Abstract
Recent advances in imaging techniques and understanding of differences in the molecular biology of adipose tissue has rendered classical anatomy obsolete, requiring a new classification of the topography of adipose tissue. Adipose tissue is one of the largest body compartments, yet a classification that defines specific adipose tissue depots based on their anatomic location and related functions is lacking. The absence of an accepted taxonomy poses problems for investigators studying adipose tissue topography and its functional correlates. The aim of this review was to critically examine the literature on imaging of whole body and regional adipose tissue and to create the first systematic classification of adipose tissue topography. Adipose tissue terminology was examined in over 100 original publications. Our analysis revealed inconsistencies in the use of specific definitions, especially for the compartment termed “visceral” adipose tissue. This analysis leads us to propose an updated classification of total body and regional adipose tissue, providing a well-defined basis for correlating imaging studies of specific adipose tissue depots with molecular processes.
Keywords: body composition, computed tomography, magnetic resonance imaging, body fat
Introduction
Increased adipose tissue mass is the primary phenotypic characteristic of obesity. The amount and distribution of adipose tissue is associated with many of the adverse consequences of obesity, such as coronary artery disease and type 2 diabetes (1–4).
Recently, it has been discovered that adipose tissue is not a single homogeneous compartment, but rather a tissue with specific regional depots with varying biological functions (5–7). Moreover, individual adipose tissue compartments have stronger associations with physiological and pathological processes than does total adipose tissue mass (6,8–11).
Although there is intense and increasing interest in regional adipose tissue compartments, there is still little available information or formal consensus on the nomenclature of regional adipose tissue depots. Whereas computerized axial tomography (CT)1 and magnetic resonance imaging (MRI) are often used to quantify adipose tissue volumes, authors vary greatly in their definition of the adipose tissue compartments they measure.
Here we review some of the complexities posed by quantification of adipose tissue by imaging methods, focusing on classification issues. The first section is an overview of differences between adipose tissue and the group of molecular-level components referred to collectively as fat. The next section explores traditional adipose tissue classification systems. We then critically examine imaging-related terminology used in metabolic research. As part of our review, in each section, we recommend what we believe is appropriate adipose tissue terminology for providing a unified imaging-based classification. We conclude with recommendations for future research.
Adipose Tissue vs. Fat
Imaging methods, CT and MRI, quantify “adipose tissue” volume as voxels or volume elements. While often referred to as “fat” according to the five-level body composition model, adipose tissue and fat are different components (12). The distinction between fat and adipose tissue in common usage is usually irrelevant, and the terms are almost always used interchangeably. However, in the body composition and metabolism field, “fat” and “adipose tissue” are distinct and different compartments (Figure 1) (12), and their taxonomic separation is important when measuring their mass and metabolic characteristics.
Figure 1.
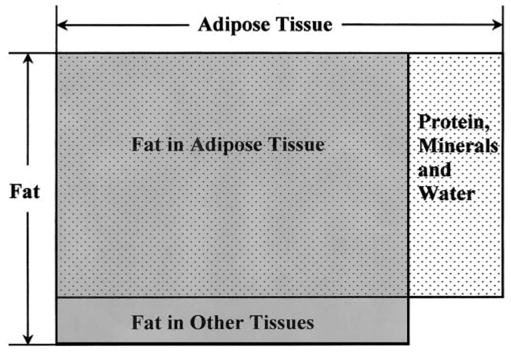
The relationship between chemical fat (or lipid) and adipose tissue.
A component at the tissue-organ body composition level (12), adipose tissue is a specialized loose connective tissue that is extensively laden with adipocytes. Adipose tissue has mainly been viewed as an energy storage depot, thermal insulator, and mechanical cushion in mammals. The 70-kg Reference Man has 15 kg of adipose tissue, representing 21% of body mass (13). The percentage is higher in women, the elderly, and overweight subjects. Adipose tissue is anatomically distributed throughout the human body, and the pattern of adipose tissue distribution is influenced by many factors, including sex, age, genotype, diet, physical activity level, hormones, and drugs (14–19).
In contrast to adipose tissue, the molecular level or chemical component fat is usually lipid in the form of triglycerides (12). Although fat is found primarily in adipose tissue, fat also exists in other tissues, especially in pathological conditions such as hepatic steatosis and various forms of lipidosis. Triglycerides in other tissues, such as in skeletal muscle, can be quantified by magnetic resonance spectroscopy (20). The most widely used current method for quantifying fat in vivo is DXA, whereas chemical analysis is used in vitro (21,22). Adipose tissue contains ~80% fat; the remaining ~20% is water, protein, and minerals (13).
Investigators in the field of metabolism often quantify fat or adipose tissue and find that the total mass of the two compartments in adults is similar, but not identical (23–27). This review concentrates on regional and total body adipose tissue, not fat or lipid, as quantified by the two main imaging methods, CT and MRI.
Traditional Adipose Tissue Classification
Classical anatomy was mainly organ-centered, without recognizing the specialized organ-like functions of different tissues. This was especially true of adipose tissue, which only recently has been recognized as an “endocrine organ” (28). We reviewed many 19th and early 20th century anatomy texts and found a conspicuous lack of detail in regard to adipose tissue classification.
Among typical approaches we did find in early texts, one is based on simple anatomic adipose tissue groupings not defined by traditional anatomic landmarks. According to this approach, adipose tissue can be typically organized into simple categories such as subcutaneous adipose tissue, organ-surrounding adipose tissue, interstitial adipose tissue, and adipose tissue in bone marrow (29). Subcutaneous adipose tissue is known to gross anatomists as superficial fascia and is defined as the adipose tissue layer found between the dermis and the aponeuroses and fasciae of the muscles. Adipose tissue is sometimes named specifically for the organ it surrounds, as in “perirenal adipose tissue.” Interstitial adipose tissue, however, is interspersed or infiltrated among the cells of different tissues so tightly that it is not readily dissectible (30).
This simple adipose tissue classification system served anatomists well for the past centuries, particularly because the main early focus was on organs, and little clinical pathology was directly attributable to or found within the adipose tissue compartment.
Adipose tissue is also named according to special biological functions, such as white, mammary gland, brown, and bone marrow adipose tissues (31). White adipose tissue functions mainly as an energy reservoir, insulator, and as a source of recently discovered hormones (32). Thermogenesis is the main function of brown adipose tissue found in many small mammals. Mammary gland adipose tissue plays an important role in epithelial cell growth and milk production, whereas bone marrow adipose tissue might participate in hematopoiesis and osteogenesis (31).
This classification provided a clear and useful approach for organizing some of the recognized biological functions of adipose tissue. However, important metabolic properties of adipose tissue depots, such as visceral adipose tissue, cannot easily be accommodated. Also, the groupings in this approach represent a hybrid that includes anatomic regions (e.g., mammary glands) and functional properties (e.g., heat production by brown adipose tissue and energy storage by white adipose tissue), with the potential for overlap.
Adipose Tissue Classification in Radiology
The prevailing confusion and, to some extent, outdated terminology concerning adipose tissue in the medical literature prompted us to review papers on imaging of adipose tissue compartments related to metabolic activity and disease. Using Medline, we examined over 100 articles with the terms “total,” “regional,” and “visceral” adipose tissue or fat published between 1979 and 2002. Two categories were identified, those that evaluated whole body and those that evaluated regional adipose tissue.
Whole-Body Imaging Studies
While investigators usually provided clear definitions of adipose tissue depots, some reports lacked adequate detail to evaluate component characteristics. Most articles did not indicate whether or how adipose tissue depots other than subcutaneous and visceral adipose tissue were measured, even though they collectively contribute to total-body adipose tissue (33,34).
Overall, the reports were concordant on a number of measurement procedures when applied to whole-body multislice CT and MRI. First, even though its boundary is clearly visible and thus easily quantified, bone marrow adipose tissue was usually not included in imaging studies of total-body adipose tissue (35). This is likely because most investigators have little interest in bone marrow adipose tissue estimates.
Second, adipose tissue in the head, feet, and hands is difficult to distinguish from adipose tissue in bone marrow with commonly applied MRI sequences, and these tissues are usually labeled as nonadipose tissue (36,37). Nevertheless, a trained analyst can isolate subcutaneous adipose tissue from bone marrow with high resolution MRI.
Third, scattered adipocytes are found within many organs and tissues, especially skeletal muscle. Unless these adipocytes clump together and form a larger mass, they may be below the commonly applied resolution of CT and MRI, relegating them to measurement within the nonadipose tissue component. While these small adipose tissue clumps are now below the current imaging threshold, it should be possible in the future with MRI to establish a separate estimate of the lipid content of scattered adipocytes by subtracting intramyocellular lipid content measured by 1H magnetic resonance spectroscopy from total tissue lipid content measured by chemical shift imaging (20,38,39). These advanced methods are revolutionizing the study of in vivo biology and redefining the study of human anatomy.
Thus, “total-body” adipose tissue measured by imaging methods in the current published literature is usually different from the actual volume of adipose tissue determined by dissection and histological analysis. Nevertheless, the potential exists with developing techniques to accurately quantify total body adipose tissue in vivo.
Some whole-body imaging studies grouped adipose tissue compartments according to metabolic activity. Barnard et al. subdivided total body adipose tissue into “subcutaneous” and “internal” (i.e., visceral, paravertebral, and intermuscular) with further partitioning of visceral adipose tissue into retro- and intraperitoneal components (33,35). This partition of total-body adipose tissue assumes that subcutaneous adipose tissue and internal adipose tissue differ in their metabolic activities. Thomas et al. (34), in their imaging studies, separated internal adipose tissue into two compartments, visceral adipose tissue and nonvisceral adipose tissue.
Although adipose tissue in the female breast functions differently from other subcutaneous regions in several respects (31), most investigators consider mammary adipose tissue a portion of the subcutaneous compartment. Localized fat pads, such as the synovia, were formerly classified as mechanical adipose tissue but are now considered by most investigators to be components of subcutaneous adipose tissue.
A number of reviews explore the well-developed technical aspects of imaging methods and their validity in quantifying total-body and subcutaneous adipose tissue (30,40–42). The coefficients of variation (CV) for repeated subcutaneous adipose tissue measurements by CT and MRI are similar and in the range of ~2% (43–45).
The subcutaneous adipose tissue of the lower trunk and the gluteal-thigh region has a thin fascial plane dividing it into superficial and deep portions, as shown in Figure 2 (46–49). In recent studies, both morphological and metabolic differences were found between these two adipose tissue layers (10,50,51). The majority of deep subcutaneous adipose tissue is located in the posterior half of the abdomen, whereas superficial subcutaneous adipose tissue is evenly distributed around the abdominal circumference (10).
Figure 2.
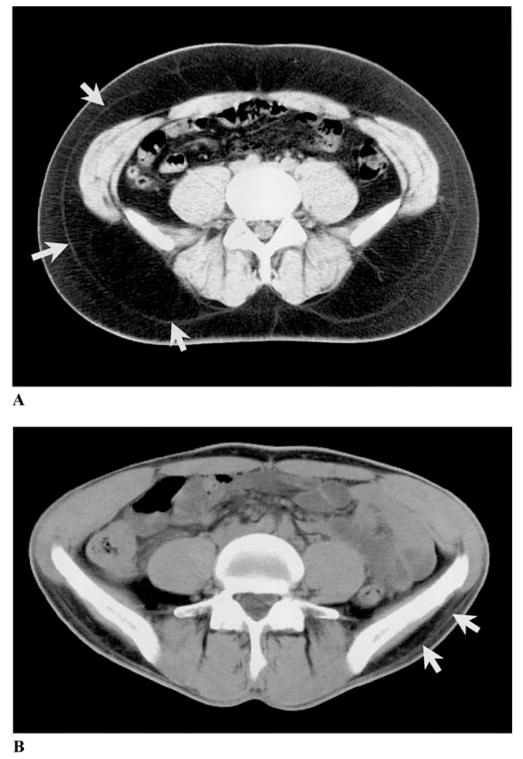
Abdominal axial CT scans of an obese (A) and a thin subject (B). Subcutaneous adipose tissue is divided into superficial and deep subcutaneous adipose tissue by a fascial plane, as indicated by the white arrows.
These collected reports led us to propose a practical total-body and regional adipose tissue classification system based on the well-defined fascial planes listed in Table 1. Total-body adipose tissue can be first divided into two main measurable components, subcutaneous and internal. Subcutaneous adipose tissue is well defined and has clear anatomic demarcations, as noted in the table. Internal adipose tissue is divided into visceral and nonvisceral components.
Table 1.
Proposed classification of total body adipose tissue
| Adipose tissue compartment | Definition |
|---|---|
| Total adipose tissue | Sum of adipose tissue, usually excluding bone marrow and adipose tissue in the head, hands, and feet. |
| Subcutaneous adipose tissue | The layer found between the dermis and the aponeuroses and fasciae of the muscles. Includes mammary adipose tissue. |
| Superficial subcutaneous adipose tissue | The layer found between the skin and a fascial plane in the lower trunk and gluteal-thigh area. |
| Deep subcutaneous adipose tissue | The layer found between the muscle fascia and a fascial plane in the lower trunk and gluteal-thigh areas. |
| Internal adipose tissue | Total adipose tissue minus subcutaneous adipose tissue. |
| Visceral adipose tissue (See Table 2) | Adipose tissue within the chest, abdomen, and pelvis. |
| Nonvisceral internal adipose tissue | Internal adipose tissue minus visceral adipose tissue. |
| Intramuscular adipose tissue | Adipose tissue within a muscle (between fascicles). |
| Perimuscular adipose tissue | Adipose tissue inside the muscle fascia (deep fascia), excluding intramuscular adipose tissue. |
| Intermuscular adipose tissue | Adipose tissue between muscles. |
| Paraosseal adipose tissue | Adipose tissue in the interface between muscle and bone (e.g., paravertebral). |
| Other nonvisceral adipose tissue | Orbital adipose tissue; aberrant adipose tissue associated with pathological conditions (e.g., lipoma). |
Among the nonvisceral components, some perimuscular adipose tissue regions are specially named. For example, when distributed among muscles, they are named as inter-muscular adipose tissue, and when adjacent to bones, they are named as paraosseal adipose tissue. The fascial planes separating perimuscular adipose tissue from adjacent adipose tissue compartments are sometimes, but not always, visible when images are prepared using typical MRI acquisition sequences (Figure 3A). These fascial planes are visible in most subjects when high-resolution images are prepared (Figure 3B). Additionally, the perimuscular and intramuscular adipose tissue depots are small and are thus not accurately measurable by traditional cadaver dissection. Recently, advanced digital photography (30) and microdissection (52) methods have provided a means of accurately estimating the areas or volumes of these difficult-to-dissect adipose tissue compartments and can be applied in human cadaver or animal studies to serve as the imaging-method criterion.
Figure 3.
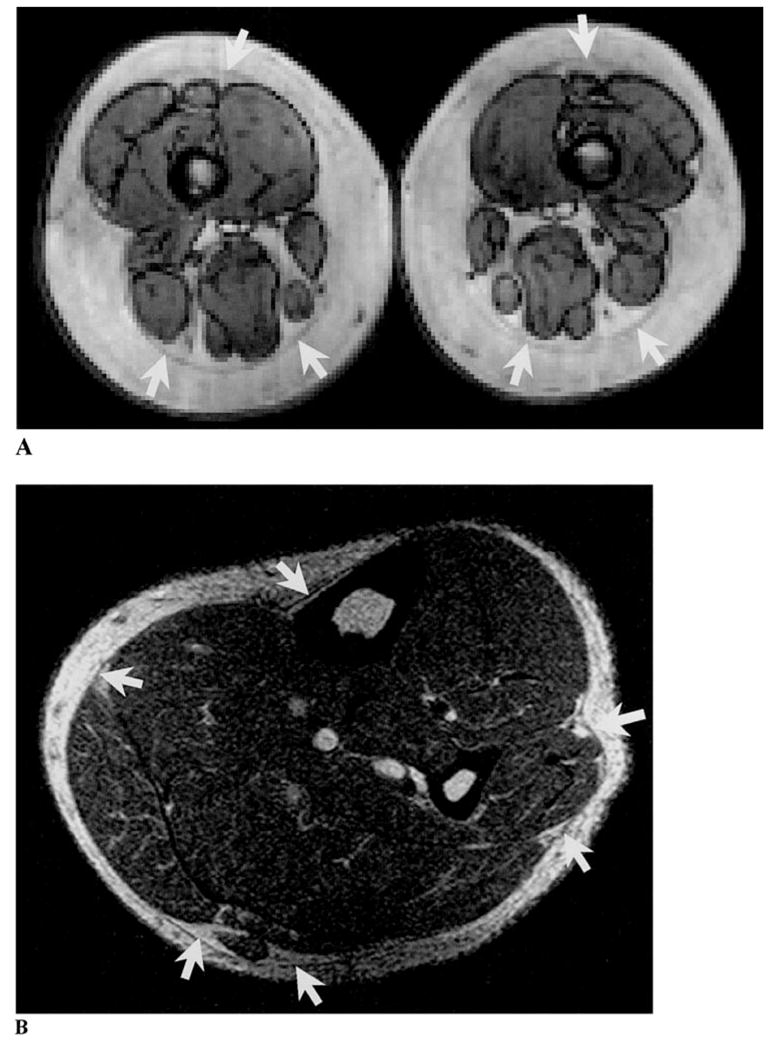
(A) Arrows indicate the fascial planes separating perimuscular from subcutaneous adipose tissue as observed on an axial leg typical resolution MRI scan. (B) Arrows indicate the fascial planes separating perimuscular from subcutaneous adipose tissue as observed on an axial lower leg high resolution MRI scan. The intramuscular adipose tissue is clearer in image B than in image A.
Absolute and relative visceral adipose tissues have been associated with the greatest health risk (53,54). Authors vary widely in their definitions and descriptions of visceral adipose tissue. Some reports did not provide any anatomic demarcations of visceral adipose tissue compartments (11,55–67). Contrary to the simple view of visceral adipose tissue held by many authors, there are important differences in the metabolic and functional properties of depots within the “visceral adipose tissue” compartment. Accordingly, in the following section, we present a critical review of previous visceral adipose tissue studies, along with a detailed classification of visceral adipose tissue.
Visceral Adipose Tissue
The word “viscera” originates from Latin (68) and is defined as “organs in the cavities of the body” (68–70). Because there are three main body cavities, it is reasonable to assume that visceral adipose tissue (VAT) consists of adipose tissue (AT) distributed in the three body cavities: intrathoracic (ITAT), intraabdominal (IAAT), and intrapelvic (IPAT). The physical location of these three cavities is from cephalad to caudad, and axial image acquisition provides the landmarks for roughly separating ITAT from IAAT and IAAT from IPAT. Accordingly, investigators have studied the metabolic characteristic of visceral adipose tissue found in these three different compartments. Most investigators report visceral adipose tissue as IAAT or the sum of IAAT and IPAT.
The CVs of visceral adipose tissue estimates by imaging methods are well studied and extensively reviewed. The CVs for VAT measurements by MRI are ~9% to 18% (44,45,71,72) and by CT are ~2% (43). The lower CV of CT is usually ascribed to a shorter image acquisition time, and CT is thus less vulnerable to image artifacts produced by peristaltic gastrointestinal tract movement (73). The signal intensity of MRI pixels from the same tissue may vary from region to region due to magnetic field heterogeneity. There may also be some sequence-related artifacts with MRI, such as chemical shift and blood flow artifacts. These effects collectively lower the accuracy and precision of MRI visceral adipose tissue estimates, particularly as image analysis requires establishing the irregular boundaries between VAT and other tissues and organs.
As a stimulus for review and as a means of evoking the prevailing confusion in the literature, we now examine earlier studies in the context of VAT as the sum of three distinct components.
VAT = ITAT + IAAT + IPAT
Although viscera are distributed throughout all body cavities, very few investigators defined VAT in humans as the sum of ITAT, IAAT, and IPAT (74–76). This definition of VAT is applied in the animal literature (77). It is not known whether the three VAT components have distinct metabolic characteristics. The least studied of these three components is ITAT. The ITAT is mainly distributed surrounding the heart, and the physiological role of ITAT is in an early stage of investigation. In animal studies, Marchington et al. (78) found that epicardial adipose tissue has a greater capacity for fatty acid release than adipose tissue elsewhere in the body. Cardiac adipose tissue may supply energy for the adjacent myocardium and serve as a buffer against toxic levels of free fatty acids.
IPAT is usually quantified together with IAAT. However, when studied separately from IAAT, the metabolic properties of IPAT and IAAT differ (49,79). Part of the reason for this metabolic difference is that IAAT represents both extra-and intraperitoneal adipose tissue, whereas IPAT represents mainly extraperitoneal adipose tissue (49).
VAT = IAAT + IPAT
The VAT compartment as defined in some reports included IAAT and IPAT (33,34,37,79) that ranged anatomically from the femoral heads to the liver dome or base of the lungs. Whole-body CT and MRI scans usually consisted of multiple slices at predefined intervals (e.g., 5 cm). With the 5-cm intervals often used in MRI protocols, VAT was frequently defined as located within the seven slices extending from two below and four above the L4–L5 level (37). The IAAT and IPAT components are anatomically connected, and it is thus reasonable to study them together.
VAT = IAAT
Most of the reviewed earlier studies defined VAT as IAAT only, with a range from 5 cm below L4–L5 to the slice corresponding to the superior border of the liver (8,9,40,80–87). Some investigators additionally divided VAT into intraperitoneal and retroperitoneal adipose tissue (9,82,83,86,87). Because the parietal peritoneum rarely is visible on cross-sectional images (73), some investigators adopted an arbitrary method in which the marker was used to draw a straight line across the anterior border of L4–L5 and the psoas muscles, continuing on a tangent toward the posterior borders of the ascending and descending colon, and extending to the abdominal wall. However, the lack of exact boundaries between the intraperitoneal and retroperitoneal space renders this subdivision only an approximation. Some investigators referred to “abdominal VAT” instead of “VAT” to indicate that they were actually measuring IAAT (34,36,88,89).
VAT = Intraperitoneal Adipose Tissue
A few studies defined VAT solely as intraperitoneal adipose tissue, which is drained by the portal vein, whereas blood from retroperitoneal adipose tissue empties into the inferior vena cava (90,91). Although limiting the definition of VAT to intraperitoneal adipose tissue is inconsistent with the term “viscera,” the relationships between intraperitoneal adipose tissue and metabolic disorders have aroused considerable research interest. Abate et al. (91) proposed that metabolic differences exist between intraperitoneal and retroperitoneal adipose tissue. Although the fatty acid component of omental and mesenteric sites is not different from subcutaneous and retroperitoneal sites (36), it is currently hypothesized that the direct exposure of liver cells through the portal circulation to high concentrations of free fatty acids and/or other metabolites derived from intraperitoneal adipose tissue is responsible for the increased frequency of dyslipidemia, hyperinsulinemia, and other metabolic complications associated with abdominal obesity (43,92).
Ideally the study of VAT should include all adipose tissue in the thoracic, abdominal, and pelvic cavities. However, many investigators are interested only in some subdivisions of VAT. Accordingly, it is reasonable to suggest that any VAT depot under study should be accurately named and characterized to avoid further confusion. Metabolic characteristics can be attributed to IAAT as a whole (9,82,83,86,87,90,91), although this may simply reflect the relatively large amount of highly active intraperitoneal adipose tissue (e.g., mesenteric and omental) found in this compartment. With increasing evidence of metabolic differences between intraperitoneal and extraperitoneal adipose tissues, it is reasonable to consider the intraperitoneal adipose tissue of both the abdominal and pelvic regions together, particularly because these compartments are contiguous.
We summarize the main VAT components in Table 2 and propose this as a classification and nomenclature for future studies. Because there is no adipose tissue adjacent to the pleura, we use pericardium instead of pleura to further separate the adipose tissue components of the thoracic cavity (Figure 4). In the studies we reviewed, rather than measuring total extraperitoneal adipose tissue, most investigators only quantified retroperitoneal adipose tissue because it could be easily separated from intraperitoneal adipose tissue by an arbitrary line.
Table 2.
Proposed classification of visceral adipose tissue
| Adipose tissue compartment |
|---|
| Visceral adipose tissue |
| Intrathoracic adipose tissue |
| Intrapericardial |
| Extrapericardial |
| Intraabdominopelvic |
| Intraperitoneal (e.g., omental and mesenteric) |
| Extraperitoneal |
| Intraabdominal |
| Preperitoneal |
| Retroperitoneal (e.g., perirenal, pararenal, periaortic, and peripancreatic) |
| Intrapelvic (e.g., parametrial, retropubic, paravesical, retrouterine, pararectal, retrorectal) |
Figure 4.
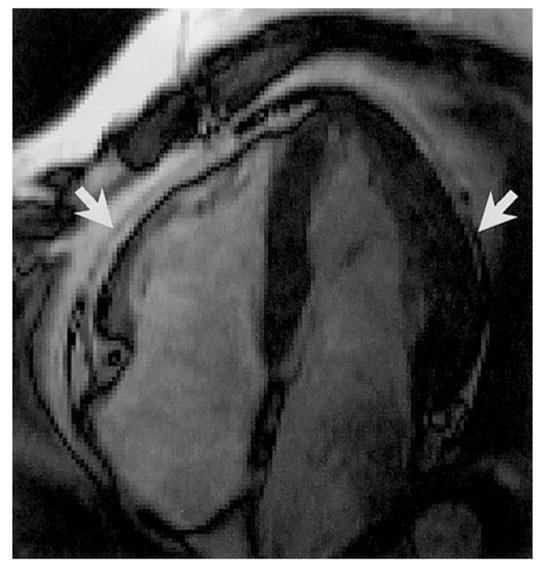
Arrows indicate the pericardium separating intrapericardial and extrapericardial adipose tissues on a gated cardiac image.
The traditional CT and MRI protocols now in use are not capable of separating all of the compartments listed in Table 2. On the other hand, retroperitoneal components such as pararenal adipose tissue are clearly visible on some conventionally acquired MRI scans (Figure 5). With a smaller field of view, higher resolution, and thinner slices, it may be possible to separate all of the adipose tissue depots from one another with the expectation of major technical advances in the future.
Figure 5.
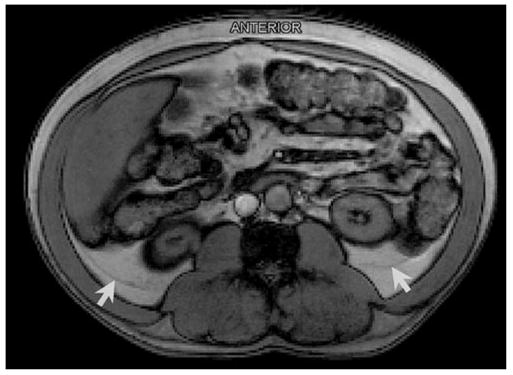
Arrows indicate the fascial planes separating pararenal from adjacent adipose tissue compartments on a typical resolution axial abdominal MRI scan.
The distribution of VAT is shown in photographs of the National Library of Medicine’s Visible Woman and Visible Man (Figures 6–8) (93,94). Because VAT seems to be metabolically heterogeneous, a reasonable future goal is to separately examine specific compartments as outlined in Table 2.
Figure 6.
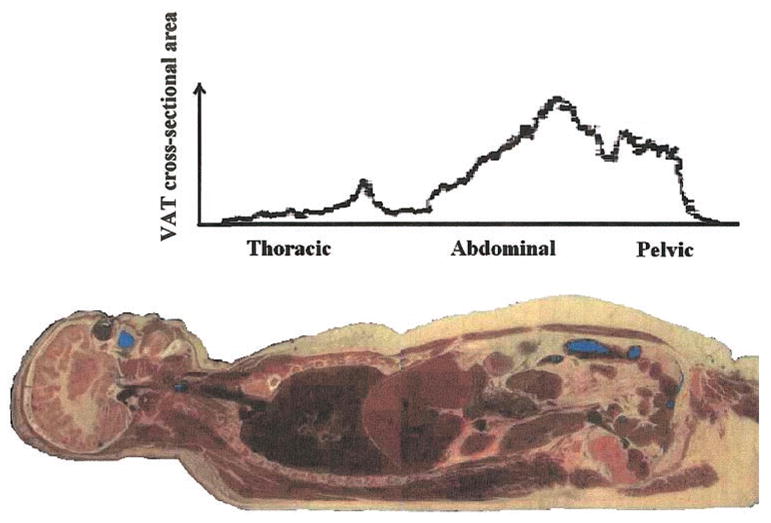
VAT distribution in the Visible Woman. Contiguous areas from 1-mm-thick slices are plotted across the thoracic, abdominal, and pelvic region. Reprinted with permission from the National Library of Medicine.
Figure 8.
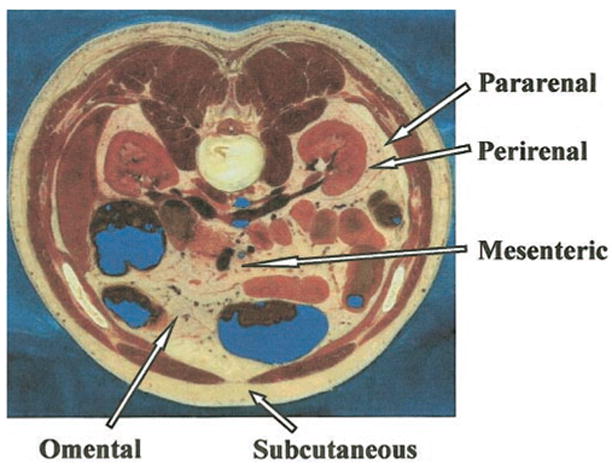
Main adipose tissue compartments in an axial section of the Visible Man. Reprinted with permission from the National Library of Medicine.
Single-Slice Studies
In addition to volume quantification of VAT by multiple-slice and whole-body imaging protocols, VAT is often reported as the area of a single slice. Because of the cost of whole-body scans and concerns over exposure to radiation, single-slice studies have often been used, although they are less accurate (95). The single-slice CT and MRI studies are usually performed at the L4–L5 level, which, in addition to omental, mesenteric, and retroperitoneal compartments, includes many other smaller adipose tissue compartments. VAT and IAAT are terms that were often used interchangeably in earlier reports. It is important to recognize that single-slice studies only provide an area when reporting “VAT,” in contrast to the volumes reported in multiple-slice studies.
There is also some inconsistency in the anatomical boundaries used in single-slice studies. Clasey et al. (96), for example, used the innermost aspect of the abdominal and oblique muscle walls, rather than the midpoint or the outermost aspect of the muscle walls, for measuring “VAT.” The internal boundary of the muscle walls was used in most of the studies we reviewed and did not include intermuscular and paravertebral adipose tissues (44,51,61,62,66,79,81,82,97–110), which we propose should be included as nonvisceral adipose tissue. These adipose tissue compartments increase in size with age (33) and can be large in obese subjects.
Summary
Investigators differ in their interests in and definitions of various adipose tissue compartments. A consistent and logical classification adapted to imaging methods will allow investigators to compare physiological and metabolic studies of adipose tissue and resolve some of the confusion in the current literature. Specifically, we propose the following:
Refer to components evaluated by CT and MRI as “adipose tissue” instead of the chemical term “fat.” Because “body fat” is also measured (e.g., by DXA), this will leave no question as to what body constituent is actually being evaluated.
Separate “total-body adipose tissue” into two categories: subcutaneous and internal. These are well demarcated and leave little room for confusion.
Separate internal adipose tissue into two discrete components: “visceral” and “nonvisceral.” VAT should appropriately include two cavities: the thoracic and abdominopelvic cavity. The abdominopelvic cavity can be further divided into intraperitoneal and extraperitoneal regions.
While there is some inconsistency in the anatomical boundaries in single-slice studies of the abdomen, we suggest that the inner boundary of the abdominal muscle wall should be used as the limit for VAT. This boundary does not include intermuscular and paravertebral adipose tissue, which should be included as nonvisceral adipose tissue.
While, at present, the main imaging focus is on the total volume of adipose tissue structures, a growing interest centers on tissue quality. Chemical shift imaging can provide information on adipose tissue lipid content (38,39), and proton spectroscopy provides estimates of tissue-free fatty acid composition (111). These advanced magnetic resonance methods have great promise in the field of obesity research. Combining these tissue quality assessment methods with high-resolution image acquisition holds great future promise in adipose tissue quantification.
It was the purpose of this paper to clearly define the adipose tissue components in published reports using a precise classification. This will, over the long term, allow elucidation of the genetic and metabolic properties of specific adipose tissue depots, their interaction, and their overall orchestrated role in energy homeostasis.
Figure 7.
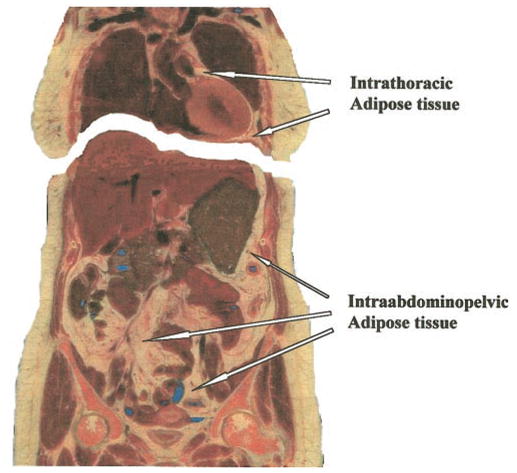
The two VAT compartments in a coronal section of the Visible Man. Reprinted with permission from the National Library of Medicine.
Acknowledgments
This work was supported by National Institutes of Health Grant NIDDK42618 and 1 R01 DK5750801.
Nonstandard abbreviations
- CT
computerized axial tomography
- MRI
magnetic resonance imaging
- CV
coefficients of variation
- VAT
visceral adipose tissue
- AT
adipose tissue
- ITAT
intrathoracic adipose tissue
- IAAT
intra-abdominal adipose tissue
- IPAT
intrapelvic adipose tissue
References
- 1.Evans DJ, Hoffmann RG, Kalkhoff RK, Kissebah AH. Relationship of body fat topography to insulin sensitivity and metabolic profiles in premenopausal women. Metabolism. 1984;33:68–75. doi: 10.1016/0026-0495(84)90164-1. [DOI] [PubMed] [Google Scholar]
- 2.Vague J. The degree of masculine differentiation of obesities: a factor determining predisposition to diabetes, atherosclerosis, gout, and uric calculous disease. 1956. Nutrition. 1999;15:89–91. doi: 10.1016/s0899-9007(98)00131-2. [DOI] [PubMed] [Google Scholar]
- 3.Colberg SR, Simoneau JA, Thaete FL, Kelley DE. Skeletal muscle utilization of free fatty acids in women with visceral obesity. J Clin Invest. 1995;95:1846–53. doi: 10.1172/JCI117864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brochu M, Poehlman ET, Ades PA. Obesity, body fat distribution, and coronary artery disease. J Cardiopulm Rehabil. 2000;20:96–108. doi: 10.1097/00008483-200003000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Vanderburgh PM. Fat distribution: its physiological significance, health implications, and its adaptation to exercise training. Mil Med. 1992;157:189–92. [PubMed] [Google Scholar]
- 6.Despres JP, Nadeau A, Tremblay A, et al. Role of deep abdominal fat in the association between regional adipose tissue distribution and glucose tolerance in obese women. Diabetes. 1989;38:304–9. doi: 10.2337/diab.38.3.304. [DOI] [PubMed] [Google Scholar]
- 7.Ross R, Fortier L, Hudson R. Separate associations between visceral and subcutaneous adipose tissue distribution, insulin and glucose levels in obese women. Diabetes Care. 1996;19:1404–11. doi: 10.2337/diacare.19.12.1404. [DOI] [PubMed] [Google Scholar]
- 8.Schoen RE, Evans RW, Sankey SS, Weissfeld JL, Kuller L. Does visceral adipose tissue differ from subcutaneous adipose tissue in fatty acid content? Int J Obes Relat Metab Disord. 1996;20:346–52. [PubMed] [Google Scholar]
- 9.Ross R, Shaw KD, Rissanen J, Martel Y, de Guise J, Avruch L. Sex differences in lean and adipose tissue distribution by magnetic resonance imaging: anthropometric relationships. Am J Clin Nutr. 1994;59:1277–85. doi: 10.1093/ajcn/59.6.1277. [DOI] [PubMed] [Google Scholar]
- 10.Kelley DE, Thaete FL, Troost F, Huwe T, Goodpaster BH. Subdivisions of subcutaneous abdominal adipose tissue and insulin resistance. Am J Physiol Endocrinol Metab. 2000;278:E941–E8. doi: 10.1152/ajpendo.2000.278.5.E941. [DOI] [PubMed] [Google Scholar]
- 11.Ronnemaa T, Koskenvuo M, Marniemi J, et al. Glucose metabolism in identical twins discordant for obesity. The critical role of visceral fat. J Clin Endocrinol Metab. 1997;82:383–7. doi: 10.1210/jcem.82.2.3763. [DOI] [PubMed] [Google Scholar]
- 12.Wang ZM, Pierson RN, Jr, Heymsfield SB. The five level model: a new approach to organizing body composition research. Am J Clin Nutr. 1992;56:19–28. doi: 10.1093/ajcn/56.1.19. [DOI] [PubMed] [Google Scholar]
- 13.Snyder WS, Cook MJ, Nasset ES, Karhausen RL, Howells GP, Tipton IH. ICRP Publication 23. Oxford, UK: Pergamon Press; 1975. Report of the Task Group on Reference Man; pp. 40–5. [Google Scholar]
- 14.An P, Rice T, Borecki IB, et al. Major gene effect on subcutaneous fat distribution in a sedentary population and its response to exercise training: the HERITAGE Family Study. Am J Human Biol. 2000;12:600–9. doi: 10.1002/1520-6300(200009/10)12:5<600::AID-AJHB4>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 15.Wu CH, Yao WJ, Lu FH, Yang YC, Wu JS, Chang CJ. Sex differences of body fat distribution and cardiovascular dysmetabolic factors in old age. Age Ageing. 2001;30:331–6. doi: 10.1093/ageing/30.4.331. [DOI] [PubMed] [Google Scholar]
- 16.Kyle UG, Genton L, Hans D, Karsegard L, Slosman DO, Pichard C. Age-related differences in fat-free mass, skeletal muscle, body cell mass and fat mass between 18 and 94 years. Eur J Clin Nutr. 2001;55:663–72. doi: 10.1038/sj.ejcn.1601198. [DOI] [PubMed] [Google Scholar]
- 17.Ramos de Marins VM, Varnier Almeida RM, Pereira RA, Barros MB. Factors associated with overweight and central body fat in the city of Rio de Janeiro: results of a two-stage random sampling survey. Public Health. 2001;115:236–42. doi: 10.1038/sj/ph/1900763. [DOI] [PubMed] [Google Scholar]
- 18.Evans EM, Van Pelt RE, Binder EF, Williams DB, Ehsani AA, Kohrt WM. Effects of HRT and exercise training on insulin action, glucose tolerance, and body composition in older women. J Appl Physiol. 2001;90:2033–40. doi: 10.1152/jappl.2001.90.6.2033. [DOI] [PubMed] [Google Scholar]
- 19.Kanaley JA, Sames C, Swisher L, et al. Abdominal fat distribution in pre- and postmenopausal women: the impact of physical activity, age, and menopausal status. Metabolism. 2001;50:976–82. doi: 10.1053/meta.2001.24931. [DOI] [PubMed] [Google Scholar]
- 20.Boesch C, Kreis R. Observation of intramyocellular lipids by 1H-magnetic resonance spectroscopy. Ann NY Acad Sci. 2000;904:25–31. [PubMed] [Google Scholar]
- 21.Meininger G, Hadigan C, Laposata M, et al. Elevated concentrations of free fatty acids are associated with increased insulin response to standard glucose challenge in human immunodeficiency virus-infected subjects with fat redistribution. Metabolism. 2002;51:260–6. doi: 10.1053/meta.2002.29999. [DOI] [PubMed] [Google Scholar]
- 22.Tang H, Vasselli JR, Wu EX, Boozer CN, Gallagher D. High-resolution magnetic resonance imaging tracks changes in organ and tissue mass in obese and aging rats. Am J Physiol Regul Integr Comp Physiol. 2002;282:R890–R9. doi: 10.1152/ajpregu.0527.2001. [DOI] [PubMed] [Google Scholar]
- 23.Clasey JL, Bouchard C, Teates CD, et al. The use of anthropometric and dual-energy X-ray absorptiometry (DXA) measures to estimate total abdominal and abdominal visceral fat in men and women. Obes Res. 1999;7:256–64. doi: 10.1002/j.1550-8528.1999.tb00404.x. [DOI] [PubMed] [Google Scholar]
- 24.Kamel EG, McNeill G, Van Wijk MC. Change in intra-abdominal adipose tissue volume during weight loss in obese men and women: correlation between magnetic resonance imaging and anthropometric measurements. Int J Obes Relat Metab Disord. 2000;24:607–13. doi: 10.1038/sj.ijo.0801204. [DOI] [PubMed] [Google Scholar]
- 25.Kamel EG, McNeill G, Van Wijk MC. Usefulness of anthropometry and DXA in predicting intra-abdominal fat in obese men and women. Obes Res. 2000;8:36–42. doi: 10.1038/oby.2000.6. [DOI] [PubMed] [Google Scholar]
- 26.Fuller NJ, Hardingham CR, Graves M, et al. Assessment of limb muscle and adipose tissue by dual-energy X-ray absorptiometry using magnetic resonance imaging for comparison. Int J Obes Relat Metab Disord. 1999;23:1295–302. doi: 10.1038/sj.ijo.0801070. [DOI] [PubMed] [Google Scholar]
- 27.Tothill P, Han TS, Avenell A, McNeill G, Reid DM. Comparisons between fat measurements by dual-energy X-ray absorptiometry, underwater weighing and magnetic resonance imaging in healthy women. Eur J Clin Nutr. 1996;50:747–52. [PubMed] [Google Scholar]
- 28.Shuldiner AR, Yang R, Gong DW. Resistin, obesity and insulin resistance—the emerging role of the adipocyte as an endocrine organ. N Engl J Med. 2001;345:1345–6. doi: 10.1056/NEJM200111013451814. [DOI] [PubMed] [Google Scholar]
- 29.Snyder WS, Cook MJ, Nasset ES, Karhausen RL, Howells GP, Tipton IH. ICRP Publication 23. Oxford, UK: Pergamon Press; 1975. Report of the Task Group on Reference Man; p. 275. [Google Scholar]
- 30.Mitsiopoulos N, Baumgartner RN, Heymsfield SB, Lyons W, Gallagher D, Ross R. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol. 1998;85:115–22. doi: 10.1152/jappl.1998.85.1.115. [DOI] [PubMed] [Google Scholar]
- 31.Klaus S. Overview: biological significance of fat and adipose tissue. In: Klaus S, editor. Adipose Tissue. Georgetown, TX: Landes Bioscience; 2001. pp. 1–10. [Google Scholar]
- 32.Trayhurn P, Beattie JH. Physiological role of adipose tissue: white adipose tissue as an endocrine and secretory organ. Proc Nutr Soc. 2001;60:329–39. doi: 10.1079/pns200194. [DOI] [PubMed] [Google Scholar]
- 33.Thomas EL, Saeed N, Hajnal JV, et al. Magnetic resonance imaging of total body fat. J Appl Physiol. 1998;85:1778–85. doi: 10.1152/jappl.1998.85.5.1778. [DOI] [PubMed] [Google Scholar]
- 34.Thomas EL, Brynes AE, McCarthy J, et al. Preferential loss of visceral fat following aerobic exercise, measured by magnetic resonance imaging. Lipids. 2000;35:769–76. doi: 10.1007/s11745-000-0584-0. [DOI] [PubMed] [Google Scholar]
- 35.Barnard ML, Schwieso JE, Thomas EL, et al. Development of a rapid and efficient magnetic resonance imaging technique for analysis of body fat distribution. NMR Biomed. 1996;9:156–64. doi: 10.1002/(SICI)1099-1492(199606)9:4<156::AID-NBM412>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 36.Busetto L, Tregnaghi A, Bussolotto M, et al. Visceral fat loss evaluated by total body magnetic resonance imaging in obese women operated with laparascopic adjustable silicone gastric banding. Int J Obes Relat Metab Disord. 2000;24:60–9. doi: 10.1038/sj.ijo.0801086. [DOI] [PubMed] [Google Scholar]
- 37.Ross R, Leger L, Morris D, de Guise J, Guardo R. Quantification of adipose tissue by MRI: relationship with anthropometric variables. J Appl Physiol. 1992;72:787–95. doi: 10.1152/jappl.1992.72.2.787. [DOI] [PubMed] [Google Scholar]
- 38.Lunati E, Marzola P, Nicolato E, Sbarbati A. In-vivo quantitative hydrolipidic map of perirenal adipose tissue by chemical shift imaging at 4.7 Tesla. Int J Obes Relat Metab Disord. 2001;25:457–61. doi: 10.1038/sj.ijo.0801262. [DOI] [PubMed] [Google Scholar]
- 39.Poon CS, Szumowski J, Plewes DB, Ashby P, Henkelman RM. Fat/water quantitation and differential relaxation time measurement using chemical shift imaging technique. Magn Reson Imaging. 1989;7:369–82. doi: 10.1016/0730-725x(89)90486-4. [DOI] [PubMed] [Google Scholar]
- 40.Ross R. Magnetic resonance imaging provides new insights into the characterization of adipose and lean tissue distribution. Can J Physiol Pharmacol. 1996;74:778–85. [PubMed] [Google Scholar]
- 41.Heymsfield SB, Ross R, Wang ZM, Forager D. Imaging techniques of body composition: advantages of measurement and new uses. In: Carlson-Newberry SJ, Costello RB, editors. Emerging Technologies for Nutrition Research. Washington, DC: National Academy Press; 1997. pp. 127–50. [Google Scholar]
- 42.Ross R. Magnetic resonance imaging (MRI): data acquisition and application in human body composition. In: Pierson RN, editor. Quality of the Body Cell Mass: Body Composition in the Third Millennium. New York: Springer; 1997. pp. 198–211. [Google Scholar]
- 43.Björntorp P. “Portal” adipose tissue as a generator of risk factors for cardiovascular disease and diabetes. Arteriosclerosis. 1990;10:493–6. [PubMed] [Google Scholar]
- 44.Elbers JM, Haumann G, Asscheman H, Seidell JC, Gooren LJ. Reproducibility of fat area measurements in young, non-obese subjects by computerized analysis of magnetic resonance images. Int J Obes Relat Metab Disord. 1997;21:1121–9. doi: 10.1038/sj.ijo.0800525. [DOI] [PubMed] [Google Scholar]
- 45.Seidell JC, Bakker CJ, van der Kooy K. Imaging techniques for measuring adipose-tissue distribution—a comparison between computed tomography and 1.5-T magnetic resonance. Am J Clin Nutr. 1990;51:953–7. doi: 10.1093/ajcn/51.6.953. [DOI] [PubMed] [Google Scholar]
- 46.Markman B, Barton FE., Jr Anatomy of the subcutaneous tissue of the trunk and lower extremity. Plast Reconstr Surg. 1987;80:248–54. doi: 10.1097/00006534-198708000-00015. [DOI] [PubMed] [Google Scholar]
- 47.Johnson D, Cormack GC, Abrahams PH, Dixon AK. Computed tomographic observations on subcutaneous fat: implications for liposuction. Plast Reconstr Surg. 1996;97:387–96. doi: 10.1097/00006534-199602000-00017. [DOI] [PubMed] [Google Scholar]
- 48.Gasperoni C, Salgarello M. Rationale of subdermal superficial liposuction related to the anatomy of subcutaneous fat and the superficial fascial system. Aesthetic Plast Surg. 1995;19:13–20. doi: 10.1007/BF00209305. [DOI] [PubMed] [Google Scholar]
- 49.Rendell M, Hulthen UL, Tornquist C, Groop L, Mattiasson I. Relationship between abdominal fat compartments and glucose and lipid metabolism in early postmenopausal women. J Clin Endocrinol Metab. 2001;86:744–9. doi: 10.1210/jcem.86.2.7260. [DOI] [PubMed] [Google Scholar]
- 50.Lovejoy JC, Smith SR, Rood JC. Comparison of regional fat distribution and health risk factors in middle-aged white and African Americans women: the Healthy Transitions Study. Obes Res. 2001;9:10–6. doi: 10.1038/oby.2001.2. [DOI] [PubMed] [Google Scholar]
- 51.Smith SR, Lovejoy JC, Greenway F, et al. Contributions of total body fat, abdominal subcutaneous adipose tissue compartments, and visceral adipose tissue to the metabolic complications of obesity. Metabolism. 2001;50:425–35. doi: 10.1053/meta.2001.21693. [DOI] [PubMed] [Google Scholar]
- 52.Guo Z, Mishra P, Macura S. Sampling the intramyocellular triglycerides from skeletal muscle. J Lipid Res. 2001;42:1041–8. [PubMed] [Google Scholar]
- 53.Björntorp P. Abdominal obesity and the metabolic syndrome. Ann Med. 1992;24:465–8. doi: 10.3109/07853899209166997. [DOI] [PubMed] [Google Scholar]
- 54.Kissebah AH, Krakower GR. Regional adiposity and morbidity. Physiol Rev. 1994;74:761–811. doi: 10.1152/physrev.1994.74.4.761. [DOI] [PubMed] [Google Scholar]
- 55.Engelson ES, Kotler DP, Tan Y, et al. Fat distribution in HIV-infected patients reporting truncal enlargement quantified by whole-body magnetic resonance imaging. Am J Clin Nutr. 1999;69:1162–9. doi: 10.1093/ajcn/69.6.1162. [DOI] [PubMed] [Google Scholar]
- 56.Hendler RG, Welle SL, Statt MC, Barnard R, Amatruda JM. The effects of weight reduction to ideal body weight on body fat distribution. Metabolism. 1995;44:1413–6. doi: 10.1016/0026-0495(95)90139-6. [DOI] [PubMed] [Google Scholar]
- 57.Snel YE, Brummer RJ, Doerga ME, et al. Adipose tissue assessed by magnetic resonance imaging in growth hormone-deficient adults: the effect of growth hormone replacement and a comparison with control subjects. Am J Clin Nutr. 1995;61:1290–4. doi: 10.1093/ajcn/61.6.1290. [DOI] [PubMed] [Google Scholar]
- 58.Ronnemaa T, Karonen SL, Rissanen A, Koskenvuo M, Koivisto VA. Relation between plasma leptin levels and measures of body fat in identical twins discordant for obesity. Ann Intern Med. 1997;126:26–31. doi: 10.7326/0003-4819-126-1-199701010-00004. [DOI] [PubMed] [Google Scholar]
- 59.Giltay EJ, Elbers JM, Gooren LJ, et al. Visceral fat accumulation is an important determinant of PAI-1 levels in young, nonobese men and women: modulation by cross-sex hormone administration. Arterioscler Thromb Vasc Biol. 1998;18:1716–22. doi: 10.1161/01.atv.18.11.1716. [DOI] [PubMed] [Google Scholar]
- 60.Szayna M, Doyle ME, Betkey JA, et al. Exendin-4 decelerates food intake, weight gain, and fat deposition in Zucker rats. Endocrinology. 2000;141:1936–41. doi: 10.1210/endo.141.6.7490. [DOI] [PubMed] [Google Scholar]
- 61.Marks SJ, Chin S, Strauss BJ. The metabolic effects of preferential reduction of visceral adipose tissue in abdominally obese men. Int J Obes Relat Metab Disord. 1998;22:893–8. doi: 10.1038/sj.ijo.0800678. [DOI] [PubMed] [Google Scholar]
- 62.Mourier A, Bigard AX, de Kerviler E, Roger B, Legrand H, Guezennec CY. Combined effects of caloric restriction and branched-chain amino acid supplementation on body composition and exercise performance in elite wrestlers. Int J Sports Med. 1997;18:47–55. doi: 10.1055/s-2007-972594. [DOI] [PubMed] [Google Scholar]
- 63.Marcus MA, Murphy L, Pi-Sunyer FX, Albu JB. Insulin sensitivity and serum triglyceride level in obese white and black women: relationship to visceral and truncal subcutaneous fat. Metabolism. 1999;48:194–9. doi: 10.1016/s0026-0495(99)90033-1. [DOI] [PubMed] [Google Scholar]
- 64.O’Connor KG, Harman SM, Stevens TE, et al. Interrelationships of spontaneous growth hormone axis activity, body fat, and serum lipids in healthy elderly women and men. Metabolism. 1999;48:1424–31. doi: 10.1016/s0026-0495(99)90154-3. [DOI] [PubMed] [Google Scholar]
- 65.Watts GF, Herrmann S, Riches FM. Effects of diet and serotonergic agonist on hepatic apolipoprotein B-100 secretion and endothelial function in obese men. QJM. 2000;93:153–61. doi: 10.1093/qjmed/93.3.153. [DOI] [PubMed] [Google Scholar]
- 66.Cefalu WT, Wang ZQ, Werbel S, et al. Contribution of visceral fat mass to the insulin resistance of aging. Metabolism. 1995;44:954–9. doi: 10.1016/0026-0495(95)90251-1. [DOI] [PubMed] [Google Scholar]
- 67.Roemmich JN, Huerta MG, Sundaresan SM, Rogol AD. Alterations in body composition and fat distribution in growth hormone-deficient prepubertal children during growth hormone therapy. Metabolism. 2001;50:537–47. doi: 10.1053/meta.2001.22510. [DOI] [PubMed] [Google Scholar]
- 68.Neilson WA. Webster’s New International Dictionary of the English Language. 2. Springfield, MA: G & C Merriam Co.; 1934. p. 2849. [Google Scholar]
- 69.Landau SI, Lovell Becker E, Butterfield WJH, McGehee Harvey A, Heptinstall RH, Thomas L. International Dictionary of Medicine and Biology. III. New York: John Wiley & Sons; 1986. p. 3156. [Google Scholar]
- 70.Considine DM. Van Nostrand’s Scientific Encyclopedia. 6. New York: Van Nostrand Reinhold Co. Inc; 1983. p. 2939. [Google Scholar]
- 71.Staten MA, Totty WG, Kohrt WM. Measurement of fat distribution by magnetic resonance imaging. Invest Radiol. 1989;24:345–9. doi: 10.1097/00004424-198905000-00002. [DOI] [PubMed] [Google Scholar]
- 72.Gerard EL, Snow RC, Kennedy DN, et al. Overall body fat and regional fat distribution in young women: quantification with MR imaging. Am J Roentgenol. 1991;157:99–104. doi: 10.2214/ajr.157.1.1646564. [DOI] [PubMed] [Google Scholar]
- 73.van der Kooy K, Seidell JC. Techniques for the measurement of visceral fat: a practical guide. Int J Obes Relat Metab Disord. 1993;17:187–96. [PubMed] [Google Scholar]
- 74.Kvist H, Sjöström L, Tylén U. Adipose tissue volume determinations in women by computed tomography: technical considerations. Int J Obes Relat Metab Disord. 1986;10:53–67. [PubMed] [Google Scholar]
- 75.Kvist H, Chowdhury B, Grangard U, Tylén U, Sjöström L. Total and visceral adipose-tissue volumes derived from measurements with computed tomography in adult men and women: predictive equations. Am J Clin Nutr. 1988;48:1351–61. doi: 10.1093/ajcn/48.6.1351. [DOI] [PubMed] [Google Scholar]
- 76.Vague J, Fenasse R. Comparative anatomy of adipose tissue. In: Renold AE, Cahill GF, editors. Adipose Tissue. Washington, DC: American Physiological Society; 1965. pp. 25–36. [Google Scholar]
- 77.Cinti S. Morphology of the adipose tissue. In: Klaus S, editor. Adipose Tissue. Georgetown, TX: Landes Bioscience; 2001. pp. 11–26. [Google Scholar]
- 78.Marchington JM, Mattacks CA, Pond CM. Adipose tissue in the mammalian heart and pericardium: structure, foetal development and biochemical properties. Comp Biochem Physiol B Biochem Mol Biol. 1989;94:225–32. doi: 10.1016/0305-0491(89)90337-4. [DOI] [PubMed] [Google Scholar]
- 79.Schoen RE, Thaete FL, Sankey SS, Weissfeld JL, Kuller LH. Sagittal diameter in comparison with single slice CT as a predictor of total visceral adipose tissue volume. Int J Obes Relat Metab Disord. 1998;22:338–42. doi: 10.1038/sj.ijo.0800591. [DOI] [PubMed] [Google Scholar]
- 80.Yanovski JA, Yanovski SZ, Filmer KM, et al. Differences in body composition of black and white girls. Am J Clin Nutr. 1996;64:833–9. doi: 10.1093/ajcn/64.6.833. [DOI] [PubMed] [Google Scholar]
- 81.Rice B, Janssen I, Hudson R, Ross R. Effects of aerobic or resistance exercise and/or diet on glucose tolerance and plasma insulin levels in obese men. Diabetes Care. 1999;22:684–91. doi: 10.2337/diacare.22.5.684. [DOI] [PubMed] [Google Scholar]
- 82.Baumgartner RN, Heymsfield SB, Roche AF, Bernardino M. Abdominal composition quantified by computed tomography. Am J Clin Nutr. 1988;48:936–45. doi: 10.1093/ajcn/48.4.936. [DOI] [PubMed] [Google Scholar]
- 83.Ross R, Rissanen J, Pedwell H, Clifford J, Shragge P. Influence of diet and exercise on skeletal muscle and visceral adipose tissue in men. J Appl Physiol. 1996;81:2445–55. doi: 10.1152/jappl.1996.81.6.2445. [DOI] [PubMed] [Google Scholar]
- 84.Janssen I, Ross R. Effects of sex on the change in visceral, subcutaneous adipose tissue and skeletal muscle in response to weight loss. Int J Obes Relat Metab Disord. 1999;23:1035–46. doi: 10.1038/sj.ijo.0801038. [DOI] [PubMed] [Google Scholar]
- 85.Brummer RJ. Effects of growth hormone treatment on visceral adipose tissue. Growth Horm IGF Res. 1998;8(Suppl B):19–23. doi: 10.1016/s1096-6374(98)80021-x. [DOI] [PubMed] [Google Scholar]
- 86.Ross R, Rissanen J. Mobilization of visceral and subcutaneous adipose tissue in response to energy restriction and exercise. Am J Clin Nutr. 1994;60:695–703. doi: 10.1093/ajcn/60.5.695. [DOI] [PubMed] [Google Scholar]
- 87.Rissanen J, Hudson R, Ross R. Visceral adiposity, androgens, and plasma lipids in obese men. Metabolism. 1994;43:1318–23. doi: 10.1016/0026-0495(94)90229-1. [DOI] [PubMed] [Google Scholar]
- 88.Wajchenberg BL, Bosco A, Marone MM, et al. Estimation of body fat and lean tissue distribution by dual energy X-ray absorptiometry and abdominal body fat evaluation by computed tomography in Cushing’s disease. J Clin Endocrinol Metab. 1995;80:2791–4. doi: 10.1210/jcem.80.9.7673425. [DOI] [PubMed] [Google Scholar]
- 89.Wajchenberg BL. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev. 2000;21:697–738. doi: 10.1210/edrv.21.6.0415. [DOI] [PubMed] [Google Scholar]
- 90.Björntorp P. Classification of obese patients and complications related to the distribution of surplus fat. Nutrition. 1990;6:131–7. [PubMed] [Google Scholar]
- 91.Abate N, Burns D, Peshock RM, Garg A, Grundy SM. Estimation of adipose tissue mass by magnetic resonance imaging: validation against dissection in human cadavers. J Lipid Res. 1994;35:1490–6. [PubMed] [Google Scholar]
- 92.Björntorp P. Adipose tissue in obesity (Willendorf lecture) In: Björntörp P, Cairella M, Howard A, editors. Recent Advances in Obesity Research. London: Libbey; 1985. pp. 163–70. [Google Scholar]
- 93.Spitzer V, Ackerman MJ, Scherzinger AL, Whitlock D. The visible human male: a technical report. J Am Med Inform Assoc. 1996;3:118–30. doi: 10.1136/jamia.1996.96236280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.The Visible Human Project. [Accessed June 2000]; Available online at http://www.nlm.nih.gov/research/visible/visible_human.html.
- 95.Greenfield JR, Samaras K, Chisholm DJ, Campbell LV. Regional intra-subject variability in abdominal adiposity limits usefulness of computed tomography. Obes Res. 2002;10:260–5. doi: 10.1038/oby.2002.35. [DOI] [PubMed] [Google Scholar]
- 96.Clasey JL, Bouchard C, Wideman L, et al. The influence of anatomical boundaries, age, and sex on the assessment of abdominal visceral fat. Obes Res. 1997;5:395–401. doi: 10.1002/j.1550-8528.1997.tb00661.x. [DOI] [PubMed] [Google Scholar]
- 97.Anderson PJ, Chan JC, Chan YL, et al. Visceral fat and cardiovascular risk factors in Chinese NIDDM patients. Diabetes Care. 1997;20:1854–8. doi: 10.2337/diacare.20.12.1854. [DOI] [PubMed] [Google Scholar]
- 98.Leung SS, Chan YL, Lam CW, Peng XH, Woo KS, Metreweli C. Body fatness and serum lipids of 11-year-old Chinese children. Acta Paediatr. 1998;87:363–7. doi: 10.1080/08035259850156896. [DOI] [PubMed] [Google Scholar]
- 99.Albu JB, Curi M, Shur M, Murphy L, Matthews DE, Pi-Sunyer FX. Systemic resistance to the antilipolytic effect of insulin in black and white women with visceral obesity. Am J Physiol. 1999;277:E551–E60. doi: 10.1152/ajpendo.1999.277.3.E551. [DOI] [PubMed] [Google Scholar]
- 100.Abe T, Kawakami Y, Sugita M, Yoshikawa K, Fukunaga T. Use of B-mode ultrasound for visceral fat mass evaluation: comparisons with magnetic resonance imaging. Appl Human Sci. 1995;14:133–9. doi: 10.2114/ahs.14.133. [DOI] [PubMed] [Google Scholar]
- 101.Snel YE, Doerga ME, Brummer RM, Zelissen PM, Koppeschaar HP. Magnetic resonance imaging-assessed adipose tissue and serum lipid and insulin concentrations in growth hormone-deficient adults. Effect of growth hormone replacement. Arterioscler Thromb Vasc Biol. 1995;15:1543–8. doi: 10.1161/01.atv.15.10.1543. [DOI] [PubMed] [Google Scholar]
- 102.van der Kooy K, Leenen R, Seidell JC, Deurenberg P, Visser M. Abdominal diameters as indicators of visceral fat: comparison between magnetic resonance imaging and anthropometry. Br J Nutr. 1993;70:47–58. doi: 10.1079/bjn19930104. [DOI] [PubMed] [Google Scholar]
- 103.Mourier A, Gautier JF, De Kerviler E, et al. Mobilization of visceral adipose tissue related to the improvement in insulin sensitivity in response to physical training in NIDDM. Effects of branched-chain amino acid supplements. Diabetes Care. 1997;20:385–91. doi: 10.2337/diacare.20.3.385. [DOI] [PubMed] [Google Scholar]
- 104.Ohsuzu F, Takayama E, Hayashi K, et al. Relation of abdominal and thigh adipose tissue distribution to serum lipids and glucose metabolism in obese males. J Atheroscler Thromb. 1997;4:34–9. doi: 10.5551/jat1994.4.34. [DOI] [PubMed] [Google Scholar]
- 105.Roelen CA, Koppeschaar HP, de Vries WR, et al. Visceral adipose tissue is associated with circulating high affinity growth hormone-binding protein. J Clin Endocrinol Metab. 1997;82:760–4. doi: 10.1210/jcem.82.3.3836. [DOI] [PubMed] [Google Scholar]
- 106.Gautier JF, Mourier A, de Kerviler E, et al. Evaluation of abdominal fat distribution in noninsulin-dependent diabetes mellitus: relationship to insulin resistance. J Clin Endocrinol Metab. 1998;83:1306–11. doi: 10.1210/jcem.83.4.4713. [DOI] [PubMed] [Google Scholar]
- 107.Lancaster JL, Ghiatas AA, Alyassin A, Kilcoyne RF, Bonora E, DeFronzo RA. Measurement of abdominal fat with T1-weighted MR images. J Magn Reson Imaging. 1991;1:363–9. doi: 10.1002/jmri.1880010315. [DOI] [PubMed] [Google Scholar]
- 108.Gronemeyer SA, Steen RG, Kauffman WM, Reddick WE, Glass JO. Fast adipose tissue (FAT) assessment by MRI. Magn Reson Imaging. 2000;18:815–8. doi: 10.1016/s0730-725x(00)00168-5. [DOI] [PubMed] [Google Scholar]
- 109.Bonora E, Micciolo R, Ghiatas AA, et al. Is it possible to derive a reliable estimate of human visceral and subcutaneous abdominal adipose tissue from simple anthropometric measurements? Metabolism. 1995;44:1617–25. doi: 10.1016/0026-0495(95)90084-5. [DOI] [PubMed] [Google Scholar]
- 110.Kockx M, Leenen R, Seidell J, Princen HM, Kooistra T. Relationship between visceral fat and PAI-1 in overweight men and women before and after weight loss. Thromb Haemost. 1999;82:1490–6. [PubMed] [Google Scholar]
- 111.Thomas EL, Taylor-Robinson SD, Barnard ML, et al. Changes in adipose tissue composition in malnourished patients before and after liver transplantation: a carbon-13 magnetic resonance spectroscopy and gas-liquid chromatography study. Hepatology. 1997;25:178–83. doi: 10.1002/hep.510250133. [DOI] [PubMed] [Google Scholar]


