 2004; Gropman 2004). Deficiencies in a variety of factors such as pyruvate dehydrogenase complex (PDHC), cytochrome c oxidase (COX), and ATPase6 give rise to LS, a genetically heterogeneous disorder (Dahl 1998). Mitochondrial copy number is a central characteristic of MDS (Gropman 2004) and is linked to MNGIE (Giordano et al. 2006).
Mutations that disrupt molecular components of biochemical pathways allow identification of specific steps in such pathways and let us study the consequences of disrupting individual steps. Genetic tools available in Drosophila make it a useful model system for such studies. Drosophila has contributed significantly to our understanding of pathways involved in neuromuscular excitability and function under normal physiological conditions (Gu and Singh 1997; Bhattacharya et al. 1999; Ganetzky 2000; Bhattacharya et al. 2004; Ueda and Wu 2006). In addition, this model system is playing a significant role in analysis of pathways involved in neuromuscular dysfunction, especially in relation to neurodegenerative disorders (Fortini and Bonini 2000; Palladino et al. 2002; Bonini and Fortini 2003; Celotto et al. 2006; Gnerer et al. 2006; Pallanck and Greenamyre 2006). Here we report mutations that disrupted COX subunit VIa and resulted in several features of mitochondrial encephalomyopathies. Analysis of these mutations, of the phenotypes produced by them, and of the rescue from these phenotypes shows that COX plays a vital role in a pathway essential in maintaining neural integrity and that disruption of this pathway leads to encephalomyopathic effects including conditional paralysis, neurodegeneration, and early death.
MATERIALS AND METHODS
Fly strains and mutant isolation:
Canton-S (CS) was used as the wild-type strain in all experiments. Flies were raised on a standard cornmeal medium at 21° and aged at 21° or 29° as described in results. Flies carrying compound chromosomes (Chovnick et al. 1970; Singh 1983; Singh et al. 1987) were fed ethylmethanesulfonate according to the mutagenesis procedure of Lewis and Bacher (Lewis and Bacher 1968; Ashburner 1989), and the progeny were screened for paralysis on exposure to 38° for 5 min. This resulted in the identification of the levy1 mutation. Additional levy alleles (levy2, levy3, and levy4) were identified using P-element mobilization. Briefly, the P element in Dcp-1k05606 was induced to mobilize by providing transposase (Δ2-3). F1 flies with both P element and transposase were crossed with w*; levy1. The F2 progeny were screened for temperature-induced paralysis. After screening ∼10,000 F2 flies, 3 individual paralytic flies were isolated and the newly generated P-element mutations were balanced with CyO. Insertion sites for P elements were determined using inverse PCR according to the methods described by the Berkeley Drosophila Genome Project (BDGP) (Bellen et al. 2004).
Behavioral assays:
For paralysis test, a clear vial containing flies was submerged in water maintained at 38°. Time was recorded for flies to paralyze at 38° and to recover from paralysis after transfer to room temperature. Wild-type flies did not paralyze for at least 15 min under these conditions. For bang sensitivity, flies were placed in a clear vial and vortexed on a Vortex Genie-2 bench mixer (VWR) at its highest speed for 10 sec, followed by quantification of the time needed for paralyzed flies to stand up. To determine life span, flies were kept at either 29° or 21°. Flies were transferred to fresh food vials three times a week. Dead flies were counted every day and removed from the vials at the time of the next transfer.
Germ-line transformation:
A 2-kb CG17280 genomic DNA fragment was cloned into the pCaSpeR4 vector and injected into early embryos produced by white-eyed levy1 mutant flies (McKay et al. 1995). Transformants were identified on the basis of red eye color. In the control, the rescue construct was replaced with vector pCaSpeR4 without an added insert.
Measurement of cytochrome c oxidase and citrate synthase activity:
Microsomal fractions were prepared by grinding 30 adults in 2 ml of an extraction buffer containing 10 mm HEPES at pH 7.5, 200 mm mannitol, 70 mm sucrose, 1 mm EGTA, 0.1 mm phenylmethyl-sulfonyl fluoride, 0.25 mm dibucaine, and 1 mm benzamidine, using five strokes on a motorized Potter-Elvehjem tissue grinder operating at 200 rpm. Homogenates were microfuged at 600 × g for 5 min at 4° and the resulting supernatants microfuged at 14,000 rpm for 20 min to collect microsomal pellets. Microsomal pellets were suspended in 200 μl of 10 mm HEPES at pH 7.5, 250 mm sucrose, 1 mm ATP, 80 μm ADP, 5 mm sodium succinate, 2 mm potassium phosphate, and 1 mm dithiothreitol and stored at −70° until used. Protein concentrations in microsomal preparations were determined using a bicinchoninic acid (BCA) protein assay kit from Pierce Biochemical. COX activity in microsomal preparations was determined using a COX assay kit (CYTOC-OX1) from Sigma-Aldrich. Citrate synthase activity in mitochondrial preparations was assayed according to instructions provided by Sigma-Aldrich.
Measurement of ATP levels:
Extracts for ATP measurements were prepared by vigorously grinding a male and a female adult in 200 μl of 6 m guanidine hydrochloride using a small pestle that fits a microfuge tube, followed by collection of the liquid fraction after microfuging homogenates at 14,000 rpm for 20 min. Protein concentrations in extracts were determined using a BCA protein assay kit from Pierce Biochemical. ATP concentration was determined by measuring relative luminescence (RLU) of 1:50 dilutions of extracts in a luminometer using an ATP bioluminescence assay kit from Roche Diagnostics.
Characterization of neurodegeneration in levy and transformants:
Plastic sectioning:
Flies were decapitated and the heads fixed overnight in 1% paraformaldehyde and 1% glutaraldehyde. Fixed head samples were embedded in Epon 812 and then cut into 1-μm thick horizontal sections, stained with toluidine blue, and visualized using bright-field microscopy.
Paraffin sectioning:
Standard paraffin embedding and sectioning techniques were followed on the basis of the protocol established by Crittenden et al. (1998). Briefly, flies were fixed in Carnoy's solution (60% vol/vol ethanol, 30% vol/vol chloroform, 10% vol/vol glacial acetic acid) for 4 hr at room temperature. After four 100% ethanol washes of 30 min each, the tissue was immersed in methyl benzoate overnight, transferred to a 1:1 solution of methyl benzoate/paraffin for 1 hr at 56°, and then transferred to a 1:2 solution of methyl benzoate/paraffin for another hour at 56°. The flies were then immersed in pure paraffin, four times for 1 hr each at 56°, and finally embedded in paraffin at room temperature. The paraffin blocks were cut into 4-μm floating serial sections that were mounted on gelatinized glass slides. Prior to histological examinations, the tissue was deparaffinized with a 3-min wash in xylene and rehydrated through an ethanol series ending in either distilled water or Tris-buffered saline as appropriate. To characterize the presence or absence or neurodegeneration in levy mutants, rehydrated paraffin sections were stained with a 1% toluidine blue/10% bromophenol blue (BPB) solution for 10 min.
RESULTS
Isolation of levy mutations and identification of the gene:
The levy1 mutation was isolated in a screen in which a number of temperature-sensitive paralytic mutants were identified in Drosophila (Hegde et al. 1999; Singh and Singh 1999; Chopra et al. 2000). The mutation was induced with EMS using compound chromosomes (Singh et al. 1987). Mutant flies paralyzed on exposure to 38°. The flies paralyzed and recovered in an age-dependent manner as follows: 2-day-old flies took up to 5 min to paralyze and recovered within 30 min, while 30-day-old flies paralyzed within 30 sec and took up to 3 hr to recover.
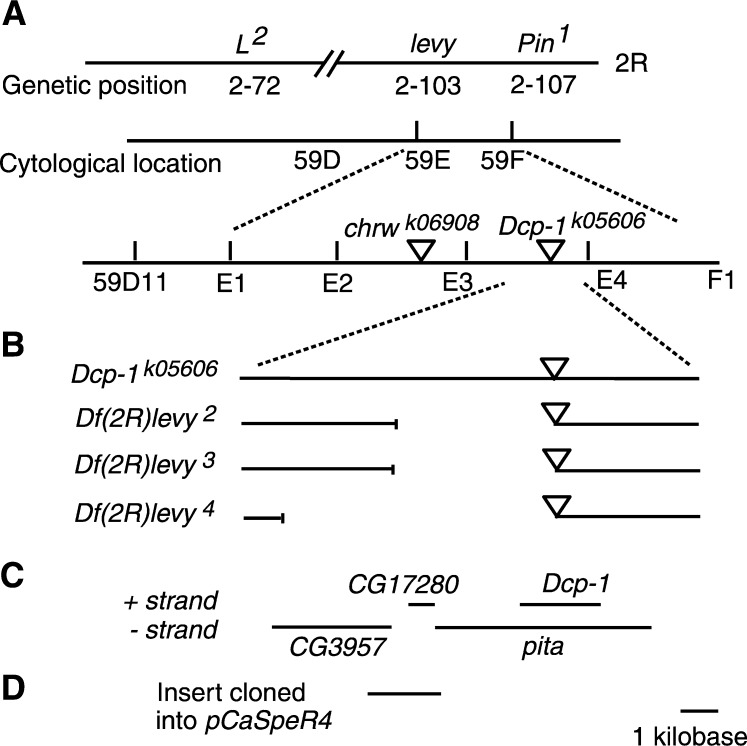
The levy gene was localized to the right arm of chromosome 2 and mapped to 103 map units by recombination with visible markers L2 and Pin1 (Figure 1A). The chromosomal location of levy was further defined using several available chromosome deficiencies in the region. Two deficiencies that were useful in localizing the levy mutation were Df(2R)bw-HB132, with break-points at 59D11 and 59F6-8, and Df(2R)egl2, with breakpoints at 59E and 60A1. These deficiencies localized the levy mutation between 59D11 and 59E on polytene chromosomes (Figure 1A), a region encompassing 30 genes (http://flybase.bio.indiana.edu/).
Figure 1.—
Molecular identification of the levy gene. Schematic of DNA encompassing the levy gene. (A) The levy gene was first localized to genetic position 2-103 using recombination mapping of the levy1 mutation with visible markers L2 and Pin1 on the right arm of chromosome 2. Deficiency mapping localized the levy gene cytologically between bands 59D11 and 59E. Site-specific recombination mapping in males localized the levy gene between two P-element insertion sites, chrwk06908 and Dcp-1k05606 (represented by triangles). (B) Results of molecular analysis of three levy deficiencies (levy2, levy3, and levy4) generated by P-element mobilization in Dcp-1k05606 flies suggested that the levy gene is CG17280, pita, or Dcp-1. Gaps in solid lines represent DNA deleted in these three levy deficiency strains. (C) The relative locations of genes in this region are shown as lines. The CG17280 gene was identified as levy by transforming a 2-kb DNA fragment encompassing CG17280 (represented by a line in D) into the germ line of levy1 mutants. These levy transformants did not exhibit temperature-induced paralytic behavior. The bar at the bottom represents 1 kb of DNA in B–D.
Site-specific recombination mapping in males (Chen et al. 1998) placed the levy gene distal to the insertion site of the chrwk06908 P element and proximal to location of the Dcp-1k05606 P element (Figure 1A). Genetic crosses were conducted to mobilize the Dcp-1k05606 P element into the levy DNA and three new levy mutant alleles (levy2, levy3, and levy4) were identified. All three alleles produced embryonic lethality in homozygous configuration and gave rise to temperature-induced paralysis when heterozygous with levy1.
Inverse PCR and sequencing of DNA flanking the P element in levy2, levy3, and levy4 revealed that all three carried small deletions (Figure 1B). The smallest of the three deletions removed a 2.2-kb region that fully included the CG17280 gene and parts of the Dcp-1 and pita genes (Figure 1C). The most likely gene among these three to be levy was CG17280 because the P-element insertion in Dcp-1k05606 complemented the levy1 mutation in genetic crosses and Dcp-1k05606 flies are known to have the insert within Dcp-1 and pita DNA (Figure 1, B and C).
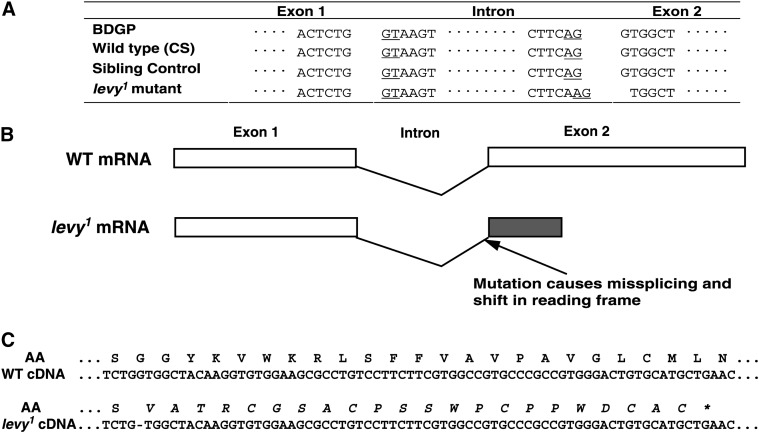
The CG17280 gene is an ortholog of the human COX subunit VIa precursor (Fabrizi et al. 1989). Sequencing of CG17280 DNA in the levy1 mutant revealed a G-to-A transition occurring at the 3′-splice junction of the single intron (Figure 2A). This base transition would presumably result in missplicing of CG17280 RNA causing the deletion of a nucleotide, in turn leading to a reading frame shift in the second exon. This misspliced RNA would encode an aberrant protein of 54 amino acids, of which the first 32 match those in the nascent wild-type Levy protein of 109 amino acids (Figure 2B). RT–PCR amplification and DNA sequencing showed that a nucleotide was missing at the relevant position in the CG17280-encoded RNA (Figure 2C). The presence of this misspliced RNA suggested that CG17280 is the levy gene.
Figure 2.—
Guanine-to-adenine transition in levy1 DNA resulted in missplicing of CG17280 RNA. (A) levy1 DNA contained an adenine nucleotide at the 3′-splice junction within the intron of CG17280 gene. This position was occupied by guanine in the wild-type gene as given in the BDGP database as well as in our own sequence determination of the region in CS and a sibling strain to levy1. The 5′- and 3′-intron splicing consensus sequences are underlined. The G-to-A transition caused the levy1 RNA to be misspliced by 1 nucleotide and resulted in a reading frame shift in exon 2, as determined by RT–PCR analysis of mutant levy RNA. (B) A schematic of missplicing of levy1 RNA. Translated regions are shown as rectangles with the wild type shown as open rectangles. The shift in reading frame would presumably lead to replacement of 77 amino acids encoded by exon 2 with 22 missense amino acids (shaded rectangle). (C) Sequencing of levy1 cDNA confirmed the occurrence of missplicing in levy1 RNA. A portion of the levy1 cDNA sequence is shown with the deduced amino acid (AA) sequence. The wild-type cDNA sequence and deduced amino acid translation are shown for comparison to those of the levy1 mutant. The aberrant amino acid sequence resulting from a shift in the reading frame is shown in italics.
To obtain proof that CG17280 is the levy gene, a 2-kb DNA fragment encompassing the CG17280 gene (see Figure 1D) was amplified from the wild-type (CS) genomic DNA and cloned into pCaSpeR4 transformation vector at KpnI and XhoI sites. The recombinant DNA construct was transferred into the genome of levy1 mutants using P-element-mediated germ-line transformation (McKay et al. 1995). As a control, transformation was performed with the pCaSpeR4 vector without the CG17280 gene. levy1 transformants carrying the wild-type CG17280 transgene were not paralytic at 38°, showing that wild-type CG17280 DNA comprises the levy gene. None of the seven independently generated transformants showed paralysis at 38° and all of the five transformant controls showed paralysis.
Reduction of cytochrome c oxidase activity in levy mutants:
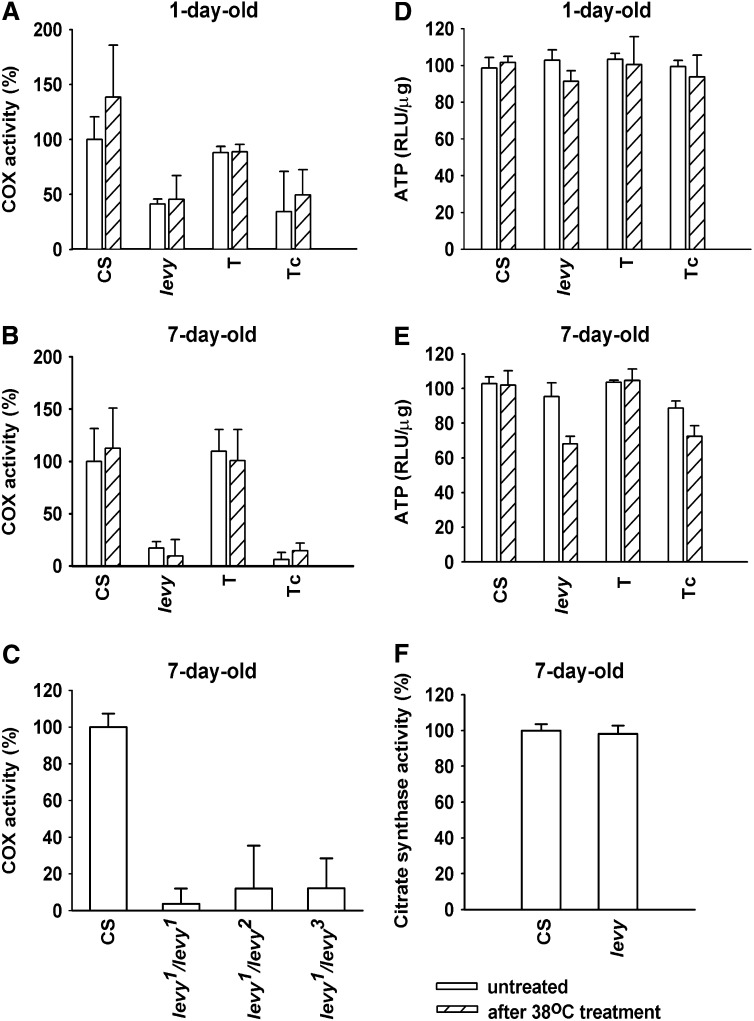
Subunit VIa plays a regulatory role in yeast and in mammalian COX (Taanman and Capaldi 1993; Taanman et al. 1994; Ludwig et al. 2001). To determine if the levy mutation affected COX activity, enzymatic assays were performed on age- and temperature-controlled flies. As shown in Figure 3 (A and B), levy1 mutants exhibited a reduction in COX activity compared to that in wild-type flies and levy transformants. Activity was reduced in levy1 mutant flies to 41% of that in the wild-type flies (Figure 3A). The reduction in COX activity was greater in 7-day-old flies (Figure 3B) than in 1-day-old flies. In this case the activity in levy1 was reduced to 17% of that in the wild type. Transformant flies carrying levy+ transgene showed enzymatic activity similar to that in the wild-type flies, whereas activity levels in the transformant control flies were similar to those in the mutant. These data support the proposal that the levy gene encodes a COX subunit. There was no further reduction in COX activity on exposing the flies to 38° for 5 min.
Figure 3.—
levy mutants exhibited age-dependent reduction in COX activity and ATP, but not in a marker for the mitochondrial matrix, citrate synthase. COX activity and levels of ATP were measured in adult flies kept at 29° for 1 or 7 days. COX activity is set to 100% in untreated wild-type (CS) flies for comparison purposes. COX activity was reduced in levy1 mutants compared to that in wild-type flies (Student's t-test, P < 0.025 for 1-day old and P < 0.050 for 7-day old) and levy transformants (P < 0.005 for 1-day old and P < 0.010 for 7-day old) (A and B). Transformant flies carrying levy+ transgene showed enzymatic activity similar to that in the wild-type flies (P ≈ 0.2), whereas activity levels in the transformant control flies were similar to those in the mutant (P ≈ 0.2). Heterozygous levy alleles exhibited a comparable reduction of COX activity with respect to wild-type (CS) flies (C). In comparison to the wild type, a reduction in ATP appeared in 7-day-old levy1 mutants (P < 0.025) and transformant controls (P < 0.025) after a temperature-shock regimen (E). Comparable reduction in ATP did not appear in 1-day-old (D) temperature-shocked levy1 mutants (P ≈ 0.2) or in 7-day-old levy1 mutants prior to being subjected to temperature shock (E) (P ≈ 0.2). There was no measurable difference between wild-type and levy mutants in regard to a classical enzymatic marker for the mitochondrial matrix, citrate synthase (F). Error bars show standard deviation from three or four measurements. T, transformant; Tc, transformant control.
Heterozygous combinations of the levy1 allele with two deletion alleles (levy2, levy3) were tested for COX activity to test a prediction that these would also exhibit a reduction in COX activity compared to wild-type flies. Indeed, levy1/levy2 and levy1/levy3 flies, aged for 7 days, exhibited a reduction in COX activity (to 12% of that in the wild type in each case) that was comparable to the reduction seen in levy1 homozygous flies (to 4% of the activity in the wild type) (Figure 3C). These data further corroborate our conclusion that the levy mutations lead to a reduction in COX activity and are in complete agreement with results showing the rescue of wild-type activity by levy+ transgene.
To test if a reduction in COX activity might be due to a reduction in the number of mitochondria in levy mutants, fly preparations were examined by assaying for citrate synthase, an enzymatic marker for the mitochondrial matrix (Ferguson et al. 2005). No measurable differences were seen in levels of citrate synthase activity between levy mutants and wild-type flies (Figure 3F). This suggests that reduced COX activity observed in levy mutants was due to a decrease in COX enzymatic activity rather than to a reduction in the overall number of mitochondria in levy tissues.
Decreased levels of ATP in levy mutants:
A defect in oxidative phosphorylation results in reduced levels of ATP in mitochondrial encephalomyopathies such as LS (Dahl 1998; Carrozzo et al. 2001). Similarly, levy1 mutants exhibited reduced ATP (Figure 3, D and E). Compared to the wild type, 7-day-old levy1 mutants that were close to dying showed 33% reduction in ATP after being subjected to temperature shock of 38° for 5 min (Figure 3E). ATP levels without the temperature shock were 103 ± 4 RLU/μg for the wild type and 95 ± 8 RLU/μg for the levy1 flies. In flies given temperature shock, the levels were 102 ± 8 RLU/μg and 68 ± 5 RLU/μg in wild type and levy1, respectively. ATP levels in levy transformants were comparable to those in wild-type flies. ATP reduction in levy1 mutants appeared to be age dependent because there was a difference in ATP between 1-day-old and 7-day-old levy1 mutants after temperature shock (Figure 3, D and E) (1-day-old levy1: 91 ± 6 RLU/μg; 7-day-old levy1: 68 ± 5 RLU/μg).
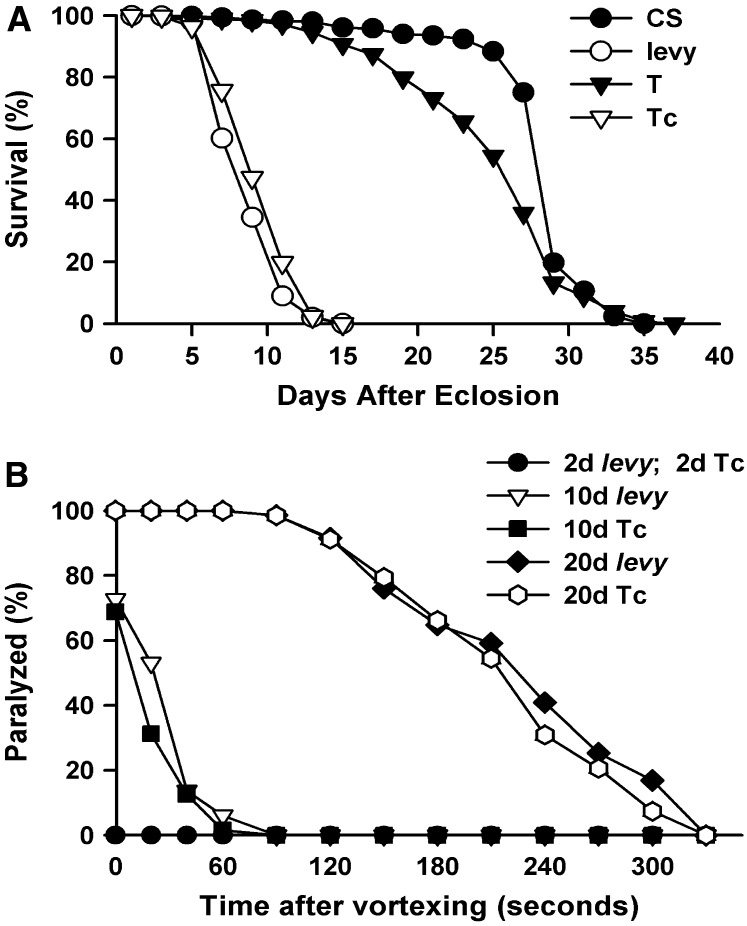
Reduced life span in levy mutants:
levy1 mutants displayed additional phenotypic characteristics that are similar to COX-deficient encephalomyopathies. A major characteristic of such disorders is early death (Leigh 1951; Rahman et al. 1996; Darin et al. 2001). This is paralleled by premature death of levy1 mutants, which showed a median life span of 8 days at 29° compared to 28 days in wild type and 26 days in transformants (Figure 4A). The median life span of transformant control (9 days) was similar to that of levy1 mutants, proving that CG17280 DNA is essential to restore normal life span in levy1 transformants. Life span of levy1 mutants was also examined at 21° and had a mean value of 51 days compared to 100 days in the wild type, 93 days in levy transformants, and 55 days in transformant control flies.
Figure 4.—
Reduced life span and progressive bang-induced paralysis in levy mutants. (A) Percentage of surviving adults kept at 29° after eclosion. levy1 flies (n = 550) died at a median age of 8 days at 29° compared to wild-type (CS) flies (n = 669), which lived to a median age of 28 days. Life span of levy transformants (n = 955) was similar to that of Canton-S flies, while transformant controls (n = 682) died about the same time as levy1 mutants. (B) Time for recovery after testing for bang-induced paralysis for 2-, 10-, and 20-day-old (after eclosion) adults kept at 21°. At this temperature, the median life span was 100 days for wild-type flies (CS), 51 days for levy1 mutants, 93 days for transformants, and 55 days for transformant controls. Two-day-old levy1 mutants did not exhibit bang-induced paralytic behavior. Only a fraction of 10-day-old levy1 mutants exhibited bang-induced paralysis, all of which recovered in <2 min. In 20-day-old levy1 mutants, all flies paralyzed in response to bang stimulus and these took longer to recover from paralysis than their younger siblings. Numbers of 2-, 10-, and 20-day-old levy flies tested were 56, 66, and 71, respectively. Numbers of 2-, 10-, and 20-day-old transformant control flies tested were 69, 64, and 68, respectively.
Bang sensitivity in levy mutants:
In another phenotypic parallel to mitochondrial encephalomyopathies, levy1 mutants exhibited motor deficiency by displaying age-dependent bang-induced paralysis (Figure 4B) (Ganetzky and Wu 1982; Pavlidis et al. 1994). Two-day-old levy1 mutants were not paralytic in response to mechanical shock, but there was an increase in the number that showed such paralysis as they aged. In flies aged for 10 days at 21°, 73% of levy1 and 69% of transformant control flies paralyzed in response to a bang shock, whereas all 20-day-old mutant and transformant control flies exhibited bang-induced paralysis. With the onset and increase in bang-induced paralysis with age, there was also a concomitant increase in the severity of paralysis, prolonging the time required for recovery from paralysis. Ten-day-old paralyzed flies took up to 90 sec to recover from bang-induced paralysis while 20-day-old flies took up to 330 sec. Bang sensitivity was also examined in levy1/levy2 and levy1/levy3 heterozygous flies and they also showed age-dependent bang-induced paralysis (data not shown). Wild-type and levy transformant flies did not show bang-induced paralysis at any of the three ages at which flies were tested.
Characterization of neurodegeneration in levy mutants:
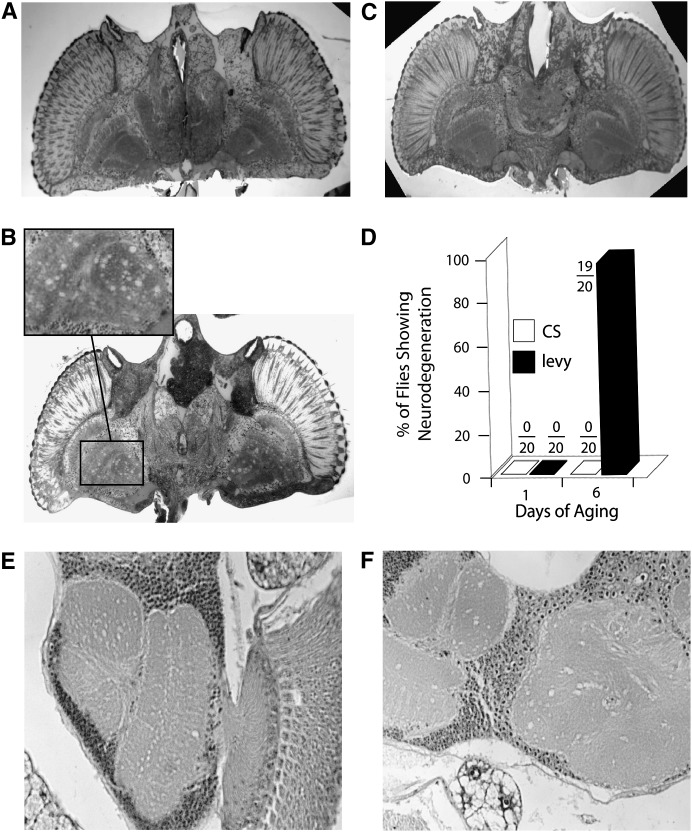
The phenotype of temperature-induced paralysis has been shown to enrich for mutations that lead to neurodegeneration (Palladino et al. 2002). In addition, several mitochondrial encephalomyopathies lead to neurodegeneration (DiMauro 2004; Gropman 2004). To determine whether or not levy1 mutants exhibited neurodegeneration, tissue sections taken through head capsules of mutant and the wild-type (CS) flies were fixed in Epon and examined microscopically. Neurodegeneration was not seen in wild-type flies (Figure 5A), but the brain and optic lobes of levy1 mutants showed Swiss cheese-like holes that are reminiscent of spongiform neurodegeneration (Figure 5B). Neurodegeneration was observed to be absent in levy transformants (Figure 5C), demonstrating that the phenomenon was produced by the levy mutation. As in the case of other phenotypes, neurodegeneration appeared to be age dependent. Paraffin sections from 20 flies each from the wild-type and the levy1 strains, aged for either 1 day or 6 days at 29°, were examined for holes in the brain. None of wild-type flies in either age category, and none of the levy1 flies aged for 1 day, showed any signs of neurodegeneration (Figure 5D). On the other hand, 19 of 20 levy1 flies aged for 6 days showed neurodegeneration.
Figure 5.—
levy mutants exhibited vacuolization of the optic lobes and brain, but were rescued by transformation with the wild-type gene. Epon-embedded horizontal brain sections were cut from head capsules of adults kept at 29° after eclosion. Slices through the heads of 7-day-old wild type (CS) (A) and levy transformant (C) are shown for comparison to that from the head of levy1 mutant (B). Swiss cheese-like holes appeared in the brain and optic lobes in 7-day-old levy1 mutants (inset). These cytological lesions also appeared in the brains and optic lobes of 7-day-old transformant control flies (data not shown). Neurodegeneration occurred in age-dependent manner (D), being absent in CS flies aged for 1 day or 6 days, and in levy1 flies aged for 1 day. On the other hand, 19 of 20 levy1 flies aged for 6 days showed clear neurodegeneration. Serial sections from paraffin processed flies were obtained from levy1/levy2 (E) and levy1/levy3 (F) flies after aging them for 6 days at 29°. Neurodegeneration was observed in flies from both strains, consistent with the phenomenon seen in levy1 mutants.
Possible occurrence of neurodegeneration in additional levy alleles was examined in levy1/levy2 and levy1/levy3 heterozygotes. Heterozygous animals aged at 29° posteclosion for 6 days were stained using hematoxylin and eosin to determine the extent of vacuolization. The levy1/levy2 (Figure 5E) and levy1/levy3 (Figure 5F) flies exhibited levy1-type neurodegeneration in the lobula and extensive vacuolization within other neural structures. The phenotype of neurodegeneration overlapped that seen in the homozygous levy1 mutants. The extent or the nature of neurodegeneration did not seem to be significantly different among the alleles examined. This is not unexpected since levy2 and levy3 are deletion mutations and levy1 itself is likely to be a null allele because of a shift in the reading frame after 32 amino acids, followed by a premature stop codon.
DISCUSSION
Mitochondrial function is integral to the healthy function of cells, particularly in cells such as neurons and muscles that are critically dependent on an abundant energy supply. A spectrum of myopathic and neuropathic symptoms in humans have been correlated to lesions in mitochondrial respiratory chain. Use of genetically tractable model systems such as Drosophila can help in identifying individual steps in the pathways leading to such disorders. Animal models are particularly useful for diseases such as mitochondrial encephalomyopathies that are intransigent to treatment and about which relatively little is known. By generating mutations in a COX subunit, analyzing the resulting phenotypes, and obtaining rescue from these phenotypes by germ-line transformation, we provide direct evidence that disruption of COX VIa results in mitochondrial encephalomyopathic effects including neurodegeneration and motor dysfunction.
Mitochondrial encephalomyopathies related to COX are characterized by enzyme deficiency, reduced ATP production, motor difficulty, neurodegeneration, and shortened life span. Phenotypes associated with levy mutants mimic all of these symptoms. Consistent with the progressive nature of these symptoms in humans, all levy phenotypes including temperature-induced paralysis, bang sensitivity, reduction in COX activity and ATP level, and neurodegeneration show age-dependent increases in severity. For example, vacuolization in the brains of levy mutants is not detected in flies aged for 1 day. However, it is widespread in flies aged for 6 days. Importantly, germ-line transformation using wild-type levy gene rescues the mutant flies from all the phenotypes described above. However, the control vector—the same vector used in transformation rescue but without the levy gene—is unable to provide such a rescue. Thus all these phenotypes can be directly linked to the disruption of subunit VIa of COX. These data reveal a COX-mediated pathway in Drosophila, disruption of which leads to mitochondrial encephalomyopathic effects including neurodegeneration, motor dysfunction, and premature death. In addition, the transformation experiments provide direct evidence for a causal link between the disruption of COX and these phenotypes.
Human COX consists of 3 mitochondrial-encoded and 10 nuclear-encoded subunits. Defects in the 3 mitochondrial-encoded subunits COXI (also called MTCO1), COXII (MTCO2), and COXIII (MTCO3) as well as in several COX assembly factors such as COX10, COX15, SURF1, SCO1, and SCO2 have been correlated with encephalomyopathies (Barrientos et al. 2002; DiMauro and Schon 2003; DiMauro and Hirano 2005). For example, >30 distinct mutations in the SURF1 gene have been associated with COX-deficient LS (Pequignot et al. 2001). In light of this, it has been noted with intrigue that no mutations in any of the nuclear-encoded structural subunits of COX have been associated with such disorders and that attempts to find such associations have not been fruitful (Shoubridge 2001; DiMauro and Schon 2003; Schon 2004; Schapira 2006). This strongly suggests that mutations in the nuclear-encoded structural subunits of COX may be lethal (DiMauro and Schon 2003; Schapira 2006). This view is reinforced by results from an efficient screen conducted for mutations in nuclear-encoded mitochondrial proteins that yielded many such mutations in Drosophila (Liao et al. 2006). The only mutation in this set to target a structural subunit of COX, the tenured mutation in subunit Va, produces lethality. Similarly, a mutation in the cyclope gene, which codes for subunit VIc of COX, leads to lethality in Drosophila (Szuplewski and Terracol 2001). The levy1 mutation discussed here provides the first case of such a mutation leading to encephalomyopathic effects rather than lethality. However, the findings presented here raise additional questions. While subunit VIa is a structural component of COX, the role of this subunit is likely to be regulatory in nature (Taanman and Capaldi 1993; Taanman et al. 1994; Ludwig et al. 2001). This is likely to be the reason for the enzyme retaining some activity even with frame-shifted and truncated subunit VIa (Figure 3).
The levy mutant was identified for its temperature-induced paralysis. However, the primary biochemical effect of the mutation—reduction in COX activity—is not temperature dependent. It implies that the COX enzyme itself does not have to be temperature sensitive to produce temperature sensitivity (paralysis) in flies. This is a common feature among temperature-paralytic mutants of Drosophila where sensitivity to high temperature arises not from the temperature sensitivity of the primary biochemical target but from a constitutive change in a biochemical or a physiological parameter. For example, temperature-induced paralysis in the parats and the napts mutants arises from a constitutive decrease in the number of sodium channels and not from channels that become temperature sensitive (Loughney et al. 1989; Kernan et al. 1991). Similarly, temperature sensitivity of ATP levels in old flies does not derive from temperature sensitivity of COX. Aged mutant flies showed a decrease in ATP levels after they were paralyzed by a temperature shock while COX activity was not affected by this treatment. While the mechanisms underlying an effect of temperature shock on ATP are unknown at this stage, it may possibly be related to the seizure activity that levy flies go through during temperature-induced paralysis. A reduction in ATP levels after seizure activity is known to occur in rat models of seizures (DeFrance and McCandless 1991; Yager et al. 2002; Darbin et al. 2005).
While the experiments reported here have identified one step in the pathway(s) leading to levy effects, and while further experimentation is needed to identify additional steps, the nature of the observed effects points to some likely mechanisms. Inhibition of COX has been shown to increase free radical generation in many systems including Drosophila (Smith and Bennett 1997; Duranteau et al. 1998; Ferguson et al. 2005). Excessive levels of free radicals can in turn lead to cell death via either apoptosis or necrosis (Beal 2000; Mattson and Kroemer 2003). There are other aspects of pathways involved in mitochondrial-mediated neurodegeneration. For example, ion channels have been implicated, either in the context of oxidative stress or outside of this context, in several types of neurodegeneration (Ueda et al. 1997; Liss et al. 2005; Burg et al. 2006; Chinopoulos and Adam-Vizi 2006). It remains to be seen if increased production of free radicals is involved in the effects observed in levy, whether levy brains show apoptotic or necrotic cell death, and if any ion channel dysfunction occurs in levy (Figure 6). Availability of levy mutations, genetic tractability of Drosophila, and the ease with which questions about oxidative stress (Ferguson et al. 2005; Dias-Santagata et al. 2007) as well as ion channel function can be explored in this model system (Chopra and Singh 1994; Gielow et al. 1995; Kraliz and Singh 1997; Kraliz et al. 1998) provide an excellent opportunity to address these questions.
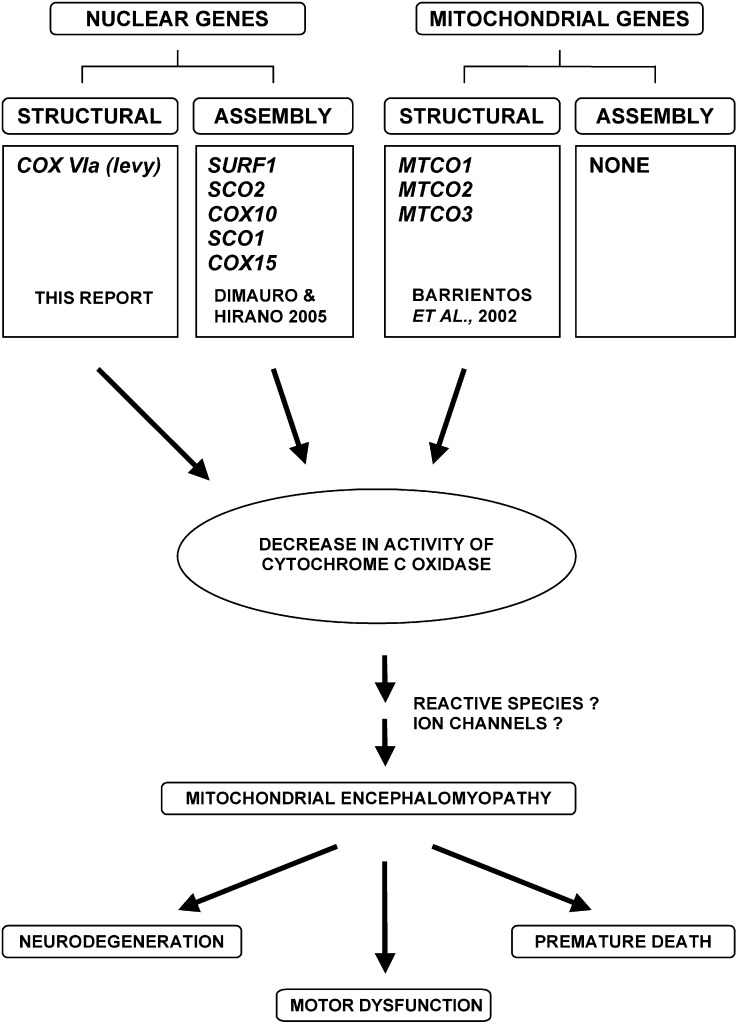
Figure 6.—
From COX deficiency to neuromuscular dysfunction. This model shows nuclear and mitochondrial genes that code for structural components of COX or its assembly factors that have been shown to be involved in mitochondrial encephalomyopathies. Until now, it has been believed that mutations in the nuclear genes coding for structural subunits of COX would be embryonic lethal. The levy1 mutation presents the first case where mutants are viable but show mitochondrial encephalomyopathic phenotypes. In addition, a general model of some possible subsequent steps via which COX deficiency could lead to neuromuscular dysfunction is presented. The data in this article establish a causal link between a reduction in COX activity and mitochondrial encephalomyopathic effects of neurodegeneration, paralytic behavior, and premature death in Drosophila.
Availability of levy mutations will particularly help in identifying steps in the pathway(s) leading to mitochondrial encephalopathy seen in the mutants. For example, it will be helpful to identify interacting genetic components by screening for suppressors or enhancers of a levy mutant phenotype. It is relatively easy to identify such modifier mutations in Drosophila, as thousands of mutagenized flies can be tested easily for phenotypes such as temperature-induced paralysis. Such modifier mutations can provide further leads into the pathways disrupted by the original mutations (the levy mutations in this case).
A significant level of our understanding on the structure, function, and regulation of COX has developed from identification and analysis of mutants in yeast (Barrientos et al. 2002). Mutations that lead to COX-related mitochondrial encephalomyopathies in organisms such as Drosophila and mice can similarly help us understand pathways leading to these disorders. Agostino et al. (2003) have generated a Surf1 knockout mouse model of Leigh syndrome that lacks Surfeit-1, an enzyme involved in COX assembly. Similarly, a Surf1 knockdown model has been generated in Drosophila by post-transcriptional silencing using dsRNA (Zordan et al. 2006). Studies comparing biochemical and physiological characteristics of various models of COX-related mitochondrial encephalomyopathies, including levy mutants and Surf1 models in mice and Drosophila, can help in understanding the mechanisms underlying the effects of such disorders. This is particularly true in regard to neurodegeneration due to a fortuitous difference between the levy flies and the Surf1 models. Neither Surf1 mice nor Surf1 flies show neurodegeneration (Agostino et al. 2003; Zordan et al. 2006). Thus, studies of similarities and differences between levy mutants and Surf1 models may enable us to address questions about what leads to neurodegeneration in one case but not in the other. These studies would lead to a better understanding of neurodegenerative processes in general and their occurrence in mitochondrial encephalomyopathies in particular. This information will also be useful in developing possible therapeutic approaches against such disorders.
Acknowledgments
This work was supported by grants MCB-0094477 and MCB-0322461 from the National Science Foundation to S.S. and R.D.S.
References
- Abou-Sleiman, P. M., M. M. Muqit and N. W. Wood, 2006. Expanding insights of mitochondrial dysfunction in Parkinson's disease. Nat. Rev. Neurosci. 7 207–219. [DOI] [PubMed] [Google Scholar]
- Agostino, A., F. Invernizzi, C. Tiveron, G. Fagiolari, A. Prelle et al., 2003. Constitutive knockout of Surf1 is associated with high embryonic lethality, mitochondrial disease and cytochrome c oxidase deficiency in mice. Hum. Mol. Genet. 12 399–413. [DOI] [PubMed] [Google Scholar]
- Ashburner, M., 1989. Drosophila: A Laboratory Handbook. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Atamna, H., and K. Boyle, 2006. Amyloid-beta peptide binds with heme to form a peroxidase: relationship to the cytopathologies of Alzheimer's disease. Proc. Natl. Acad. Sci. USA 103 3381–3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos, A., M. H. Barros, I. Valnot, A. Rotig, P. Rustin et al., 2002. Cytochrome oxidase in health and disease. Gene 286 53–63. [DOI] [PubMed] [Google Scholar]
- Beal, M. F., 2000. Energetics in the pathogenesis of neurodegenerative diseases. Trends Neurosci. 23 298–304. [DOI] [PubMed] [Google Scholar]
- Bellen, H. J., R. W. Levis, G. Liao, Y. He, J. W. Carlson et al., 2004. The BDGP gene disruption project: single transposon insertions associated with 40% of Drosophila genes. Genetics 167 761–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benchoua, A., Y. Trioulier, D. Zala, M. C. Gaillard, N. Lefort et al., 2006. Involvement of mitochondrial complex II defects in neuronal death produced by N-terminus fragment of mutated huntingtin. Mol. Biol. Cell 17 1652–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya, A., G. G. Gu and S. Singh, 1999. Modulation of dihydropyridine-sensitive calcium channels in Drosophila by a cAMP-mediated pathway. J. Neurobiol. 39 491–500. [DOI] [PubMed] [Google Scholar]
- Bhattacharya, A., S. S. Lakhman and S. Singh, 2004. Modulation of L-type calcium channels in Drosophila via a pituitary adenylyl cyclase-activating polypeptide (PACAP)-mediated pathway. J. Biol. Chem. 279 37291–37297. [DOI] [PubMed] [Google Scholar]
- Bonini, N. M., and M. E. Fortini, 2003. Human neurodegenerative disease modeling using Drosophila. Annu. Rev. Neurosci. 26 627–656. [DOI] [PubMed] [Google Scholar]
- Burg, E. D., C. V. Remillard and J. X. Yuan, 2006. K+ channels in apoptosis. J. Membr. Biol. 209 3–20. [DOI] [PubMed] [Google Scholar]
- Carrozzo, R., A. Tessa, M. E. Vazquez-Memije, F. Piemonte, C. Patrono et al., 2001. The T9176G mtDNA mutation severely affects ATP production and results in Leigh syndrome. Neurology 56 687–690. [DOI] [PubMed] [Google Scholar]
- Celotto, A. M., A. C. Frank, S. W. McGrath, T. Fergestad, W. A. Van Voorhies et al., 2006. Mitochondrial encephalomyopathy in Drosophila. J. Neurosci. 26 810–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, B., T. Chu, E. Harms, J. P. Gergen and S. Strickland, 1998. Mapping of Drosophila mutations using site-specific male recombination. Genetics 149 157–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinopoulos, C., and V. Adam-Vizi, 2006. Calcium, mitochondria and oxidative stress in neuronal pathology. Novel aspects of an enduring theme. FEBS J. 273 433–450. [DOI] [PubMed] [Google Scholar]
- Choo, Y. S., G. V. Johnson, M. MacDonald, P. J. Detloff and M. Lesort, 2004. Mutant huntingtin directly increases susceptibility of mitochondria to the calcium-induced permeability transition and cytochrome c release. Hum. Mol. Genet. 13 1407–1420. [DOI] [PubMed] [Google Scholar]
- Chopra, M., and S. Singh, 1994. Developmental temperature selectively regulates a voltage-activated potassium current in Drosophila. J. Neurobiol. 25 119–126. [DOI] [PubMed] [Google Scholar]
- Chopra, M., G.-G. Gu and S. Singh, 2000. Mutations affecting the delayed rectifier potassium current in Drosophila. J. Neurogenet. 14 107–123. [DOI] [PubMed] [Google Scholar]
- Chovnick, A., G. H. Ballantyne, D. L. Baillie and D. G. Holm, 1970. Gene conversion in higher organisms: half-tetrad analysis of recombination within the rosy cistron of Drosophila melanogaster. Genetics 66 315–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crittenden, J. R., E. M. Skoulakis, K. A. Han, D. Kalderon and R. L. Davis, 1998. Tripartite mushroom body architecture revealed by antigenic markers. Learn. Mem. 5 38–51. [PMC free article] [PubMed] [Google Scholar]
- Dahl, H. H., 1998. Getting to the nucleus of mitochondrial disorders: identification of respiratory chain-enzyme genes causing Leigh syndrome. Am. J. Hum. Genet. 63 1594–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darbin, O., J. J. Risso, E. Carre, M. Lonjon and D. K. Naritoku, 2005. Metabolic changes in rat striatum following convulsive seizures. Brain Res. 1050 124–129. [DOI] [PubMed] [Google Scholar]
- Darin, N., A. Oldfors, A. R. Moslemi, E. Holme and M. Tulinius, 2001. The incidence of mitochondrial encephalomyopathies in childhood: clinical features and morphological, biochemical, and DNA anbormalities. Ann. Neurol. 49 377–383. [PubMed] [Google Scholar]
- DeFrance, J. F., and D. W. McCandless, 1991. Energy metabolism in rat hippocampus during and following seizure activity. Metab. Brain Dis. 6 83–91. [DOI] [PubMed] [Google Scholar]
- Dias-Santagata, D., T. A. Fulga, A. Duttaroy and M. B. Feany, 2007. Oxidative stress mediates tau-induced neurodegeneration in Drosophila. J. Clin. Invest. 117 236–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMauro, S., 2004. Mitochondrial diseases. Biochim. Biophys. Acta 1658 80–88. [DOI] [PubMed] [Google Scholar]
- DiMauro, S., and M. Hirano, 2005. Mitochondrial encephalomyopathies: an update. Neuromuscul. Disord. 15 276–286. [DOI] [PubMed] [Google Scholar]
- DiMauro, S., and E. A. Schon, 2003. Mitochondrial respiratory-chain diseases. N. Engl. J. Med. 348 2656–2668. [DOI] [PubMed] [Google Scholar]
- Duranteau, J., N. S. Chandel, A. Kulisz, Z. Shao and P. T. Schumacker, 1998. Intracellular signaling by reactive oxygen species during hypoxia in cardiomyocytes. J. Biol. Chem. 273 11619–11624. [DOI] [PubMed] [Google Scholar]
- Esposito, L., J. Raber, L. Kekonius, F. Yan, G. Q. Yu et al., 2006. Reduction in mitochondrial superoxide dismutase modulates Alzheimer's disease-like pathology and accelerates the onset of behavioral changes in human amyloid precursor protein transgenic mice. J. Neurosci. 26 5167–5179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabrizi, G. M., R. Rizzuto, H. Nakase, S. Mita, B. Kadenbach et al., 1989. Sequence of a cDNA specifying subunit VIa of human cytochrome c oxidase. Nucleic Acids Res. 17 6409. [DOI] [PMC free article] [PubMed]
- Ferguson, M., R. J. Mockett, Y. Shen, W. C. Orr and R. S. Sohal, 2005. Age-associated decline in mitochondrial respiration and electron transport in Drosophila melanogaster. Biochem. J. 390 501–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortini, M. E., and N. M. Bonini, 2000. Modeling human neurodegenerative diseases in Drosophila: on a wing and a prayer. Trends Genet. 16 161–167. [DOI] [PubMed] [Google Scholar]
- Ganetzky, B., 2000. Genetic analysis of ion channel dysfunction in Drosophila. Kidney Int. 57 766–771. [DOI] [PubMed] [Google Scholar]
- Ganetzky, B., and C. F. Wu, 1982. Indirect suppression involving behavioral mutants with altered nerve excitability in Drosophila melanogaster. Genetics 100 597–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gielow, M. L., G. G. Gu and S. Singh, 1995. Resolution and pharmacological analysis of the voltage-dependent calcium channels of Drosophila larval muscles. J. Neurosci. 15 6085–6093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano, C., M. Sebastiani, G. Plazzi, C. Travaglini, P. Sale et al., 2006. Mitochondrial neurogastrointestinal encephalomyopathy: evidence of mitochondrial DNA depletion in the small intestine. Gastroenterology 130 893–901. [DOI] [PubMed] [Google Scholar]
- Gnerer, J. P., R. A. Kreber and B. Ganetzky, 2006. wasted away, a Drosophila mutation in triosephosphate isomerase, causes paralysis, neurodegeneration, and early death. Proc. Natl. Acad. Sci. USA 103 14987–14993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gropman, A. L., 2004. The neurological presentations of childhood and adult mitochondrial disease: established syndromes and phenotypic variations. Mitochondrion 4 503–520. [DOI] [PubMed] [Google Scholar]
- Gu, G. G., and S. Singh, 1997. Modulation of the dihydropyridine-sensitive calcium channels in Drosophila by a phospholipase C-mediated pathway. J. Neurobiol. 33 265–275. [DOI] [PubMed] [Google Scholar]
- Hegde, P., G. G. Gu, D. Chen, S. J. Free and S. Singh, 1999. Mutational analysis of the Shab-encoded delayed rectifier K+ channels in Drosophila. J. Biol. Chem. 274 22109–22113. [DOI] [PubMed] [Google Scholar]
- Keeney, P. M., J. Xie, R. A. Capaldi and J. P. Bennett, Jr., 2006. Parkinson's disease brain mitochondrial complex I has oxidatively damaged subunits and is functionally impaired and misassembled. J. Neurosci. 26 5256–5264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kernan, M. J., M. I. Kuroda, R. Kreber, B. S. Baker and B. Ganetzky, 1991. napts, a mutation affecting sodium channel activity in Drosophila, is an allele of mle, a regulator of X chromosome transcription. Cell 66 949–959. [DOI] [PubMed] [Google Scholar]
- Kraliz, D., and S. Singh, 1997. Selective blockade of the delayed rectifier potassium current by tacrine in Drosophila. J. Neurobiol. 32 1–10. [PubMed] [Google Scholar]
- Kraliz, D., A. Bhattacharya and S. Singh, 1998. Blockade of the delayed rectifier potassium current in Drosophila by quinidine and related compounds. J. Neurogenet. 12 25–39. [DOI] [PubMed] [Google Scholar]
- Leigh, D., 1951. Subacute necrotizing encephalomyelopathy in an infant. J. Neurochem. 14 216–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis, E. B., and F. Bacher, 1968. Method of feeding ethylmethanesulfonate (EMS) to Drosophila males. Drosoph. Inf. Serv. 43 193.
- Liao, T. S., G. B. Call, P. Guptan, A. Cespedes, J. Marshall et al., 2006. An efficient genetic screen in Drosophila to identify nuclear-encoded genes with mitochondrial function. Genetics 174 525–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liss, B., O. Haeckel, J. Wildmann, T. Miki, S. Seino et al., 2005. K-ATP channels promote the differential degeneration of dopaminergic midbrain neurons. Nat. Neurosci. 8 1742–1751. [DOI] [PubMed] [Google Scholar]
- Loughney, K., R. Kreber and B. Ganetzky, 1989. Molecular analysis of the para locus, a sodium channel gene in Drosophila. Cell 58 1143–1154. [DOI] [PubMed] [Google Scholar]
- Ludwig, B., E. Bender, S. Arnold, M. Huttemann, I. Lee et al., 2001. Cytochrome C oxidase and the regulation of oxidative phosphorylation. Chembiochem 2 392–403. [DOI] [PubMed] [Google Scholar]
- Mattson, M. P., and G. Kroemer, 2003. Mitochondria in cell death: novel targets for neuroprotection and cardioprotection. Trends Mol. Med. 9 196–205. [DOI] [PubMed] [Google Scholar]
- McFarland, R., R. W. Taylor and D. M. Turnbull, 2002. The neurology of mitochondrial DNA disease. Lancet Neurol. 1 343–351. [DOI] [PubMed] [Google Scholar]
- McKay, R. R., D. M. Chen, K. Miller, S. Kim, W. S. Stark et al., 1995. Phospholipase C rescues visual defect in norpA mutant of Drosophila melanogaster. J. Biol. Chem. 270 13271–13276. [DOI] [PubMed] [Google Scholar]
- Palladino, M. J., T. J. Hadley and B. Ganetzky, 2002. Temperature-sensitive paralytic mutants are enriched for those causing neurodegeneration in Drosophila. Genetics 161 1197–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallanck, L., and J. T. Greenamyre, 2006. Neurodegenerative disease: pink, parkin and the brain. Nature 441 1058. [DOI] [PubMed]
- Pavlidis, P., M. Ramaswami and M. A. Tanouye, 1994. The Drosophila easily shocked gene: a mutation in a phospholipid synthetic pathway causes seizure, neuronal failure, and paralysis. Cell 79 23–33. [DOI] [PubMed] [Google Scholar]
- Pequignot, M. O., R. Dey, M. Zeviani, V. Tiranti, C. Godinot et al., 2001. Mutations in the SURF1 gene associated with Leigh syndrome and cytochrome C oxidase deficiency. Hum. Mutat. 17 374–381. [DOI] [PubMed] [Google Scholar]
- Rahman, S., R. B. Blok, H. H. Dahl, D. M. Danks, D. M. Kirby et al., 1996. Leigh syndrome: clinical features and biochemical and DNA abnormalities. Ann. Neurol. 39 343–351. [DOI] [PubMed] [Google Scholar]
- Schapira, A. H., 2006. Mitochondrial disease. Lancet 368 70–82. [DOI] [PubMed] [Google Scholar]
- Schon, E. A., 2004. Complements of the house. J. Clin. Invest. 114 760–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoubridge, E. A., 2001. Cytochrome c oxidase deficiency. Am. J. Med. Genet. 106 46–52. [DOI] [PubMed] [Google Scholar]
- Singh, S., 1983. A mutagenesis scheme for obtaining autosomal mutations in Drosophila. Indian J. Exp. Biol. 21 635–636. [Google Scholar]
- Singh, A., and S. Singh, 1999. Unmasking of a novel potassium current in Drosophila by a mutation and drugs. J. Neurosci. 19 6838–6843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, S., P. Bhandari, M. J. S. Chopra and D. Guha, 1987. Isolation of autosomal mutations in Drosophila melanogaster without setting up lines. Mol. Gen. Genet. 208 226–229. [Google Scholar]
- Smith, T. S., and J. P. Bennett, Jr., 1997. Mitochondrial toxins in models of neurodegenerative diseases. I: In vivo brain hydroxyl radical production during systemic MPTP treatment or following microdialysis infusion of methylpyridinium or azide ions. Brain Res. 765 183–188. [DOI] [PubMed] [Google Scholar]
- Szuplewski, S., and R. Terracol, 2001. The cyclope gene of Drosophila encodes a cytochrome c oxidase subunit VIc homolog. Genetics 158 1629–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taanman, J. W., and R. A. Capaldi, 1993. Subunit VIa of yeast cytochrome c oxidase is not necessary for assembly of the enzyme complex but modulates the enzyme activity. Isolation and characterization of the nuclear-coded gene. J. Biol. Chem. 268 18754–18761. [PubMed] [Google Scholar]
- Taanman, J. W., P. Turina and R. A. Capaldi, 1994. Regulation of cytochrome c oxidase by interaction of ATP at two binding sites, one on subunit VIa. Biochemistry 33 11833–11841. [DOI] [PubMed] [Google Scholar]
- Ueda, A., and C. F. Wu, 2006. Distinct frequency-dependent regulation of nerve terminal excitability and synaptic transmission by IA and IK potassium channels revealed by Drosophila Shaker and Shab mutations. J. Neurosci. 26 6238–6248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda, K., S. Shinohara, T. Yagami, K. Asakura and K. Kawasaki, 1997. Amyloid beta protein potentiates Ca2+ influx through L-type voltage-sensitive Ca2+ channels: a possible involvement of free radicals. J. Neurochem. 68 265–271. [DOI] [PubMed] [Google Scholar]
- Yager, J. Y., E. A. Armstrong, H. Miyashita and E. C. Wirrell, 2002. Prolonged neonatal seizures exacerbate hypoxic-ischemic brain damage: correlation with cerebral energy metabolism and excitatory amino acid release. Dev. Neurosci. 24 367–381. [DOI] [PubMed] [Google Scholar]
- Zordan, M. A., P. Cisotto, C. Benna, A. Agostino, G. Rizzo et al., 2006. Post-transcriptional silencing and functional characterization of the Drosophila melanogaster homolog of human Surf1. Genetics 172 229–241. [DOI] [PMC free article] [PubMed] [Google Scholar]