Abstract
We have studied a naturally occurring small-molecule antimitotic called diazonamide A. Diazonamide A is highly effective at blocking spindle assembly in mammalian cell culture and does so through a unique mechanism. A biotinylated form of diazonamide A affinity purifies ornithine δ-amino transferase (OAT), a mitochondrial enzyme, from HeLa cell and Xenopus egg extracts. In the latter system, the interaction between diazonamide A and OAT is regulated by RanGTP. We find that specific OAT knockdown in human cervical carcinoma and osteosarcoma cells by RNA interference blocks cell division and causes cell death, the effects largely phenocopying diazonamide A treatment in these cell lines. Our experiments reveal an unanticipated, paradoxical role for OAT in mitotic cell division and identify the protein as a target for chemotherapeutic drug development.
Keywords: mitochondria, mitosis, mitotic spindle, natural, product
A variety of small molecules block progression through M phase of the cell cycle. The most common are tubulin ligands. Tubulin-binding toxins have helped elucidate the structure and organization of the mitotic spindle, and certain of these toxins are clinically effective as cancer chemotherapy. However, systemic disruption of the tubulin cytoskeleton has drawbacks. Microtubule poisons disturb nonmitotic functions of the cytoskeleton in both replicating cells and differentiated nondividing cells. Wasting, neutropenia, and peripheral neuropathy are severe dose-limiting toxicities common to this family of drugs in vivo (1). As a result, considerable effort has been made to identify alternative antineoplastics that target mitotic regulatory factors or components of the spindle other than tubulin (2). The development of specific inhibitors of the aurora kinases (3) and the kinesin motor protein Eg5 (2) are notable recent examples. The product of the aurora A oncogene mediates centrosomal duplication, whereas the mitotic kinesin Eg5 transports replicated centrosomes to opposing spindle poles. Antagonists of either activity cause mitotic arrest broadly, both in vitro and in vivo.
In an attempt to understand how spindle assembly might be blocked even more selectively, we have studied an antimitotic natural product called diazonamide A (1; Fig. 1). This molecule was isolated from extracts of a marine organism, initially as a constituent that inhibited human cancer cell growth in culture (4). At low-nanomolar concentrations, diazonamide A induces an M-phase growth arrest in a variety of cancer cell types. Diazonamide A's effects are reminiscent of those caused by tubulin poisons (5), yet we could find no evidence of a direct interaction between the compound and tubulin/microtubules in vitro. Moreover, experiments showed that, at i.v. doses sufficient to regress human tumor xenografts in nude mice, diazonamide-treated animals showed neither weight loss nor evidence of neutropenia, side effects common to tubulin-interacting drugs (6).
Fig. 1.
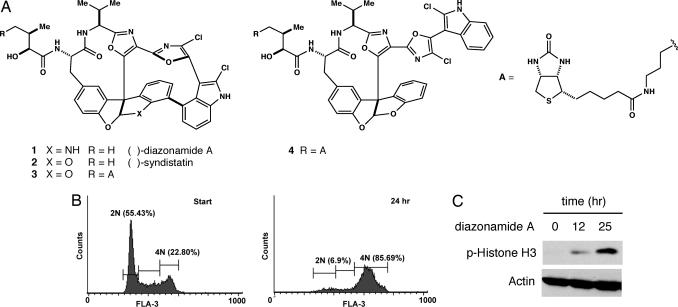
Diazonamide A arrests cell cycle progression at the G2/M transition. (A) Chemical structure of diazonamide A and synthetic derivatives used in this study. Biotin conjugate 3 retains potent antimitotic activity in cell culture whereas modified form 4 is a thousandfold less active. (B) FACS analysis of HeLa cells treated with 30 nM diazonamide A for the indicated times. (C) HeLa cells were dosed with 30 nM diazonamide A for the indicated times. Extracts prepared from these cells were immunoblotted with antibodies against phosphorylated histone H3 and actin.
The structure initially assigned to diazonamide A was incorrect (4). We were able to revise that proposal through chemical synthesis (7, 8). These efforts also provided access to analogs and tagged derivatives. Using certain of these as reagents, we have identified the cellular receptor that mediates diazonamide antimitotic effects. Surprisingly, that receptor is a previously characterized mitochondrial enzyme.
Results
Impact of Diazonamide A on Spindle Assembly in HeLa Cells and Xenopus Egg Extracts.
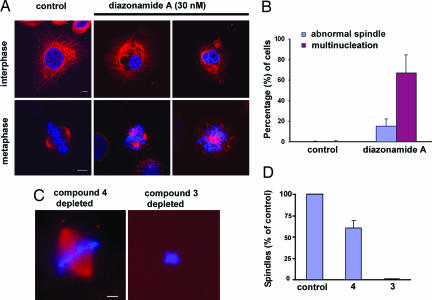
Diazonamide A induces a classic G2/M phase growth arrest in a variety of human cancer cell lines. For example, HeLa cells dosed with low concentrations of 1 accumulate in a tetraploid state (Fig. 1B), coincident with high levels of the mitotic marker phosphorylated Histone H3 (Fig. 1C). As reported previously, growth arrest results from aberrant mitotic spindle formations (5, 8). In the presence of 30 nM diazonamide A, HeLa cells assemble both mono- and multipolar spindle structures (Fig. 2A) that do not support proper cell division. The percentage of mitotic cells tabulated in Fig. 2B is a lower estimate; rounded, dying cells are readily dislodged from chamber slides during the immunostaining procedure. In addition, a high proportion of cells show a multinucleation phenotype, perhaps the result of disregulated cytokinesis (Fig. 2A).
Fig. 2.
Impact of diazonamide A on spindle assembly in HeLa cells and Xenopus egg extracts. (A and B) HeLa cells were grown in the absence and presence of 30 nM compounds 1. After 24 h, spindle and nuclear morphology were analyzed by immunostaining with an antibody against α-tubulin (red). DNA is 4′,6′-diamidino-2-phenylindole (DAPI, blue) stained. (A) Representative images of normal (Control) and defective (diazonamide-treated) cells in interphase (Upper) and metaphase (Lower). (Scale bar: 5 μm.) (B) Quantification of abnormal cells in control and diazonamide treatment shown in (A). (C and D) CSF-arrested Xenopus egg extracts were incubated with active compound 3 or inactive compound 4-coated avidin agarose at 4°C for 60 min. Beads were pelleted and the supernatant was assayed for spindle assembly stimulated by sperm centrioles. (C) Representative images of chromosome and spindle. Microtubules are rhodamine labeled (red) and DNA stained with Hoechst 33342 (blue). (Scale bar: 10 μm.) Spindles were quantified in D. Similar results were obtained in three independent experiments.
Synthetic probes such as biotinylated substance 3 (Fig. 1A) proved invaluable in elucidating diazonamide's mode of action. Like the natural product, compound 3 is potently antimitotic in HeLa cell culture (data not shown). As a structurally related, negative control, seco analog 4 was synthesized. This molecule is identical to 3, except that it lacks the σ-bond connecting carbons 16 and 18. This modification allows the bis(oxazoyl)indole motif to adopt an extended conformation and results in a thousandfold loss of cellular activity.
Data from the NCI's COMPARE screen implied a mechanistic similarity between diazonamide A and microtubule depolymerizing agents such as maytansine and vinblastine. Hamel et al. (5) subsequently found that diazonamide A inhibits tubulin polymerization at high concentrations in vitro. However, neither we nor they could demonstrate competitive binding at a previously characterized small-molecule-interacting site on heterodimeric tubulin. It was possible these molecules did interact with tubulin, but in a manner not seen before. To address this issue, biotinylated compound 3 and a radiolabeled congener (data not shown) were used for direct binding measurements. Neither interacted specifically with tubulin or microtubules in vitro (Fig. 3B, and data not shown), yet both potently induced spindle abnormalities during mitosis indistinguishable from those caused by the natural product. It seemed that the tubulin interaction was either low-affinity, nonspecific, or indirect and at diazonamide concentrations sufficient to block mitosis in cell culture (EC50 ≈2–10 nM, depending on the cell type), another target(s) may be relevant. In support of this idea, diazonamide A had no discernable impact on HeLa interphase microtubules at doses exceeding its GI50 (Fig. 2A). More importantly, compound 3-depleted Xenopus egg extracts no longer supported sperm centromere-initiated spindle assembly reactions, and supplementing those depleted extracts with purified tubulin did not restore the activity (Fig. 2 C and D). This experiment strongly suggested that probe 3 removed a factor(s), other than tubulin, essential for spindle assembly in this in vitro system.
Fig. 3.
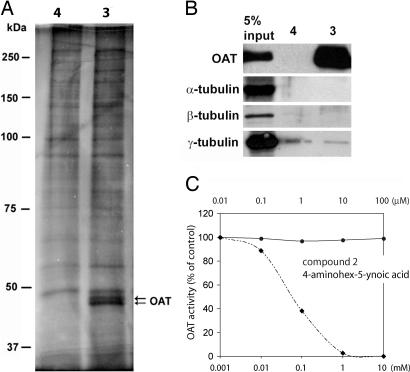
OAT is a specific diazonamide-binding protein in vitro. (A) Hitrap-Q column fractions obtained from HeLa nuclear extract were incubated with compounds 3 or 4 bound to avidin agarose. Proteins subsequently recovered from the affinity matrices were analyzed by silver-stained SDS/PAGE. Arrows indicate specific diazonamide-binding proteins, both identified as OAT by mass spectrometry. (B) The affinity matrices of compounds 3 or 4 were analyzed by Western blotting with the indicated antibodies. (C) Compound 2 does not inhibit mouse liver-derived OAT enzymatic activity in vitro. Assay measures conversion of l-ornithine to Δ1-pyrroline-5-carboxylic acid as determined through its reaction with o-aminobenzaldehyde in situ. Crude OAT enzyme was prepared from mouse liver as described (9). The standard incubation mixture contained 70 mM l-ornithine, 7.5 mM potassium α-ketoglutarate, and 100 mM potassium phosphate, pH 7.1, in a total volume of 2 ml. The enzyme was added last with dilution series of compound 2 or 4-aminohex-5-ynoic acid, and the mixture was incubated with shaking at 37°C for 20 min. The pyrroline-5-carboxylate formed was determined by reaction with o-aminobenzaldehyde, and the absorbance was determined at 443 mm.
Identification of Ornithine δ-Amino Transferase (OAT) as a Diazonamide-Binding Protein.
To isolate diazonamide-binding protein(s), HeLa extracts were fractionated by Hitrap Q anion-exchange chromatography. Each eluted column fraction was then probed with affinity matrices prepared from both compounds 3 (active) and 4 (inactivated control). Affinity-purified proteins were visualized by silver-stained SDS/PAGE wherein two bands ≈50 kDa in size were recovered specifically by the active compound matrix (Fig. 3A). Mass spectrometry identified both as OAT. The biochemical nature that causes OAT to migrate as two species on SDS/PAGE is not known.
To confirm that compound 3 specifically binds to OAT, polyclonal antibodies against full-length human OAT (hOAT) and Xenopus OAT (xOAT) were subsequently raised and used for Western blotting analysis of proteins affinity-purified from both crude HeLa cells and Xenopus egg extracts with compound 3. OAT was specifically detected in the compound 3-associated fraction from both sources (Figs. 3B and 4C). Pretreatment of extracts with an excess of nonbiotinylated substance 2 competitively blocked affinity-purifications of OAT by compound 3 (data not shown). Compound 3 also interacts with purified recombinant OAT (data not shown), suggesting a direct interaction between the two. However, the molecule does not inhibit OAT enzymatic activity when assayed in crude mitochondrial extracts from mouse liver (9) (Fig. 3C). The conversion of l-ornithine to l-Δ1-pyrroline-5-carboxylic acid in this system was unaffected by compounds 1 or 2, whereas 4-aminohex-5-ynoic acid, a characterized OAT inhibitor (10), suppressed the activity in a dose-dependent manner.
Fig. 4.
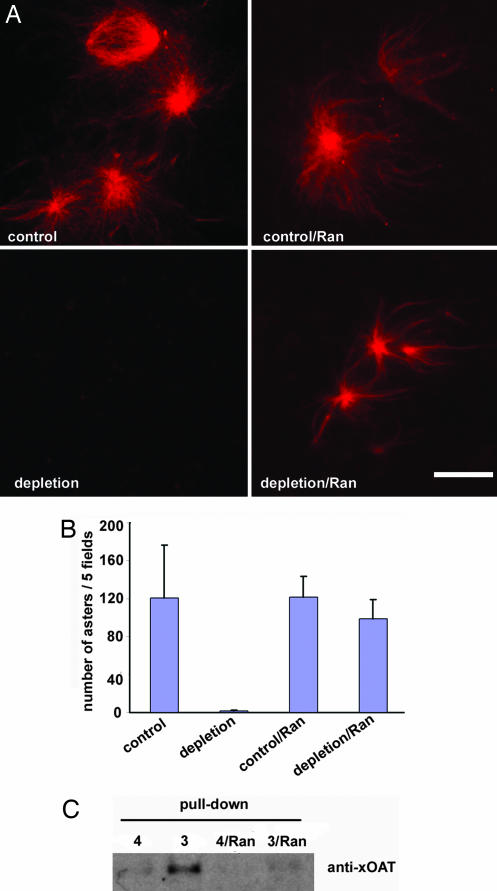
Ran stimulation attenuates the interaction between compound 3 and OAT. (A) Compound 3 or 4 was coupled to avidin agarose beads. The beads were washed extensively and incubated with extracts at 4°C for 60 min. The resultant supernatant from Compound 4 depletion served as “control” extracts, and that from Compound 3 depletion was indicated as “depletion” extracts. Then 24 μmM RanL43E and 0.05 mg/ml rhodamine-labeled tubulin were added to 10 μl of the above extracts. After incubating at room temperature for 30 min, these reactions were observed for Ran aster formation under a microscope as shown (Left). In a parallel set of experiments (Right), extracts were first treated with 24 μM RanL43E for 30 min at room temperature before being incubated with avidin beads coupled with either inactive compound 4 or active compound 3 at 4°C for 60 min. The resultant supernatants were designated as control/Ran, and depletion/Ran, respectively. Note that only 0.05 mg/ml rhodamine-labeled tubulin but no additional Ran was added to these reactions. The visible rhodamine-labeled asters were induced by presupplemented RanL43E. (Scale bar: 10 μm.) (B) Quantification of asters observed in A. (C) The extracts in A were diluted 10-fold with buffer B supplemented with 0.1% Nonidet P-40 and incubated with compound 3 or 4 coupled avidin beads at 4°C overnight. The affinity-purified proteins were probed with antibodies to Xenopus OAT.
OAT is well characterized as a mitochondrial matrix enzyme. In this context, OAT regulates flux through the urea cycle and couples, indirectly, proline biosynthesis to the availability of fumarate for consumption in the TCA cycle (11). Of the extensive biochemical and genetic information on OAT, none had previously connected the protein to cell division. The idea that OAT binding underpinned the antimitotic property of diazonamide A was therefore approached cautiously.
Ran Disrupts the Interaction Between Diazonamide Probe 3 and OAT.
Recent studies have shown that RanGTP stimulates release of a subset of sequestered spindle assembly factors from transport receptors importin β and thereby has broad actions in microtubule nucleation and stabilization during spindle assembly (12–14, 22). Given that spindle assembly is impeded by diazonamide A in cells, we sought to determine whether the compound affects RanGTP-stimulated microtubule aster formations in Xenopus egg extract. Addition of a constitutively active Ran mutant having a GTP hydrolysis deficiency (RanL43E) is sufficient to induce the formation of asters and pseudospindles. As shown in Fig. 4A Left, Ran asters formed normally in egg extracts depleted with inactive compound 4 coupled to avidin agarose beads (control). In contrast, no asters were seen in extracts similarly depleted with active compound 3 (depletion). These results implied that diazonamide A inhibits a downstream effector(s) of RanGTP. Interestingly, when Xenopus egg extracts were preincubated with a constitutively active form of Ran, RanL43E, before addition of compound-coupled agarose beads, depletion with active compound 3 had little effect on the number and size of Ran-induced asters relative to a mock depletion with control compound 4 (Fig. 4A Right and 4B). Consistent with this result, we observed that diazonamide A has no effect on preexisting spindles in cultured cells (data not shown). Importantly, Western blotting analyses of proteins bound to beads retrieved in the above experiment revealed a marked decrease of OAT binding to probe 3 after Ran pretreatment. However, levels of OAT in extracts were unaffected (Fig. 4C, data not shown). The Ran signal, by some means, appeared to mask or eliminate the diazonamide A-binding site on OAT. This observation is consistent with the notion that OAT, as the target of diazonamide, works downstream of Ran signaling in mitotic spindle assembly.
OAT Is Important for Cell Division in HeLa and U2OS Cells.
The identification of OAT as a binding partner for diazonamide A indicated that the protein might have a second function in mitosis. We attempted to immunodeplete OAT with an anti-xOAT antibody to examine whether OAT is required for proper spindle assembly in Xenopus egg extracts. Unfortunately, this effort obtained maximally a 50% reduction in OAT levels; wherein sperm-induced spindle assembly and Ran aster formations were unaffected. We therefore knocked down endogeneous OAT in HeLa cells by siRNA specifically targeting OAT mRNA. Eliminating OAT expression resulted in massive cell death 3 days after OAT-specific siRNA transfection but not in cells transfected with a control siRNA. The observed cell death was likely not due to lack of endogenous OAT enzymatic activity because supplementing culture media with either l-arginine or l-ornithine failed to attenuate the rate of death in OAT siRNA-treated cells [supporting information (SI) Fig. 6]. Notably, a 2-day treatment led to accumulation of cells arrested in the G2/M phase of the cell cycle and an increase in mitotic spindle defects (SI Fig. 6). In that case, mitotic cells assembled a characteristic multipole spindle structure having misaligned chromosomes, a phenomenon highly reminiscent of that seen upon diazonamide A treatment in this cell line.
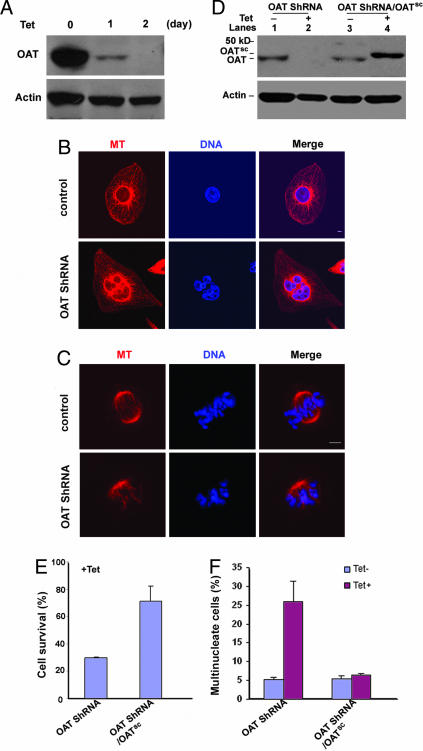
To exclude the possibility that the above phenotypes were due to nonspecific transfection toxicity or siRNA off-target effects, we established U2OS cell lines that express small hairpin RNA (shRNA) against OAT mRNA upon tetracycline induction. The growth of parental U2OS cells was not affected by tetracycline treatment. However, elimination of OAT protein and pronounced cell death was observed in OAT shRNA stable cell lines 2 days after induction (Fig. 5A). To ask whether cell-cycle progression through mitosis was perturbed after OAT depletion, we stained nuclei of live cells with Hoechst 33342 and followed nucleus morphology changes by time-lapse microscopy. Beginning 30 h after tetracycline induction, cells were photographed every 5 min for 24 h. Thirty-four percent of cells showed severe chromosome misalignments, whereas only 8% of control cells showed this phenotype under identical imaging conditions (SI Movies 1 and 2, control and RNAi). Immunostaining of OAT knockdown cells with an antitubulin antibody and DAPI staining revealed inhibition of bipolar spindle formation and chromosome misalignments in >80% of mitotic cells. Multinucleate cells with macro- and micronuclei were also observed four times as frequently in tetracycline-treated stable cell lines relative to their nontreated counterparts (Fig. 5 B–F). This effect is likely due to improper chromosome segregation and a subsequent failure of cytokinesis. Notably, U2OS cells displayed similar abnormalities upon diazonamide A treatment (SI Fig. 7).
Fig. 5.
Inducible expression of OAT shRNA knocked down OAT in U2OS cells. (A) A tetracycline-inducible OAT ShRNA cell line was generated by following the protocol from Oligoengine (see Materials and Methods for details). Expression of OAT shRNA was induced by adding 1 μg/ml tetracycline to the culture medium of the cell line. Treated cells were harvested, and the cell extracts were prepared for Western blotting against OAT and actin antibody at the indicated time. (B and C) Representative images of multinucleate interphase (B) and metaphase (C) cells in OAT ShRNA cells. OAT ShRNA cells were treated with tetracycline for 48 h. Cells were stained for DNA (DAPI, blue) and MTs (α-tubulin antibody, red). (Scale bar: 5 μm.) (D) The inducible OAT expression vector (OATsc) was constructed to scramble the corresponding siRNA sequence. OATsc was transfected into the inducible OAT ShRNA cell line to obtain the stable cell line (OAT shRNA/OATsc) that exogenously expresses OATsc resistant to the shRNA knock down. OATsc protein is slightly larger than endogenous OAT because it contains a six-histidine tag at its C terminus. OAT shRNA and OAT shRNA/ OATsc cell lines were cultured in the absence (lanes 1 and 3) and presence (lanes 2 and 4) of tetracycline for 48 h. Cells were collected and analyzed by Western blot using OAT and actin antibodies. (E) The percentage of surviving cells upon tetracycline treatment compared with untreated counterpart. OAT shRNA and OAT shRNA/OATsc cell lines were cultured in the absence and presence of 1 μg/ml tetracycline for 70 h. The cells were collected for trypan blue staining, and the number of living cells was counted. (F) Frequency of multinucleate cells in OAT shRNA and OAT shRNA/OATsc cell lines after 48-h tetracycline induction or noninduction. At least 300 cells in each sample were analyzed. n = 3 independent experiments in E and F. All error bars are mean ± SD.
To control for off-target effects of this shRNA and to ensure that the observed cell division defect was due specifically to OAT knockdown, we further introduced an OAT expression construct controlled by the same tetracycline induction but wherein the OAT mRNA harbored silent mutations that scramble the shRNA targeting sequence into the OAT shRNA stable cell lines. In OAT shRNA/OATsc cells, tetracycline induction causes concurrent loss of endogenous OAT and the appearance of the OAT transgene product (Fig. 5D). As shown in Fig. 5 E and F, this exogenously expressed OAT reversed the cell death and mitosis defects (i.e., abnormal spindle formations and multinucleation) caused by OAT ablation, indicating that the phenotype is specifically associated with loss of OAT.
Discussion
The mitotic spindle is a molecular machine assembled transiently to segregate replicated chromosomes and to promote cytokinesis within dividing cells. The mitotic spindle is a dynamic network of microtubules harboring various motor assemblies, accessory proteins, and regulatory factors. Proper coordination of these components is required to establish spindle bipolarity and to ensure fidelity in the separation of sister chromatids at anaphase (15, 16).
The synthesis of tagged diazonamide derivatives retaining the biological activity of the parent has allowed us to identify a known mitochondrial enzyme, OAT, as an unexpected player in spindle formation. The finding that OAT mediates the mitotic arrest caused by diazonamide A in human cancer cells is surprising for two reasons: (i) the previously characterized biochemical activity and cellular location of OAT did not anticipate this discovery and (ii) genetic data from OAT-null mice and OAT-deficient human patients (17, 18) did not indicate an essential function, as might be expected for a regulator of cell division. Nonetheless, our data clearly demonstrate that OAT has a role in regulating mitotic cell division. Although the activity may not be essential for normal development, it is critical for cell division in human cancer cells sensitive to diazonamide A. Our experiments show that OAT is required for proper spindle assembly in human cancer cells and Xenopus egg extracts. Details of how this mitochondrial protein functions in mitosis, and how Ran and diazonamide regulate its activity, remain to be elucidated.
Diazonamide A performs especially well as a cancer chemotherapeutic in animal models without causing systemic toxicities typical of antimitotic drug treatments. We propose that, unlike tubulin-binding compounds, diazonamide A inhibits the mitotic functions of OAT, which itself facilitates spindle assembly in human and murine tumor cell lines. Our data suggest that this second function of OAT is unrelated to its role in amino acid metabolism. Diazonamide A does not impede OAT enzymatic activity (Fig. 3C), and a known small-molecule inhibitor of OAT has no effect on cell growth (SI Fig. 8). It is therefore anticipated that diazonamide A will inhibit mitosis in cells using OAT for spindle assembly yet not induce the progressive retinal degeneration (gyrate atrophy) associated with loss of OAT enzymatic activity in rodents and humans (17, 18).
OAT appears to not be essential for cellular proliferation during normal development, as evidenced by the viability of OAT-null mice (6, 17). It is possible that there are redundancies in normal cells able to compensate for OAT loss during mitosis or, more provocatively, that rapidly proliferating cancer cells may exploit OAT more prominently during mitosis than normal cells. Not surprisingly, freshly isolated mouse embryonic fibroblasts are not sensitive to diazonamide treatment (data not shown), nor do mice treated with the compound (at doses sufficient to eliminate human tumor xenographs) show signs of cell growth defects such as weight loss or neutropenia (6). On the other hand, a wide variety of human cancer cell lines are exquisitely sensitive to diazonamide A treatment, and acute ablation of OAT in HeLa and U2OS cells causes mitotic defects and subsequent cell death.
Inappropriately activating the Wnt/β-catenin signaling pathway by introducing an oncogenic form of β-catenin into transgenic mice specifically induced OAT overexpression in liver. This observation is consistent with the role of Wnt signaling in cell division and is perhaps relevant to hepatocarcinogenesis (19). In this particular case, it is possible that up-regulation of OAT drives hepatocellular hyperproliferation.
Because OAT-null mice are viable, it is now possible to test the involvement of OAT in tumorgenesis and tumor maintenance directly by breeding tumor-prone mice into an OAT-null background. In doing so, it will be particularly interesting to examine the onset, rate, and extent of tumorigenesis in mice lacking OAT. If tumorigenesis is in fact suppressed, the implications for cancer-selective drug development by targeting the mitotic functions of OAT would be significant.
Materials and Methods
In Vitro Binding Studies.
Human HeLa S3 cells were obtained from National Cell Culture Center (Minneapolis, MN). The cell pellets were resuspended in buffer A [20 mM Hepes-KOH (pH 7.5)/10 mM KCl/1.5 mM MgCl2/1 mM sodium EDTA/1 mM sodium EGTA/1 mM DTT/0.1 mM PMSF). HeLa nuclear extracts (NE) were prepared as described (20). Biotinylated diazonamide were precoupled to avidin beads (Sigma) at 3 nmol/50 ml of beads, and incubated with 1 ml of HeLa NE, which had been dialyzed against buffer B (buffer A containing 150 mM NaCl) and supplemented with 0.1% Nonidet P-40, overnight at 4°C. The pulldown by compounds was also carried out in a series of Q fractions from NE to get rid of much nonspecific background binding. Binding of recombinant proteins to compounds was performed essentially as above, except that avidin beads were blocked with 5 mg/ml BSA before coupling with compounds.
In Vitro Ran Aster Assays and Sperm-Spindle Assembly Assays.
Ten thousand × g cytoplasmic extracts from cytostatic factor (CSF)-arrested Xenopus egg were prepared as described (21). Rhodamine-labeled tubulin (Cytoskeleton, Denver, CO) was added to a final concentration of 0.05 mg/ml to 10 μl of egg extracts. For aster assay, GST-RanL43E [a gift from Y. Zheng, Carnegie Institution of Washington, Baltimore, MD (22)] was added at a final concentration of 25 μM unless indicated otherwise. In the cycled spindle-assembly assay, ≈300 sperm nuclei were added to each reaction. Sperm chromatin was visualized by Hoechst 33342 staining.
Depletion with Diazonamide.
Compounds 3 or 4 were coupled to avidin beads as described above. The beads were washed four times with buffer B, followed by two washes in CSF-XB buffer (10 mM K-Hepes, pH 7.5/100 mM KCl/2 mM MgCl2/0.1 mM CaCl2/5 mM EGTA/50 mM sucrose). Fifteen microliters of beads were incubated with 50-ml extracts at 4°C for 60 min. Beads were pelleted by centrifugation at 1,000 × g. The supernatant was assayed for Ran aster assembly or for sperm-spindle assembly.
Cloning, Production of Recombinant OAT in Bacteria, and the Antibodies.
The human OAT (hOAT) cDNA was amplified by PCR from HeLa cDNA library and cloned into the bacterial expression vector pET-21a (Novagen, San Diego, CA) to express C-terminally 6× His-tagged OAT proteins. The recombinant protein was purified by using NiTA-agarose (Qiagen, Valencia, CA). The same procedure was applied to make recombinant Xenopus OAT, whose cDNA was amplified from image clone (Clone ID 5543139; Open Biosystems, Huntsville, AL). The rabbit antibodies were raised against recombinant hOAT or xOAT (Rockland, Gilbertsville, PA) and were affinity-purified.
Transfection and Immunostaining of Cells.
The cells were fixed in 4% formaldehyde and stained with a mouse monoclonal antibody against α-tubulin (1:1,000; Molecular Probes, Eugene, OR). The slides were mounted in Vectashield Mounting medium with DAPI (Vector Laboratories, Burlingame, CA). Images of spindle and chromatin structures were captured by using a LSM 510 META laser scanning confocal microscope (Zeiss, Thornwood, NY) with Chameleon XR NIR laser (University of Texas Southwestern Live Cell Imaging Core Facility). For RNAi of hOAT in HeLa cells, double-strand siRNA 5′-GUAUGGUGCACACAACUACdTdT-3′ (selected by siDESIGN Center; Dharmacon, Lafayette, CO), corresponding to a region of hOAT mRNA, was synthesized by University of Texas Southwestern Medical Center RNA Oligonucleotide Core to disrupt OAT mRNA in HeLa cells. Double-strand siRNA, 5′-AACGUACGCGGAAUACUUCGAdTdT-3′, corresponding to a region of luciferase was used as a control. Transfection of siRNA was performed by using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) according to standard procedure.
Stable Cell Lines for Inducible OAT Knockdown and Rescue in U2OS Cells.
Six copies of OAT shRNA (sequence as GTATGGTGCACACAACTAC) were cloned into pSuperior.puro vector according to ref. 23 and the protocol from Oligoengine (Seattle, WA). The OAT shRNA construct was transfected into U2OS cells stably expressing tetracycline repressor by using Lipofectamine 2000 transfection reagent (Invitrogen). Individual clones resistant to 1 μg/ml blasticidine and 2 μg/ml puromycin were picked up and treated with 1 μg/ml tetracycline for 2–3 days. Nine of 24 clones show morphology change and cell death. Cell extracts were subjected to Western blotting against OAT antibody. OAT was depleted in all nine clones. Subsequently, one OAT shRNA cell line was selected for further generation of inducible rescue line (OAT shRNA/OATsc). OATsc is an OAT expression plasmid on modified pCDNA vector with inducible H1 promoter. The OATsc expression is shRNA resistant because six scrambling mutations (GTACGGCGCCCATAATTAT) (the italicized letters indicate a mutated sequence) were introduced to full-length OAT cDNA with C-terminal 6× His-tag. The OATsc was transfected into the OAT shRNA cell line, and the procedure for selection of inducible OAT shRNA/OATsc cell lines is essentially similar to that of the OAT shRNA cell line, except that culture medium contains an additional selection reagent, 1 μg/ml neomycin. In these cell lines, expressions of OAT shRNA and OATsc were induced by 1 μg/ml tetracycline treatment.
Supplementary Material
Abbreviations
- OAT
ornithine δ-amino transferase
- shRNA
small hairpin RNA.
Footnotes
Conflict of interest statement: P.G.H. and X.W. are cofounders of a company, Joyant Pharmaceutical Inc., that is in the process of developing diazonamide as an anticancer drug.
This article contains supporting information online at www.pnas.org/cgi/content/full/0610832104/DC1.
References
- 1.Wood KW, Cornwell WD, Jackson JR. Curr Opin Pharmacol. 2001;1:370–377. doi: 10.1016/s1471-4892(01)00064-9. [DOI] [PubMed] [Google Scholar]
- 2.Mayer TU, Kapoor TM, Haggarty SJ, King RW, Schreiber SL, Mitchison TJ. Science. 1999;286:971–974. doi: 10.1126/science.286.5441.971. [DOI] [PubMed] [Google Scholar]
- 3.Harrington EA, Bebbington D, Moore J, Rasmussen RK, Ajose-Adeogun AO, Nakayama T, Graham JA, Demur C, Hercend T, Diu-Hercend A, et al. Nat Med. 2004;10:262–267. doi: 10.1038/nm1003. [DOI] [PubMed] [Google Scholar]
- 4.Lindquist N, Fenical W, Van Duyne GD, Clardy J. J Am Chem Soc. 1991;113:2303–2304. [Google Scholar]
- 5.Cruz-Monserrate Z, Vervoort HC, Bai R, Newman DJ, Howell SB, Los G, Mullaney JT, Williams MD, Pettit GR, Fenical W, Hamel E. Mol Pharmacol. 2003;63:1273–1280. doi: 10.1124/mol.63.6.1273. [DOI] [PubMed] [Google Scholar]
- 6.Williams NS, Burgett AWG, Atkins A, Wang X, Harran P, McKnight SL. Proc Natl Acad Sci USA. 2007;104:2074–2079. doi: 10.1073/pnas.0611340104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li J, Jeong S, Esser L, Harran PG. Angew Chem Intl Ed Engl. 2001;40:4765–4770. doi: 10.1002/1521-3773(20011217)40:24<4765::aid-anie4765>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 8.Li J, Burgett AWG, Esser L, Amezcua C, Harran PG. Angew Chem Int Ed Engl. 2001;40:4770–4773. doi: 10.1002/1521-3773(20011217)40:24<4770::aid-anie4770>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 9.Strecker HJ. J Biol Chem. 1965;240:1225–1230. [PubMed] [Google Scholar]
- 10.Jung MJ, Seiler N. J Biol Chem. 1978;253:7431–7439. [PubMed] [Google Scholar]
- 11.Seiler N. Curr Drug Targets. 2000;1:119–153. doi: 10.2174/1389450003349254. [DOI] [PubMed] [Google Scholar]
- 12.Carazo-Salas RE, Guarguaglini G, Gruss OJ, Segref A, Karsenti E, Mattaj IW. Nature. 1999;400:178–181. doi: 10.1038/22133. [DOI] [PubMed] [Google Scholar]
- 13.Ems-McClung SC, Zheng Y, Walczak CE. Mol Biol Cell. 2004;15:46–57. doi: 10.1091/mbc.E03-07-0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gruss OJ, Carazo-Salas RE, Schatz CA, Guarguaglini G, Kast J, Wilm M, Le Bot N, Vernos I, Karsenti E, Mattaj IW. Cell. 2001;104:83–93. doi: 10.1016/s0092-8674(01)00193-3. [DOI] [PubMed] [Google Scholar]
- 15.Desai A, Mitchison TJ. Annu Rev Cell Dev Biol. 1997;13:83–117. doi: 10.1146/annurev.cellbio.13.1.83. [DOI] [PubMed] [Google Scholar]
- 16.Karsenti E, Vernos I. Science. 2001;294:543–547. doi: 10.1126/science.1063488. [DOI] [PubMed] [Google Scholar]
- 17.Wang T, Lawler AM, Steel G, Sipila I, Milam AH, Valle D. Nat Genet. 1995;11:185–190. doi: 10.1038/ng1095-185. [DOI] [PubMed] [Google Scholar]
- 18.Brody LC, Mitchell GA, Obie C, Michaud J, Steel G, Fontaine G, Robert MF, Sipila I, Kaiser-Kupfer M, Valle D. J Biol Chem. 1992;267:3302–3307. [PubMed] [Google Scholar]
- 19.Cadoret A, Ovejero C, Saadi-Kheddouci S, Souil E, Fabre M, Romagnolo B, Kahn A, Perret C. Cancer Res. 2001;61:3245–3249. [PubMed] [Google Scholar]
- 20.Jiang X, Kim HE, Shu H, Zhao Y, Zhang H, Kofron J, Donnelly J, Burns D, Ng SC, Rosenberg S, Wang X. Science. 2003;299:223–226. doi: 10.1126/science.1076807. [DOI] [PubMed] [Google Scholar]
- 21.Blower MD, Nachury M, Heald R, Weis K. Cell. 2005;121:223–234. doi: 10.1016/j.cell.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 22.Wilde A, Zheng Y. Science. 1999;284:1359–1362. doi: 10.1126/science.284.5418.1359. [DOI] [PubMed] [Google Scholar]
- 23.Zhong Q, Gao W, Du F, Wang X. Cell. 2005;121:1085–1095. doi: 10.1016/j.cell.2005.06.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.