Abstract
Intramyocellular lipid (IMCL) plays an important role in the study of metabolism in vivo. Magnetic resonance spectroscopy (MRS) studies of IMCL are usually performed with clinical 1.5-T magnetic resonance imaging (MRI) systems and have employed the single-voxel MRS technique. The present study reports the results of our systematic evaluation of the ability of single- and multi-voxel MRS to yield high-quality, contamination-free IMCL levels from the tibialis anterior (TA) muscle. A clinical, 1.5-T, whole-body MRI scanner was used to measure IMCL with a standard knee coil, head coil, or a 3-cm receive-only surface coil with a body coil transmit. Excellent IMCL spectra were obtained in healthy males in only 8 min from multiple 0.25-cm3 voxels using the surface coil receive/body coil transmit in conjunction with the standard PRESS spectroscopic imaging (SI) technique. This approach provided the spatial resolution and voxel placement flexibility permitting optimal separation of IMCL and extramyocellular lipid. Our findings demonstrate the potential of the SI approach.
Keywords: Intramyocellular lipid, Magnetic resonance spectroscopy
Introduction
Intramyocellular lipid (IMCL) provides an important source of cellular energy for skeletal muscle that can be metabolized under conditions of increased demand. Investigation of the interplay between IMCL and insulin resistance is providing an improved understanding of the etiology of obesity and diabetes mellitus.
Most previous attempts to establish correlations between IMCL and insulin resistance in obesity and diabetes mellitus were based on analysis of muscle biopsy samples [1]. However, measures of IMCL derived in this manner may be of limited value due to the possibility of contamination by extramyocellular lipid (EMCL). Also, IMCL and EMCL are not easily separated on biopsy specimens.
Recently, it has been demonstrated that single-voxel magnetic resonance spectroscopy (MRS) techniques are capable of distinguishing IMCL from EMCL in vivo, thereby offering the potential to derive more reliable IMCL measures [2–5]. Furthermore, MRS is noninvasive, which is a clear and distinct advantage over biopsy in longitudinal studies. Existing single-voxel MRS-based methods for measuring in vivo IMCL are limited in spatial resolution, which leads to degraded spectral resolution. In the present study we evaluated single-voxel and multi-voxel IMCL measurement methods.
Methods
Single-voxel MRS and spectroscopic imaging (SI) methods for evaluating IMCL were applied to the tibialis anterior (TA) muscle of healthy male subjects. A clinical 1.5-T whole-body scanner (SIGNA, General Electric, Milwaukee, WI, USA) was applied in all studies with the standard system knee coil, head coil, or a 3-cm receive-only surface coil with a body coil transmit.
Single-voxel MRS
A 1-cm3 voxel was positioned in the TA muscle using an optimized PRESS sequence (repetition time, 2 s; echo time, 35 ms; 128 acquisitions; water presaturation).
Two-dimensional MRS
Two dimensions of in-plane phase encoding (32×32) over 16.0×16.0 cm2 resulted in a nominal voxel resolution of 0.25 cm3 with the multi-voxel version of the PRESS technique (repetition time, 2 s; echo time, 35 ms; 128 acquisitions; water pre-saturation).
All data were processed with XsOs NMR software, developed in-house by two of the investigators (XM, DCS), for postprocessing of MRS studies.
Results
A spectrum obtained with single-voxel MRS is shown in Fig 1. Though the IMCL and EMCL resonances have been resolved, the peak separation is poor, despite use a spectral resolution-enhancing filter. Figure 2 shows an SI data set obtained in 8 minutes using a surface coil receive/body coil transmit arrangement. The SI data have been overlaid on a magnetic resonance image (MRI) of the calf of a male subject from which they were recorded. A spectrum extracted from SI voxel within the subject’s TA muscle is shown in Fig. 3, showing a significantly improved separation of IMCL and IMCL; also note the significant decrease of the intensity of the EMCL peak in the SI spectrum compared to that in the single voxel MRS spectrum.
Fig. 1.
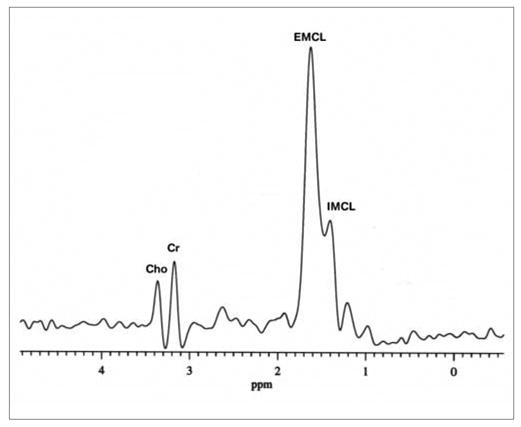
Spectrum of single-voxel magnetic resonance spectroscopy (MRS). Cho, choline; Cr, creatine; IMCL, intramyocellular lipid; EMCL, extramyocellular lipid
Fig. 2.
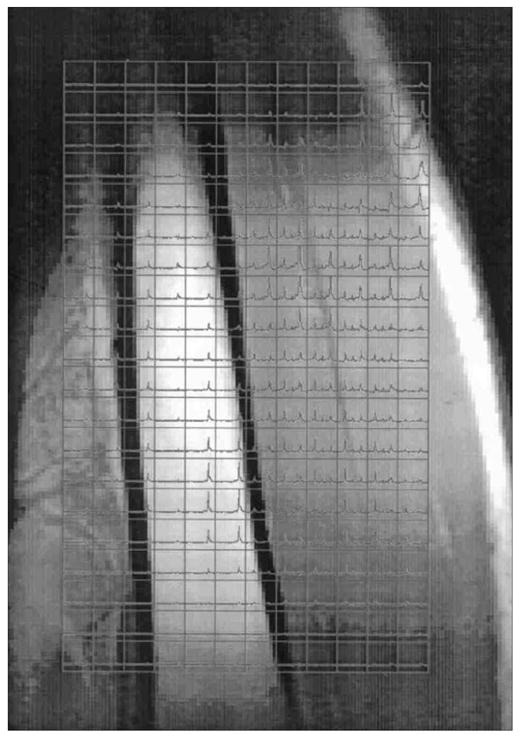
Overlay of 2-D magnetic resonance spectroscopy (MRS) on a coronal magnetic resonance imaging (MRI) scan of a male subject’s right calf. The bone in the middle of the image is the tibia, the muscle to the right of the bone is the tibialis muscle
Fig. 3.
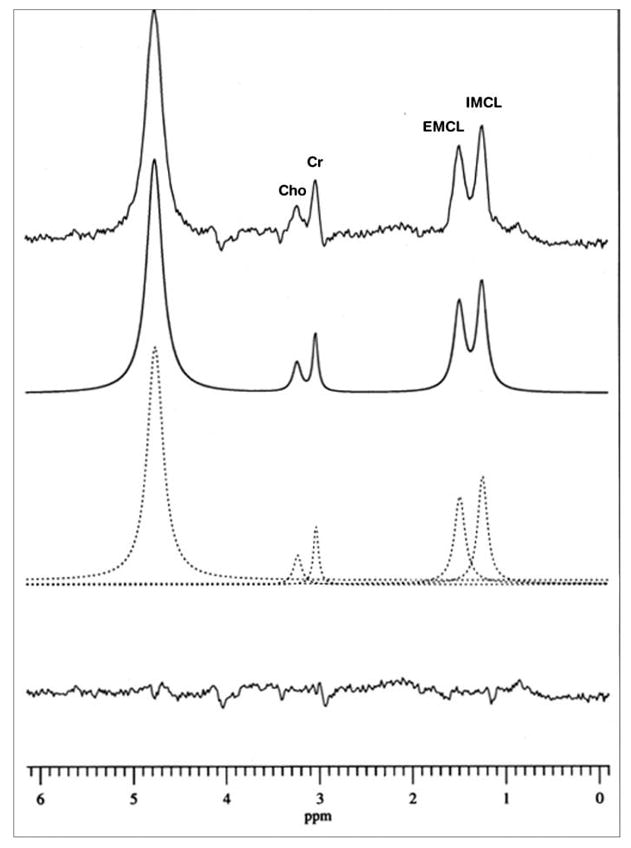
Spectrum from a SI voxel in the tibialis anterior muscle of a male subject. The experimental spectrum has been fitted to a sum of Lorentzian lines. Cho, choline; Cr, creatine; IMCL, intramyocellular lipid; EMCL, extramyocellular lipid
Discussion
Except for two studies which were performed using experimental high magnetic field MR systems [6, 7], all previously reported MRS studies of IMCL in human muscle were conducted on standard clinical 1.5 T MRI systems using the single-voxel technique [2, 8–10]. In the present study, we have demonstrated that at 1.5 T the multi-voxel or magnetic resonance spectroscopic imaging (MRSI) approach holds two critically important advantages over single-voxel MRS with respect to in vivo detection of IMCL: (a) higher spatial resolution and (b) increased flexibility in placing the voxel of interest. By virtue of their relatively small sizes, MRSI voxels can be found that are contained almost exclusively within the desired muscle tissue. As a result IMCL spectra within such a voxel will exhibit minimal contamination by EMCL from surrounding or adjacent adipose tissue. Moreover, because MRSI is able to record spectra simultaneously from multiple relatively small voxels encompassing the tissue of interest (Fig. 2), there is no need for prior careful voxel placement, which, in addition to requiring an experienced technician [8], could be quite time-consuming for obese subjects [9]. With the flexibility of the multi-voxel MRSI approach, voxels of interest exhibiting the least amount of adipose tissue contamination can simply be selected after data acquisition and processing. Clearly, this flexibility afforded by MRSI for post-acquisition voxel selection would minimize set up time in comparison with the single-voxel technique, in which careful and possibly time-consuming voxel placement is necessary before acquisition to ensure minimal adipose tissue contamination.
In summary, we have demonstrated that MRSI may be better suited than single-voxel MRS for use in studies of IMCL at 1.5T.
Acknowledgments
Supported by National Institutes of Health Grants NIDDK 42618 and 1 R01 DK57508-01.
Contributor Information
W. Shen, Obesity Research Center, St. Luke’s-Roosevelt Hospital, Institute of Human Nutrition, College of Physicians and Surgeons, Columbia University, New York, USA
X. Mao, Department of Radiology, The Hatch NMR Research Center, Columbia University, College of Physicians and Surgeons, New York, USA
Z. Wang, Obesity Research Center, St. Luke’s-Roosevelt Hospital, Institute of Human Nutrition, College of Physicians and Surgeons, Columbia University, New York, USA
M. Punyanitya, Obesity Research Center, St. Luke’s-Roosevelt Hospital, Institute of Human Nutrition, College of Physicians and Surgeons, Columbia University, New York, USA
S.B. Heymsfield, Obesity Research Center, St. Luke’s-Roosevelt Hospital, Institute of Human Nutrition, College of Physicians and Surgeons, Columbia University, New York, USA
D.C. Shungu, Department of Radiology, The Hatch NMR Research Center, Columbia University, College of Physicians and Surgeons, New York, USA
References
- 1.Pan AD, Lillioja S, Kriketos AD, et al. Skeletal muscle triglyceride levels are inversely related to insulin action. Diabetes. 1997;46:983–988. doi: 10.2337/diab.46.6.983. [DOI] [PubMed] [Google Scholar]
- 2.Bachmann OP, Dahl DB, Brechtel K, et al. Effects of intravenous and dietary lipid challenge on intramyocellular lipid content and the relation with insulin sensitivity in humans. Diabetes. 2001;50:2579–2584. doi: 10.2337/diabetes.50.11.2579. [DOI] [PubMed] [Google Scholar]
- 3.Boden G, Lebed B, Schatz M, Homko C, Lemieux S. Effects of acute changes of plasma free fatty acids on intramyocellular fat content and insulin resistance in healthy subjects. Diabetes. 2001;50:1612–1617. doi: 10.2337/diabetes.50.7.1612. [DOI] [PubMed] [Google Scholar]
- 4.Greco AV, Mingrone G, Giancaterini A, et al. Insulin resistance in morbid obesity: reversal with intramyocellular fat depletion. Diabetes. 2002;51:144–151. doi: 10.2337/diabetes.51.1.144. [DOI] [PubMed] [Google Scholar]
- 5.Krssak M, Petersen KF, Dresner A, et al. Intramyocellular lipid concentrations are correlated with insulin sensitivity in humans: a 1H NMR spectroscopy study. Diabetologia. 1999;42:113–116. doi: 10.1007/s001250051123. [DOI] [PubMed] [Google Scholar]
- 6.Larson-Meyer DE, Newcomer BR, Hunter GR. Influence of endurance running and recovery diet on intramyocellular lipid content in women: a 1H NMR study. Am J Physiol Endocrinol Metab. 2002;282:95–106. doi: 10.1152/ajpendo.2002.282.1.E95. [DOI] [PubMed] [Google Scholar]
- 7.Hwang JH, Pan JW, Heydari S, Hetherington HP, Stein DT. Regional differences in intramyocellular lipids in humans observed by in vivo 1H-MR spectroscopic imaging. J Appl Physiol. 2001;90:1267–1274. doi: 10.1152/jappl.2001.90.4.1267. [DOI] [PubMed] [Google Scholar]
- 8.Boesch C, Decombaz J, Slotboom J, Kreis R. Observation of intramyocellular lipids by means of 1H magnetic resonance spectroscopy. Proc Nutr Soc. 1999;58:841–850. doi: 10.1017/s0029665199001147. [DOI] [PubMed] [Google Scholar]
- 9.Thomas EL, Saeed N, Hajnal JV, et al. Magnetic resonance imaging of total body fat. J Appl Physiol. 1998;85:1778–1785. doi: 10.1152/jappl.1998.85.5.1778. [DOI] [PubMed] [Google Scholar]
- 10.Brechtel K, Dahl DB, Machann J, et al. Fast elevation of the intramyocellular lipid content in the presence of circulating free fatty acids and hyperinsulinemia: a dynamic 1H-MRS study. Magn Reson Med. 2001;45:179–183. doi: 10.1002/1522-2594(200102)45:2<179::aid-mrm1023>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]


