Abstract
Objectives
Investigators and clinicians almost always rely on Diagnostic and Statistical Manual of Mental Disorder, 4th edition’s (DSM-IV) somatoform disorders (and its derivative diagnoses) to characterize and identify patients with medically unexplained symptoms (MUS). Our objective was to evaluate this use by determining the prevalence of DSM-IV somatoform and nonsomatoform disorders in patients with MUS proven by a gold standard chart review.
Methods
In a community-based staff model HMO, we identified subjects for a clinical trial using a systematic and reliable chart rating procedure among high-utilizing MUS patients. Only baseline data are reported here. The World Health Organization Composite International Diagnostic Interview provided full and abridged DSM-IV diagnoses. Patients with full or abridged DSM-IV somatoform diagnoses were labeled “DSM somatoform-positive,” whereas those without them were labeled “DSM somatoform-negative.”
Results
Two hundred six MUS patients averaged 13.6 visits in the year preceding study, 79.1% were females, and the average age was 47.7 years. We found that 124 patients (60.2%) had a nonsomatoform (“psychiatric”) DSM-IV diagnosis of any type; 36 (17.5%) had 2 full nonsomatoform diagnoses, and 41 (19.9%) had >2; 92 (44.7%) had some full anxiety diagnosis and 94 (45.6%) had either full depression or minor depression diagnoses. However, only 9 of 206 (4.4%) had any full DSM-IV somatoform diagnosis, and only 39 (18.9%) had abridged somatization disorder. Thus, 48 (23.3%) were “DSM somatoform-positive” and 158 (76.7%) were “DSM somatoform-negative.” The latter exhibited less anxiety, depression, mental dysfunction, and psychosomatic symptoms (all p< .001) and less physical dysfunction (p = .011). Correlates of this DSM somatoform-negative status were female gender (p = .007), less severe mental (p = .007), and physical dysfunction (p = .004), a decreased proportion of MUS (p< .10), and less psychiatric comorbidity (p < .10); c-statistic = 0.77.
Conclusion
We concluded that depression and anxiety characterized MUS patients better than the somatoform disorders. Our data suggested radically revising the somatoform disorders for DSM-V by incorporating a new, very large group of now-overlooked DSM somatoform-negative patients who were typically women with less severe dysfunction.
Keywords: somatization, medically unexplained symptoms, DSM-V, chart review, primary care, somatoform
INTRODUCTION
Without an organic disease explanation for their illness, patients with medically unexplained symptoms (MUS) present a difficult problem for clinicians (1–5). Despite high utilization and much medical attention, MUS patients do poorly with their predominantly personal, psychologic problems (2–4,6). Moreover, there are no evidence-based treatment guidelines for the primary care clinicians who care for most MUS patients (7–9).
One reason for the absence of treatment guidelines is that diagnostic understanding has not been established (1,10–15). The Somatoform Disorders in the Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV) (16), and their abridged derivatives (not contained in DSM-IV), have provided virtually all guidance for identifying and naming MUS patients (10), but only the rare somatization disorder (SD) (16) has been validated (12–15). Because of overlap in diagnostic criteria, neither the remaining DSM-IV diagnoses nor the various medical MUS syndromes (eg, chronic fatigue and fibromyalgia) have been validated or shown to be comprehensive (1,11,17,18).
Plans for revising DSM-IV are underway with the aim of producing DSM-V by approximately 2010 (19). Especially in light of recommendations for dropping the somatoform group altogether (20), research data on MUS can inform decisions about how to address MUS patients in DSM-V.
As part of a clinical trial treating 206 high-utilizing MUS patients, we obtained extensive baseline data that allowed us to evaluate the role of the somatoform and nonsomatoform DSM-IV diagnoses in primary care. We identified MUS patients by a systematic chart rating procedure (21) and thus had a gold standard against which DSM-IV diagnoses could be compared. Based on our previous experience with a large number of MUS patients who had what we called minor acute illness and on the Epidemiologic Catchment Area (ECA) Study findings, we hypothesized that most MUS subjects would not have full or abridged DSM-IV somatoform diagnoses, and that those without these diagnoses would exhibit less dysfunction mentally and physically than those who had them (5,22,23).
METHOD
Study Design
We report here on just the baseline data of a clinical trial conducted from May 2000 to January 2003 (21,24–26). We identified primary care patients with at least 2 consecutive years of high utilization and then, among high-utilizers, we used medical chart review to identify subjects with MUS as their primary problem (21). We obtained baseline psychiatric diagnoses (full or abridged) from the World Health Organization Composite International Diagnostic Interview (WHO-CIDI) (27). This provided standard DSM-IV somatoform diagnoses (somatization disorder [SD], pain disorder, hypochondriasis, conversion) and many nonsomatoform (“psychiatric”) diagnoses. We then combined all full somatoform diagnoses into 1 category called somatoform disorder (16), and we identified the abridged form of SD as MUS patients not meeting SD criteria but having at least 4 (men) or 6 (women) of the DSM-IV symptoms for SD (28). Finally, to enhance analysis and meaning, we combined somatoform disorder and abridged SD to define “DSM somatoform-positive” status. Patients lacking both diagnoses were called “DSM somatoform-negative.” These 2 diagnoses were compared on the following measures: Short-Form 36 (SF-36) (29), Spielberger State Anxiety Scale (SSAS) (30), Center for Epidemiological Studies Depression (CES-D) (31), and the Psychosomatic Symptom Checklist (PSC) (32). We used logistic regression to identify the correlates of DSM somatoform-positive/negative status.
Subject Identification
Outlined in Figure 1, we screened patients between 18 and 65 years old for high utilization through the HMO’s information system. For at least the preceding 2 years, potential patients had 8 or more visits per year to primary care providers, consulting physicians, urgent care, or emergency rooms. Among high-utilizers, patients’ charts were rated by clinicians who used a review procedure developed for this project (21). Raters were trained to achieve a high degree of reliability for their ratings of symptoms as documented organic disease, documented nonorganic disease (measure of severe MUS), and undocumented (measure of mild MUS). Documentation meant that meaningful laboratory or consultative investigation had occurred while undocumented indicated that none took place (21). Chart ratings occurred as much as 9 to 12 months before entry into recruitment, and the chart scoring system was very sensitive to the presence of MUS (thus, a high false-positive rate for organic diseases) (21). Consequently, patient charts that met the study entry criterion for primary MUS (a high proportion of undocumented and documented nonorganic symptoms combined) (21) were independently reviewed a final time by 1 of the authors (R.C.S.) just before entry to ensure continued high utilization and that organic disease had not become the primary basis for high utilization. The details of the chart rating procedure are available from the authors.
Figure 1.
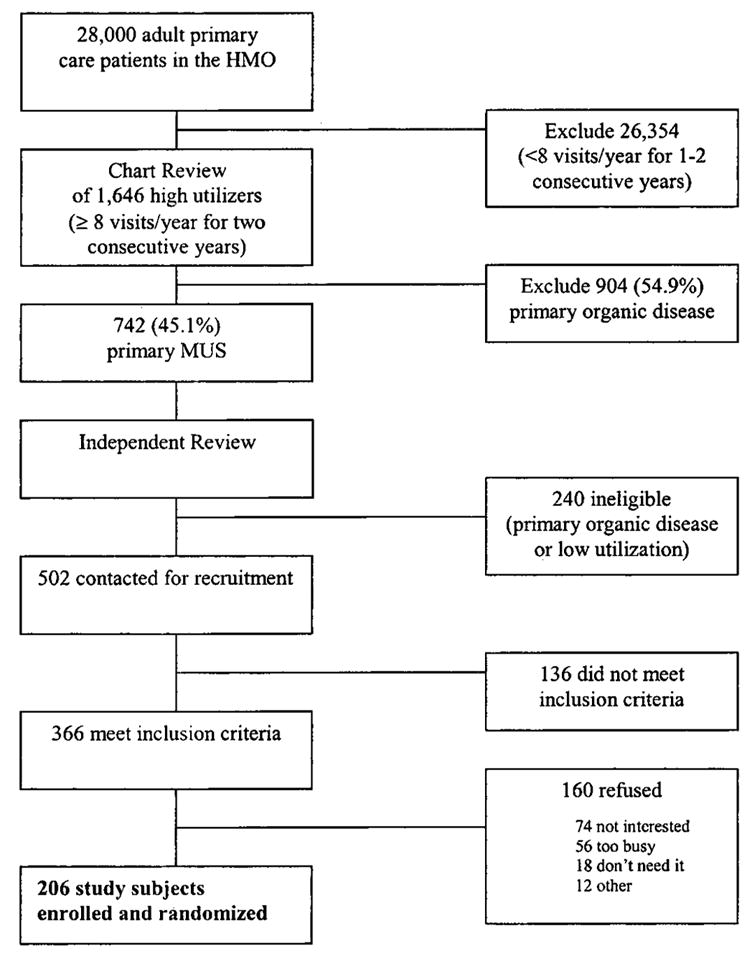
Participant flow to identify 206 subjects.
Participants
Inclusion criteria for patients with MUS recruited into the trial were being 18 to 65 years old, a member of the HMO for at least 1 year, fluent with English, access to a telephone, literate, not under care by a mental health professional more often than once per month, and planning to be in the HMO for at least 1 year. Exclusion criteria were pregnancy, substance use disorders, suicidal ideation, organic mental syndromes, and psychosis. The study was approved by the HMO and the Michigan State University Institutional Review Board, and subjects signed informed consent.
Study Measures
Research interviewers independent of the project, trained specifically for it, and monitored to ensure fidelity to task, obtained through telephone-administered interviews the SF-36 (29), CES-D (31), SSAS (30), and the PSC (32,33). The Mental Component Summary (MCS) and Physical Component Summary (PCS) were the only dimensions of the SF-36 used for this study (34). The same interviewers also collected the WHO-CIDI (27) and demographic data. The CIDI provided the DSM-IV diagnoses: Somatoform (SD, hypochondriasis, pain disorder, and conversion) and nonsomatoform (major depression, bipolar, dysthymia, obsessive-compulsive disorder, posttraumatic stress, panic, agoraphobia, social phobia, generalized anxiety disorder, and specific phobias); following DSM-IV criteria for somatization disorder, somatoform and nonsomatoform diagnoses were not mutually exclusive (16). In addition to determining entry into the study as primary MUS, chart raters’ classification of symptoms was also used for explanatory analyses: documented organic disease, documented nonorganic disease, and undocumented disease. In this population of primary MUS patients, documented organic was used to represent comorbid organic disease, whereas documented nonorganic and undocumented represented, respectively, severe and mild types of MUS.
In addition to standard DSM-IV somatoform diagnoses, we identified abridged SD (defined earlier) (28). As a measure of psychiatric comorbidity, we also obtained a count of all full (major) nonsomatoform CIDI diagnoses. Similarly, we derived a count of subthreshold (minor, abridged) diagnoses of depression, obsessive-compulsive disorder, posttraumatic stress, panic disorder with and without agoraphobia, social phobia, and generalized anxiety disorder (35–37), eg, minor depression was represented by 2 or more criteria for major depression (16). Minor diagnoses were made only in patients who had no similar major diagnosis, ie, no overlap.
Statistical Method
We created a variable, “DSM somatoform-positive,” for the presence of any 1 of the 4 full somatoform diagnoses or abridged SD; “DSM somatoform-negative” had no full or abridged somatoform diagnosis. Correlates of DSM somatoform-positive status were examined among 3 sets of variables. Demographics were age, gender, years of education (≤12 years vs. >12 years), and marital status (married vs. not married); psychologic and physical function were determined by CES-D, SSAS, PSC, MCS, and PCS; and disease severity was classified as presence or absence of any full nonsomatoform CIDI diagnoses, presence or absence of subthreshold nonsomatoform diagnoses, and symptom counts of documented organic disease, documented nonorganic disease, and undocumented disease. In addition, we considered 2 other variables: a count of all primary care visits in the past 12 months and the proportion of MUS (documented nonorganic and undocumented) to all symptoms (documented organic, documented nonorganic, and undocumented).
Logistic regression analysis was used to assess the association of potential correlates of DSM somatoform-positive/negative status. Initial analyses screened for variables that were significant at p ≤ .20. These were used in a backward elimination procedure that retained variables significant at p ≤ .10. Variables excluded at the initial stage were then added to the model to ascertain if its predictive power could be appreciably improved. Odds ratios and 95% confidence intervals were computed for all correlates of DSM somatoform-positive status in the final model. The Hosmer-Lemeshow goodness-of-fit test and c-statistic were used to gauge the reliability of the model.
RESULTS
Rater Reliability
Agreement among raters with 1 of the authors (R.C.S.) for a primary MUS problem was 97.6% (40 of 41 cases were similarly evaluated); in the 1 rater without perfect agreement, the kappa was 0.84 with agreement on 12 of 13 charts (92.3%). Interrater reliability was assessed on the basic disease category rating from 10 charts rated by all 3 raters. The following are the percentage of agreement: organic disease categories—92%; nonorganic disease categories—96%; and undocumented disease categories—92% (21).
Participant Flow
Figure 1 describes participant flow. Of 502 subjects who entered recruitment as primary MUS subjects for the clinical trial, 136 (27.1%) were determined ineligible during the interview screening (eg, changed residence, no longer in the HMO). Of the remaining 366 subjects meeting inclusion criteria, 206 were enrolled and randomized—a 56.3% recruitment rate for the RCT (38); 160 eligible subjects refused (43.7%): not interested—74; too busy—56; don’t need/want treatment—18; satisfied with doctor—4; miscellaneous or unreachable—8. There was no statistically significant clinical or demographic difference between the 206 subjects enrolled and those subjects who refused on the following measures obtained from the HMO information system and chart review procedure: age, gender, copay status, mean number of visits, and percentage of MUS symptoms.
Diagnostic and Statistical Manual of Mental Disorders, 4th Edition Profile of Medically Unexplained Symptoms Patients Other Information
Table 1 presents the profile of MUS patients.
TABLE 1.
Characteristics of High-Utilizing Patients With Medically Unexplained Symptoms (n = 206)
| Characteristic | Mean or Percent | SD | Range |
|---|---|---|---|
| Gender, female (%) | 79.1 | — | — |
| Married (%) | 71.8 | — | — |
| Education, ≤ 12 yr | 36.4 | — | — |
| Age (years) | 47.7 | 8.9 | 21–65 |
| No. visits in the past 12 months | 13.6 | 4.7 | 8–35 |
| Percent medically unexplained symptoms | 60.8 | 18.0 | 25–100 |
| CES–Depression | 15.6 | 12.2 | 0–55 |
| Psychosomatic Symptom checklist | 23.0 | 15.2 | 0–75.9 |
| Spielberger State Anxiety | 39.1 | 19.8 | 3.3–88.3 |
| SF-36 Mental Component Summary | 47.6 | 11.9 | 12.4–67.3 |
| SF-36 Physical Component Summary | 36.4 | 10.3 | 13.8–61.2 |
CES = Center for Epidemiological Studies.
Table 2 shows all nonsomatoform (“psychiatric”) diagnoses in 206 patients; 124 (60.2%) had any nonsomatoform diagnosis; 36 (17.5%) had 2 full nonsomatoform diagnoses and 41 (19.9%) had >2; 92 (44.7%) had any full anxiety diagnosis and 94 (45.6%) had either major (full) depression or minor depression diagnoses. Among patients with any depression (major or minor), 6 had a full somatoform diagnosis and 24 had abridged SD. Among patients with generalized anxiety disorder, 3 had a full somatoform diagnosis and 12 had abridged SD.
TABLE 2.
DSM-IV Diagnoses in High-Utilizing Patients With Medically Unexplained Symptoms
| Diagnosis | No. (Percent) |
|---|---|
| Nonsomatoform (“Psychiatric”) | |
| Major depression | 60 (29.1) |
| Minor depression | 34 (16.5) |
| Bipolar disorder | 7 (3.4) |
| Dysthymia | 7 (3.4) |
| Generalized anxiety disorder | 46 (22.3) |
| Agoraphobia | 8 (3.9) |
| Social phobia | 10 (4.9) |
| Specific phobia | 47 (22.8) |
| Posttraumatic stress disorder | 17 (8.3) |
| Obsessive compulsive disorder | 14 (6.8) |
| Panic disorder | 11 (5.3) |
| Somatoform | |
| Somatization disorder | 3 (1.5) |
| Hypochondriasis | 4 (1.9) |
| Chronic pain* | 2 (1.0) |
| Conversion disorder | 1 (0.5) |
| Abridged somatization disorder | 39 (18.9) |
| DSM somatoform-positive | 48 (23.3) |
One patient was positive for both chronic pain and conversion disorder.
DSM-IV = Diagnostic and Statistical Manual of Mental Disorders, 4th edition.
Table 2 also shows all somatoform disorders. Only 9 (4.4%) MUS patients had at least 1 full DSM-IV somatoform diagnosis, and another 39 (18.9%) had abridged SD. Thus, only 48 (23.3%) were “DSM somatoform-positive” and 158 (76.7%) were “DSM somatoform-negative.” Among the 48 DSM somatoform-positive patients, 11 (23%) had one full nonsomatoform diagnosis, and another 26 (54.2%) had at least 2 full nonsomatoform diagnoses. Among these 48 DSM somatoform-positive patients, 30 (62.5%) had full or minor depression, and 28 (58.3%) had any full anxiety diagnosis. Of 158 DSM somatoform-negative patients, 64 (40.5%) had any depression.
Comparison of Diagnostic and Statistical Manual of Mental Disorders Somatoform-Positive and Diagnostic and Statistical Manual of Mental Disorders Somatoform-Negative Patients
Indicating good discriminating power of DSM-IV diagnoses, Table 3 shows that DSM somatoform-positive patients were more severely dysfunctional than DSM somatoform-negative patients on all 5 psychologic and physical measures (CES-D, SSAS, MCS, PCS, and PSC). DSM somatoform-negative patients were more severely dysfunctional than general population normals (29–34) on 2 measures, CES-D and PCS.
TABLE 3.
Comparing DSM Somatoform-Positive With DSM Somatoform-Negative States, and DSM Somatoform-Negative With General Population Normal States*
| Scale | DSM-Positive (N = 48) | DSM-Negative (N = 158) | p Value | General Population Normals† | p Value‡ |
|---|---|---|---|---|---|
| CES–Depression− (CES-D) | 21.8
18.1–25.5 |
13.8
12.0–15.6 |
<.001 | 9.3
8.9–9.6 |
<.001 |
| Psychosomatic Symptom Checklist− (PSC) | 30.7
26.8–34.7 |
20.7
18.4–23.0 |
<.001 | 23.7
22.1–25.3 |
.645 |
| Spielberger State Anxiety− (SSAS) | 48.8
43.7–53.9 |
36.2
33.1–39.3 |
<.001 | 35.5
35.0–36.0 |
.665 |
| Mental Component Summary+ (MCS) | 42.0
38.6–45.5 |
49.2
47.5–51.0 |
<.001 | 50.0
49.6–50.4 |
.416 |
| Physical Component Summary+ (PCS) | 33.2
30.3–36.0 |
37.4
35.8–39.0 |
.011 | 50.0
49.6–50.4 |
<.001 |
Entries are mean and 95% confidence interval; combined, DSM-positive and DSM-negative comprise all MUS subjects.
Comparison of DSM somatoform-negative with population normals.
Positively scored scale, higher scores indicate less severe problem in the SF-36.
Negatively scored scale, higher scores indicate more severe problem.
DSM = Diagnostic and Statistical Manual of Mental Disorders; CES = Center for Epidemiological Studies; MUS = medically unexplained symptoms.
Of 43 men, 16 were DSM somatoform-positive (37.2%); of 163 women, 32 were DSM somatoform-positive (19.6%). The following variables were associated with DSM somatoform-positive status on univariable evaluation: male gender (p = .02), CES-D (p < .0001), PSC (p < .0001), SSAS (p = .0001), MCS (p = .0003), PCS (p = .012), documented nonorganic symptoms (p = .02), count of visits in the past 12 months (p = 0.07), proportion of MUS relative to all symptoms (p = .11), and the presence of any nonsomatoform CIDI diagnosis (p = 0.002). Shown in Table 4, the final logistic model for DSM somatoform-positive status contained gender, MCS, PCS, presence of any nonsomatoform CIDI diagnosis, and proportion of MUS. The c-statistic was 0.77.
TABLE 4.
Correlates of DSM Somatoform-Positive Status*
| Variable | Odds Ratio (95% CI) | p Value |
|---|---|---|
| Gender, male vs. female | 3.2 (1.4–7.5) | .007 |
| MCS, SD = 11.9 | 1.7 (1.2–2.5)† | .007 |
| PCS, SD = 10.3 | 1.7 (1.2–2.6)† | .004 |
| Nonsomatoform CIDI diagnoses, present vs. absent | 2.1 (0.9–5.1) | .094 |
| Proportion MUS, SD = 0.18 | 1.4 (0.9–1.9)‡ | .094 |
c-statistic = 0.77.
OR for a 1 SD lower score.
OR for a 1 SD higher score.
DSM = Diagnostic and Statistical Manual of Mental Disorders; CI = confidence interval; MCS = Mental Component Summary; PCS = Physical Component Summary; CIDI = Composite International Diagnostic Interview; SD = standard deviation; OR = odds ratio.
DISCUSSION
We identified 206 high-utilizing primary care patients with MUS from a medical chart review. Only 23.3% had a DSM-IV-derived somatoform diagnosis (“DSM somatoform-positive”). Patients without a full or abridged somatoform diagnosis (“DSM somatoform-negative”) constituted 76.7% of the entire MUS population. Compared with DSM somatoform-negative patients, DSM somatoform-positive patients were more severely distressed on all measures of mental and physical function. Although this was expected from the ECA Study (22,23), the surprise was the large prevalence of the DSM somatoform-negative population. Furthermore, although we did not ourselves study a normal group for comparison, the DSM somatoform-negative group was more severely distressed than general population normals suggesting, as others have (1,23,28,39–41), a spectrum of severity for MUS patients. To further differentiate the 2 groups, we identified the clinical profile of DSM somatoform-negative patients: females with less psychiatric comorbidity, less mental and physical dysfunction, and a lower proportion of MUS. In a review of the literature (25), we found little study of this population and no data on its clinical features or prevalence (22,23). In the nonresearch literature, some may have referred to these patients as the “worried well.”
We also found that depression and anxiety better characterized the entire MUS population: 60.2% had some type of DSM-IV nonsomatoform (“psychiatric”) diagnosis. This finding is consistent with research that strongly supports the association of MUS with depression/anxiety (42–45). In addition, a linear relationship of the number of nonorganic symptoms and the severity of depression/anxiety has been found (42,44,45). Data show also that the number of symptoms correlates, independently, with a personality trait of harm avoidance (46). We agree with others that MUS represents a general warning (“stress”) signal that points to underlying psychologic distress (22,47–49).
Potential Limitations
It is conceivable that our interviewers performed the WHO-CIDI incorrectly. However, we found no evidence of this on review of interviews of DSM somatoform-negative patients. Moreover, interviewers met ongoing quality control and fidelity standards multiple times during the study, the supervisor was fully trained in the WHO-CIDI (she conducted most interviews), and interviewers met regularly with the research team to discuss questions during the study.
One might try to explain our results as simply having identified a population with mild MUS, but all had a high proportion of unexplained symptoms, at least 2 consecutive years of high utilization, and they averaged 13.6 visits per year in the year before study. This degree of utilization was well beyond the 85th percentile in the HMO and, therefore, represented only the more severe tip of the primary care iceberg. We would thus expect that the majority of patients, those with lower utilization, would have even fewer DSM-IV-derived diagnoses.
Finally, although our recruitment rate of 56.3% was good for a clinical trial (38), the 43.7% who refused could have differed from the study population. Nevertheless, considerable baseline clinical and demographic data showed no significant differences from the 206 study patients, suggesting that there was no systematic bias or threat to generalization. However, the possibility of selection bias cannot altogether be excluded.
Key Issues
It is erroneous to say that DSM-IV misses over three fourths of MUS patients. Nearly 85% of our subjects were classified when we included the DSM-IV miscellaneous categories of somatoform disorder not otherwise specified and undifferentiated somatoform disorder (16); others also have found a high prevalence of undifferentiated somatoform disorder in MUS patients (50). However, these categories have not been studied or used in primary care, and we excluded them (11,51,52). That these miscellaneous categories contain the largest number of subjects suggests an inadequate nomenclature (53,54). Moreover, they typically are discarded in clinical trials and other studies and, hence, of limited value (53,54). Furthermore, the extensive use of alternatives to DSM such as the International Classification of Diseases, 10th Revision (55) and Multi-Somatoform Disorder (MSD) (51) also suggest problems with the use of the DSM-IV. On the other hand, although not included in DSM-IV, but derived from it, abridged SD is well recognized and well studied (28), and we included it among DSM somatoform-positives. Indeed, because of its much greater prevalence in our sample, it appears to be the most useful DSM-IV-derived construct. Nevertheless, it accounted for only 18.9% of our subjects, so it does not begin to provide a comprehensive description of MUS patients.
Katon et al. found a much higher prevalence of somatoform disorders in a distressed group with similar utilization (56). However, their study population included only the 51.1% who had the most severe depression and anxiety as well as the most severe somatization itself. Making this population sicker still, 62% also had moderate to severe comorbid organic diseases (56). We know that such prominent comorbid disease has an additional adverse impact on both depressed and MUS patients (57–60). Thus, severe comorbid physical disease may have rendered this already sicker population even worse. Not only do more severe patients report more physical symptoms (42,44,45), but, with the addition of serious comorbid organic disease, they would be expected to report even more symptoms—those resulting from the organic disease itself and/or those precipitated by the stress it creates. These patients would thus have many more physical symptoms that could influence DSM diagnoses. Our population differed considerably: we used the entire population of high-utilizing MUS patients, and they were not confounded by severe organic diseases, making them less severe with fewer physical symptoms and, therefore, fewer DSM diagnoses. At least for classification purposes, we believe this provides a more accurate picture of the true, more pure MUS patient.
The Role of Chart Review
The findings of this study depended on a new chart review method to define MUS patients (21). To our knowledge, it represents the only reliable chart rating procedure that provides a clinical picture by classifying physical symptoms based on documented medical evidence (21). We propose that it can provide a gold standard not previously available (61) for identifying MUS patients. DSM-IV somatoform diagnoses now depend only on patients’ interpretations of their doctors’ diagnoses to determine symptom classifications (16). There is no external validation of whether the symptom is organic or medically unexplained.
A related attribute is the rating method’s ability to identify comorbid medical conditions. The chart rating method not only can quantify medical comorbidity, but it also can be adjusted to select patients in whom comorbid organic diseases are not a primary problem. Alternatively, if one wanted to include more patients with organic diseases, the investigator simply adjusts the scoring rule. The flexibility of the rating method and its scoring rule can allow investigators, for the first time, to quantify comorbid medical disease.
The chart rating procedure is labor- and cost-intensive, requires physicians or nurse practitioners as raters, and is designed only for research. Nevertheless, the chart data provide several potential research advantages for identifying patients with MUS in comparison to studies that rely on DSM-IV (21). Chart data: 1) derive from a total of 245 possible physical symptoms (vs. 41 contained in DSM-IV (16)); others also have questioned whether such a restricted list of symptoms is useful (13); 2) involve symptoms prompting health care-seeking (HCS), as recorded at the time of actual HCS; 3) do not “forget” symptoms; data show that, with assessment of DSM somatoform criteria 12 months apart, approximately one half of subjects have forgotten their previous complaints (14); and 4) provide longitudinal evaluation, compared with cross-sectional DSM-IV data (13,14,62); DSM provides no information on the natural course of MUS patients (63).
Implications for the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition
We call for change in DSM-V in 3 ways: 1) The newly recognized, less severe MUS patients in this study will need to be incorporated (23,53,64,65). Access to the full continuum of MUS can benefit practitioners and researchers alike. Those patients at the less severe end of the spectrum can lead to greater power in studies of theoretical constructs, genetic influence, environmental risk factors, and comorbidity. For clinicians, specific diagnostic recognition of the less severe MUS patients could allow them to prevent progression along the continuum (65). A cost-effective form of treatment would therefore need to be identified (23).
2) DSM-V also will need to reflect the spectrum into which all the various disorders seem to fall (from most to least severe): somatization disorder → other somatoform disorders → MSD/abridged somatization disorder → DSM somatoform-negative → normals. DSM-V could define the spectrum as a diagnosis of “MUS spectrum disorder,” qualified, respectively, as very severe, severe, moderate, mild, and normal variant—in terms of both mental and physical severity. Those formulating DSM-V also will need to consider the placement of other newly defined MUS syndromes such as multisomatoform disorder (MSD) (51) and abridged somatization disorder (28). Importantly, recent data suggest that MUS spectrum disorder also should be classified according to the stage of illness (acute, subacute, chronic) and to the extent of comorbid organic diseases (66).
3) For research purposes, DSM-V somatoform disorders should be anchored in a gold standard means for excluding organic diseases rather than relying on patients’ reports (21). In the current absence of a gold standard (61), we propose that our recently developed chart rating method can be used (21) or that others be developed. Directly examining and evaluating individual patients to document comorbid organic disease would be a still better way, but expense likely will preclude this. Whatever the method, the field will benefit by being able to effectively identify MUS patients (by the absence of an organic disease explanation) and to identify and quantify their comorbid organic diseases.
Other Implications
Although there are no data on the management of DSM somatoform-negative patients, this study indicates that they are less severe and, therefore, perhaps amenable to little or no investigation and to the less-intensive aspects of treatment outlined in a recent review (25). Much study of the practical diagnosis and treatment of this new group will be needed, and, at the same time, we will need to begin exploring its public health implications (eg, utilization of resources), how to effectively screen for it, and when these patients should, if ever, be referred.
Glossary
- HMO
health maintenance organization
- DSM-IV
Diagnostic and Statistical Manual of Mental Disorders, 4th edition
- DSM-V
planned for approximately 2010, the Diagnostic and Statistical Manual of Mental Disorders, 5th edition
- MUS
medically unexplained symptoms
- ECA
epidemiologic catchment area
- SD
somatization disorder
- SF-36
Short-Form 36
- MCS
Mental Component Summary of the SF-36
- PCS
Physical Component Summary of the SF-36
- SSAS
Spielberger State Anxiety Scale
- CES-D
Center for Epidemiological Studies Depression inventory
- PSC
Psychosomatic Symptom Checklist
- WHO-CIDI
World Health Organization Composite International Diagnostic Interview
Footnotes
This research was supported by NIMH grant MH57099.
The authors are grateful for the support and cooperation from colleagues at Blue Cross Network, Lansing, Michigan. The authors also thank the Michigan Public Health Institute for their always effective and friendly assistance in the data gathering.
References
- 1.Escobar JI, Manu P, Matthews D, Lane T, Swartz M, Canino G. Medically unexplained physical symptoms, somatization disorder and abridged somatization: studies with the Diagnostic Interview Schedule. Psychiatric Development. 1989;3:235–45. [PubMed] [Google Scholar]
- 2.Escobar JI, Waitzkin H, Silver RC, Gara M, Holman A. Abridged somatization: a study in primary care. Psychosom Med. 1998;60:466–72. doi: 10.1097/00006842-199807000-00012. [DOI] [PubMed] [Google Scholar]
- 3.Katon W, Russo J. Somatic symptoms and depression. J Fam Pract. 1989;29:65–9. [PubMed] [Google Scholar]
- 4.deGruy F, Crider J, Hashimi DK, Dickinson P, Mullins HC, Troncale J. Somatization disorder in a university hospital. J Fam Pract. 1987;25:579–84. [PubMed] [Google Scholar]
- 5.Smith RC, Gardiner JC, Lyles JS, Johnson M, Rost KM, Luo Z, Goddeeris J, Lein C, Given WE, Given B, Dwamena FC, Collins C, Van Egeren LF, Korban E, Kanj M, Haddad R. Minor acute illness: a preliminary research report of the ‘worried well’. J Fam Pract. 2002;51:24–9. [PubMed] [Google Scholar]
- 6.deGruy F, Columbia L, Dickinson P. Somatization disorder in a family practice. J Fam Pract. 1987;25:45–51. [PubMed] [Google Scholar]
- 7.Kroenke K, Arrington ME, Mangelsdorff AD. The prevalence of symptoms in medical outpatients and the adequacy of therapy. Arch Intern Med. 1990;150:1685–9. doi: 10.1001/archinte.150.8.1685. [DOI] [PubMed] [Google Scholar]
- 8.Kroenke K, Mangelsdorff AD. Common symptoms in ambulatory care: incidence, evaluation, therapy, and outcome. Am J Med. 1989;86:262–6. doi: 10.1016/0002-9343(89)90293-3. [DOI] [PubMed] [Google Scholar]
- 9.Connelly JE, Smith GR, Philbrick JT, Kaiser DL. Healthy patients who perceive poor health and their use of primary care services. J Gen Intern Med. 1991;6:47–51. doi: 10.1007/BF02599392. [DOI] [PubMed] [Google Scholar]
- 10.Pincus HA. The future of behavioral health and primary care: drowning in the mainstream or left on the bank? Psychosomatics. 2003;44:1–11. doi: 10.1176/appi.psy.44.1.1. [DOI] [PubMed] [Google Scholar]
- 11.Kroenke K, Spitzer RL, deGruy FV, Swindle R. A symptom checklist to screen for somatoform disorders in primary care. Psychosomatics. 1998;39:263–72. doi: 10.1016/S0033-3182(98)71343-X. [DOI] [PubMed] [Google Scholar]
- 12.Rief W, Hiller W, Geissner E, Fichter MM. A two-year follow-up study of patients with somatoform disorders. Psychosomatics. 1995;36:376–86. doi: 10.1016/S0033-3182(95)71647-4. [DOI] [PubMed] [Google Scholar]
- 13.Fink P. Physical complaints and symptoms of somatizing patients. J Psychosom Res. 1992;36:125–36. doi: 10.1016/0022-3999(92)90021-s. [DOI] [PubMed] [Google Scholar]
- 14.Simon GE, Gureje O. Stability of somatization disorder and somatization symptoms among primary care patients. Arch Gen Psychiatry. 1999;56:90–5. doi: 10.1001/archpsyc.56.1.90. [DOI] [PubMed] [Google Scholar]
- 15.Cloninger CR, Martin RL, Guze SB, Clayton PJ. A prospective follow-up and family study of somatization in men and women. Am J Psychiatry. 1986;143:873–8. doi: 10.1176/ajp.143.7.873. [DOI] [PubMed] [Google Scholar]
- 16.Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 17.Boland RJ. How could the validity of the DSM-IV Pain Disorder be improved in reference to the concept that it is supposed to identify? Curr Pain Headache Rep. 2002;6:23–9. doi: 10.1007/s11916-002-0020-y. [DOI] [PubMed] [Google Scholar]
- 18.Wessely S, Nimnuan C, Sharpe M. Functional somatic syndromes: one or many? Lancet. 1999;354:936–9. doi: 10.1016/S0140-6736(98)08320-2. [DOI] [PubMed] [Google Scholar]
- 19.Kupfer DJ, First MB, Regier DE. Introduction. In: Kupfer DJ, First MB, Regier DA, editors. A Research Agenda for DSM-V. Washington, DC: American Psychiatric Association; 2002. pp. xv–xxii. [Google Scholar]
- 20.Phillips KA, Price LH, Greenberg BD, Rasmussen SA. Should the DSM diagnostic groupings be changed? In: Phillips KA, First MB, Pincus HA, editors. Advancing DSM—Dilemmas in Psychiatric Diagnoses. Washington, DC: American Psychiatric Association; 2003. pp. 57–84. [Google Scholar]
- 21.Smith RC, Korban E, Kanj M, Haddad R, Lyles JS, Lein C, Gardiner JC, Hodges A, Dwamena FC, Coffey J, Collins C. A method for rating charts to identify and classify patients with medically unexplained symptoms. Psychother Psychosom. 2004;73:36–42. doi: 10.1159/000074438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Swartz M, Landerman R, George LK, Blazer DG, Escobar J. Somatization disorder. In: Robins LN, Regier DA, editors. Psychiatric Disorders in America—The Epidemiologic Catchment Area Study. New York: The Free Press (Macmillan, Inc); 1991. pp. 220–57. [Google Scholar]
- 23.Kessler RC, Merikangas KR, Berglund P, Eaton WW, Koretz DS, Walters EE. Mild disorders should not be eliminated from the DSM-V. Arch Gen Psychiatry. 2003;60:1117–22. doi: 10.1001/archpsyc.60.11.1117. [DOI] [PubMed] [Google Scholar]
- 24.Lyles JS, Hodges A, Collins C, Lein C, Given CW, Given B, D’Mello D, Osborn GG, Goddeeris J, Gardiner JC, Smith RC. Using nurse practitioners to implement an intervention in primary care for high utilizing patients with medically unexplained symptoms. Gen Hosp Psychiatry. 2003;25:63–73. doi: 10.1016/s0163-8343(02)00288-8. [DOI] [PubMed] [Google Scholar]
- 25.Smith RC, Lein C, Collins C, Lyles JS, Given B, Dwamena FC, Coffey J, Hodges A, Gardiner JC, Goddeeris J, Given CW. Treating patients with medically unexplained symptoms in primary care. J Gen Intern Med. 2003;18:478–89. doi: 10.1046/j.1525-1497.2003.20815.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lein C, Collins C, Lyles JS, Hillman D, Smith RC. Building research relationships with managed care organizations: issues and strategies. Families, Systems & Health. 2003;21:205–14. doi: 10.1037/h0089607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sartorius N. Composite International Diagnostic Interview (CIDI)—Core Version 1.1. Geneva: World Health Organization; [Google Scholar]
- 28.Escobar JI, Swartz M, Rubio-Stipec M, Manu P. Medically unexplained symptoms: distribution, risk factors, and comorbidity. In: Kirmayer LJ, Robbins JM, editors. Current Concepts of Somatization: Research and Clinical Perspectives. Washington, DC: American Psychiatric Press, Inc; 1991. pp. 63–78. [Google Scholar]
- 29.Ware JJE, Snow KK, Kosinski M, Gandek B. SF-36 Health Survey—Manual and Interpretation Guide. Boston: The Health Institute, New England Medical Center; 1993. [Google Scholar]
- 30.Spielberger CD, Gorsuch RL, Lushene PR, Jacobs GA. State-Trait Anxiety Inventory (Form Y) (‘Self-Evaluation Questionnaire’) Palo Alto, CA: Consulting Psychologists Press, Inc; 1983. [Google Scholar]
- 31.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Measur. 1977;1:385–401. [Google Scholar]
- 32.Chibnall J, Tait R. The Psychosomatic Symptom Checklist revisited: reliability and validity in a chronic pain population. J Behav Med. 1989;12:297–307. doi: 10.1007/BF00844873. [DOI] [PubMed] [Google Scholar]
- 33.Andrasik F, Blanchard E, Arena J, Teders S, Teevan R, Rodichok L. Psychological functioning in headache sufferers. Psychosom Med. 1982;44:171–82. doi: 10.1097/00006842-198205000-00003. [DOI] [PubMed] [Google Scholar]
- 34.Ware JE, Jr, Kosinski M, Keller SD. SF-36 Physical and Mental Health Summary Scales: A User’s Manual. Boston: The Health Institute, New England Medical Center; 1994. [Google Scholar]
- 35.Katon W, Robinson P, von Korff M, Lin E, Bush T, Ludman E, Simon G, Walker E. A multifaceted intervention to improve treatment of depression in primary care. Arch Gen Psychiatry. 1996;53:924–32. doi: 10.1001/archpsyc.1996.01830100072009. [DOI] [PubMed] [Google Scholar]
- 36.Olfson M, Broadhead WE, WEissman MM, Leon AC, Farber L, Hove C, Kathol R. Subthreshold psychiatric symptoms in a primary care group practice. Arch Gen Psychiatry. 1996;53:880–6. doi: 10.1001/archpsyc.1996.01830100026004. [DOI] [PubMed] [Google Scholar]
- 37.Marshall RD, Olfson M, Hellman F, Blanco C, Guardino M, Struening EL. Comorbidity, impairment, and suicidality in subthreshold PTSD. Am J Psychiatry. 2001;158:1467–73. doi: 10.1176/appi.ajp.158.9.1467. [DOI] [PubMed] [Google Scholar]
- 38.Barsky AJ, Ahern DK. Cognitive behavior therapy for hypochondriasis—a randomized controlled trial. JAMA. 2004;291:1464–70. doi: 10.1001/jama.291.12.1464. [DOI] [PubMed] [Google Scholar]
- 39.Murphy MR. Classification of the somatoform disorders. In: Bass CM, editor. Somatization: Physical Symptoms and Psychological Illness. Oxford: Blackwell; 1990. pp. 10–39. [Google Scholar]
- 40.Katon W, Lin E, von Korff M, Russo J, Lipscomb P, Bush T. Somatization: a spectrum of severity. Am J Psychiatry. 1991;148:34–40. doi: 10.1176/ajp.148.7.A34. [DOI] [PubMed] [Google Scholar]
- 41.Katon W, Sullivan M, Walker E. Medical symptoms without identified pathology: relationship to psychiatric disorders, childhood and adult trauma, and personality traits. Ann Intern Med. 2001;134:917–25. doi: 10.7326/0003-4819-134-9_part_2-200105011-00017. [DOI] [PubMed] [Google Scholar]
- 42.Katon WJ, Walker EA. Medically unexplained symptoms in primary care. J Clin Psychiatry. 1998;59(suppl 20):15–21. [PubMed] [Google Scholar]
- 43.Kroenke K, Jackson JL, Chamberlin J. Depressive and anxiety disorders in patients presenting with physical complaints: clinical predictors and outcome. Am J Med. 1997;103:339–47. doi: 10.1016/s0002-9343(97)00241-6. [DOI] [PubMed] [Google Scholar]
- 44.Kroenke K, Spitzer RL, Williams JBW, Linzer M, Hahn SR, deGruy FV, III, Brody D. Physical symptoms in primary care—predictors of psychiatric disorders and functional impairment. Arch Fam Med. 1994;3:774–9. doi: 10.1001/archfami.3.9.774. [DOI] [PubMed] [Google Scholar]
- 45.Kisely S, Goldberg D, Simon G. A comparison between somatic symptoms with and without clear organic cause: results of an international study. Psychol Med. 1997;27:1011–9. doi: 10.1017/s0033291797005485. [DOI] [PubMed] [Google Scholar]
- 46.Russo J, Katon W, Sullivan M, Clark M, Buchwald D. Severity of somatization and its relationship to psychiatric disorders and personality. Psychosomatics. 1994;35:546–56. doi: 10.1016/S0033-3182(94)71723-0. [DOI] [PubMed] [Google Scholar]
- 47.Simon G, Gater R, Kisely S, Piccinelli M. Somatic symptoms of distress: an international primary care study. Psychosom Med. 1996;58:481–8. doi: 10.1097/00006842-199609000-00010. [DOI] [PubMed] [Google Scholar]
- 48.Simon GE, VonKorff M. Somatization and psychiatric disorder in the NIMH Epidemiologic Catchment Area Study. Am J Psychiatry. 1991;148:1494–500. doi: 10.1176/ajp.148.11.1494. [DOI] [PubMed] [Google Scholar]
- 49.Henningsen P, Zimmermann T, Sattel H. Medically unexplained physical symptoms, anxiety, and depression: a meta-analytic review. Psychosom Med. 2003;65:528–33. doi: 10.1097/01.psy.0000075977.90337.e7. [DOI] [PubMed] [Google Scholar]
- 50.De Wall MWM, Arnold IA, Eekhof JAH, Van Hemert AM. Somatoform disorders in general practice—prevalence, functional impairment and comorbidity with anxiety and depressive disorders. Brit J Psychiatry. 2004;184:470–6. doi: 10.1192/bjp.184.6.470. [DOI] [PubMed] [Google Scholar]
- 51.Kroenke K, Spitzer RL, deGruy FV, Hahn SR, Linzer M, Williams JBW, Brody D, Davies M. Multisomatoform disorder—an alternative to undifferentiated somatoform disorder for the somatizing patient in primary care. Arch Gen Psychiatry. 1997;54:352–8. doi: 10.1001/archpsyc.1997.01830160080011. [DOI] [PubMed] [Google Scholar]
- 52.Mayou R, Levenson J, Sharpe M. Somatoform disorders in DSM-V. Psychosomatics. 2003;44:449–51. doi: 10.1176/appi.psy.44.6.449. [DOI] [PubMed] [Google Scholar]
- 53.Widiger TA, Clark LA. Toward DSM-V and the classification of psychopathology. Psychol Bull. 2000;126:946–63. doi: 10.1037/0033-2909.126.6.946. [DOI] [PubMed] [Google Scholar]
- 54.Helmuth L. In sickness or in health? Science. 2003;302:808–10. doi: 10.1126/science.302.5646.808. [DOI] [PubMed] [Google Scholar]
- 55.The ICD-10 Classification of Mental and Behavioral Disorders—Clinical Descriptions and Diagnostic Guidelines. Geneva: World Health Organization; 1992. [Google Scholar]
- 56.Katon W, von Korff M, Lin E, Lipscomb P, Russo J, Wagner E, Polk E. Distressed high utilizers of medical care: DSM-III-R diagnoses and treatment needs. Gen Hosp Psychiatry. 1990;12:355–62. doi: 10.1016/0163-8343(90)90002-t. [DOI] [PubMed] [Google Scholar]
- 57.Koike AK, Unutzer J, Wells KB. Improving the care for depression in patients with comorbid medical illness. Am J Psychiatry. 2002;159:1738–45. doi: 10.1176/appi.ajp.159.10.1738. [DOI] [PubMed] [Google Scholar]
- 58.Brown C, Schulberg HC, Prigerson HG. Factors associated with symptomatic improvement and recovery from major depression in primary care patients. Gen Hosp Psychiatry. 2000;22:242–50. doi: 10.1016/s0163-8343(00)00086-4. [DOI] [PubMed] [Google Scholar]
- 59.Goodwin RD, Kroenke K, Hoven CW, Spitzer RL. Major depression, physical illness, and suicidal ideation in primary care. Psychosom Med. 2003;65:501–5. doi: 10.1097/01.psy.0000041544.14277.ec. [DOI] [PubMed] [Google Scholar]
- 60.Khan AA, Khan A, Harezlak J, Tu W, Kroenke K. Somatic symptoms in primary care: etiology and outcome. Psychosomatics. 2003;44:471–8. doi: 10.1176/appi.psy.44.6.471. [DOI] [PubMed] [Google Scholar]
- 61.Faraone SV, Tsuang MT. Measuring diagnostic accuracy in the absence of a ‘gold standard’. Am J Psychiatry. 1994;151:650–7. doi: 10.1176/ajp.151.5.650. [DOI] [PubMed] [Google Scholar]
- 62.Lynch DJ, McGrady A, Nagel R, Zsembik C. Somatization in family practice: comparing 5 methods of classification. Primary Care Companion J Clin Psychiatry. 1999;1:85–9. doi: 10.4088/pcc.v01n0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gureje O, Simon GE. The natural history of somatization in primary care. Psychol Med. 1999;29:669–76. doi: 10.1017/s0033291799008417. [DOI] [PubMed] [Google Scholar]
- 64.deGruy F., III . Mental health care in the primary care setting. In: Donaldson MS, Yordy KD, Lohr KN, Vanselow NA, editors. Primary Care—America’s Health in a New Era. Washington, DC: National Academy Press; 1996. pp. 285–311. [PubMed] [Google Scholar]
- 65.Norquist G, Hyman SE. Advances in understanding and treating mental illness: implications for policy. Health Aff (Millwood) 1999;18:32–47. doi: 10.1377/hlthaff.18.5.32. [DOI] [PubMed] [Google Scholar]
- 66.Klinkman MS, Coyne JC, Gallo S, Schwenk TL. False positives, false negatives, and the validity of the diagnosis of major depression in primary care. Arch Fam Med. 1998;7:451–61. doi: 10.1001/archfami.7.5.451. [DOI] [PubMed] [Google Scholar]


