Abstract
Nerve growth factor (NGF) and neurotrophin-3 (NT-3) are target-derived proteins that regulate innervating sympathetic neurons. Here, we used western blot analysis to investigate changes in NGF and NT-3 protein in several peripheral tissues following loss of sympathetic input. Following removal of the superior cervical ganglion (SCG), large molecular weight (MW) NGF species, including proNGF-A, were increased in distal intracranial SCG targets, such as pineal gland and extracerebral blood vessels (bv). Mature NGF was a minor species in these tissues and unchanged following sympathectomy. Large MW NGF species also were increased when sympathectomy was followed by in vivo NGF administration. Mature NT-3, which was abundant in controls, was significantly decreased in these targets following sympathetic denervation. The decrease in mature NT-3 was enhanced following NGF administration. The trigeminal ganglion, which provides sensory input to these targets, showed increased NGF, but decreased NT-3, in these treatments, demonstrating that decreased NT-3 at the targets did not result from enhanced NT-3 uptake. Unlike pineal gland and extracerebral bv, the external carotid artery, an extracranial proximal SCG target, showed no change in NGF following denervation, and mature NT-3 was significantly increased. Following NGF administration, NT-3 was significantly decreased. We provide evidence for sympathetic regulation of NGF and NT-3 in peripheral targets and that elevated NGF can depress NT-3. The differential response in distal and proximal adult targets is consistent with the idea that neurons innervating proximal and distal targets may serve different roles in regulating neurotrophin protein. In addition, we conclude that previous ELISA results showing increased NGF protein following sympathetic denervation may have resulted from increases in large MW species, rather than an increase in mature NGF.
Keywords: superior cervical ganglion, SCG, extracerebral blood vessels, pineal gland, external carotid artery, sympathectomy, sympathetic denervation
1. Introduction
The interactions of peripheral neurons and their target tissues are not well understood, although the survival functions of target-derived neurotrophins such as nerve growth factor (NGF) and neurotrophin-3 (NT-3) have been well-characterized. In the periphery, NGF and NT-3 are produced by target tissues, internalized by the innervating sympathetic and/or sensory neuron, and retrogradely transported to the cell body (Thoenen and Barde 1980; Schwab et al. 1982; Zhou and Rush 1996; Zhou et al. 1997; Kuruvilla et al. 2004), where they carry out their survival activities. NGF is essential for the survival of sympathetic neurons during development (Gorin and Johnson 1980; Crowley et al. 1994) as well as in adulthood (Ruit et al. 1990; Ghasemlou et al. 2004). NT-3 also is important for the survival of sympathetic neurons (Zhou and Rush 1995; Rush et al. 1997; Francis et al. 1999). Severe deficits in sensory and sympathetic neuronal populations have been demonstrated in NT-3 null mutant mice (Farinas et al. 1994). A possible cooperative role between endogenous NGF and NT-3 in sympathetic neuron survival has been suggested, and the administration of NGF during NT-3 antiserum treatment, or vice versa, inhibited neuronal death (Tafreshi et al. 1998), although it is unclear exactly how these two neurotrophins interact to regulate sympathetic neurons.
The exact role of sympathetic input in the regulation of target-derived NGF and NT-3 is unknown. Humpel and colleagues (1993) showed that unilateral removal of the SCG resulted in increased NGF mRNA levels in the submandibular gland. Consistent with these results, the removal of the SCG resulted in increased NGF protein, determined using ELISA, in the submandibular gland (Ekstrom and Reinhold 2004). In addition, the administration of 6-OHDA resulted in increased NGF protein in the submandibular gland as well as in the iris (Korsching and Thoenen 1985). These reports suggest a potential regulatory role by sympathetic input in the expression of target-derived neurotrophins in the peripheral nervous system. Indeed, norepinephrine (NE), the major neurotransmitter utilized by postganglionic sympathetic neurons, was shown to decrease NGF content in cardiac myocytes (Qin et al. 2002).
In these previous reports, the changes in NGF following sympathetic removal were determined using ELISA, which measures total NGF protein. With the recent findings that the mature NGF species may not be the prevalent NGF form in most peripheral tissues (Bierl et al. 2005), it is not clear which NGF species are altered in peripheral targets following sympathetic denervation. In addition, there have been no studies examining the influence of sympathetic innervation on NT-3 protein expression in peripheral targets. Thus, in the present study, we carried out a detailed NGF and NT-3 western blot analysis of several peripheral targets following a three week removal of the SCG. The effects of in vivo NGF administration following the removal of the SCG also was examined.
2. Results
NGF protein expression in peripheral tissues: effects of denervation
NGF western analysis of peripheral targets from young adult Sprague Dawley rats revealed a characteristic pattern of NGF protein expression similar to that described previously in the Fischer strain of rats (Bierl et al. 2005). For example, the mature NGF species was only weakly detected in the pineal gland, extracerebral bv, or the external carotid artery, though it was evident that the NGF antibody readily recognized the mature NGF peptide from Harlan as well as the mature NGF form that was abundant in the male mouse submandibular gland (smg; Fig. 1A). In the peripheral tissues examined, NGF species were present in varying abundance. The 22–24, 34, 55, and 75kDa NGF forms were observed in the pineal gland (Fig. 1A) while a 32kDa, rather than the 34kDa species, was predominant in the extracerebral blood vessels, and a 150kDa band also was present (Fig. 1B). In contrast, the 75 and 150kDa NGF species were prevalent in the external carotid artery (Fig. 1C). No specific staining was associated with the trigeminal, the smg, or NGF Harlan peptide following the preadsorption of the NGF antibody with excess NGF blocking peptide (Fig. 1D).
Figure 1.
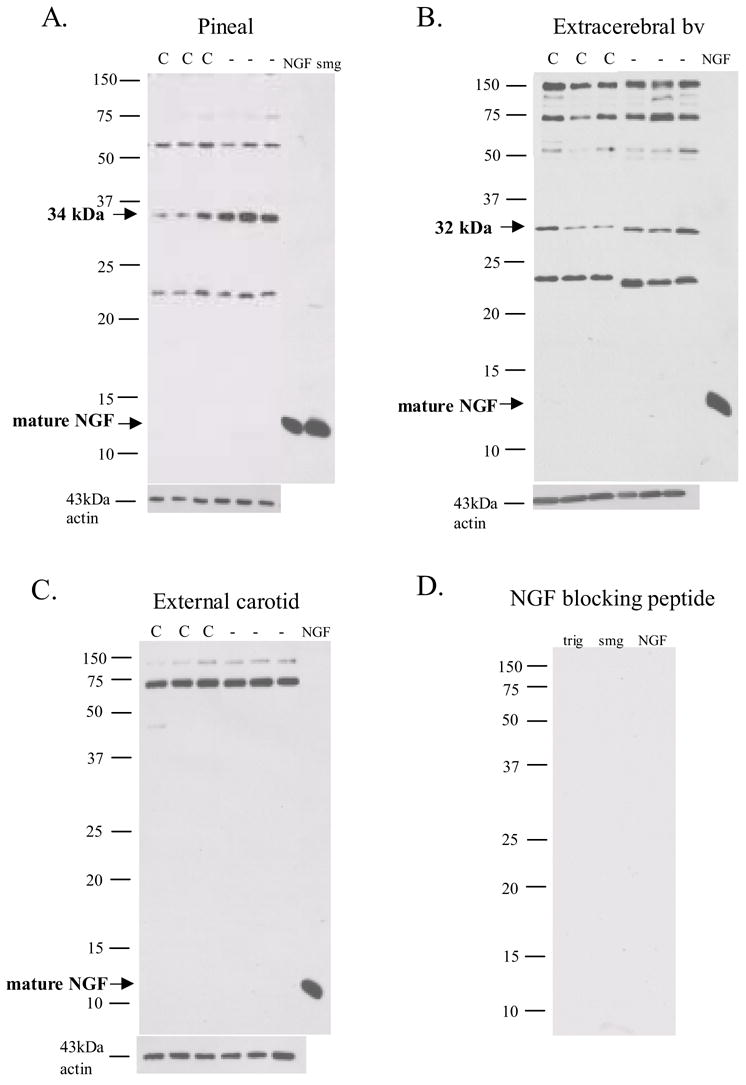
NGF western blot analysis of pineal gland (A.), extracerebral bv (B.), and external carotid artery (C.) reveal various molecular weight species but with no mature NGF species evident. Following sympathetic denervation (-), significant changes in NGF isoforms were observed when compared with control (C). 20 μg total protein loaded. NGF = 2.5S NGF (Harlan), smg = male mouse submandibular gland (5μg loaded). No staining was observed when antibody was incubated overnight with excess NGF peptide (D.).
The removal of sympathetic input (gcont) resulted in specific changes in NGF species associated with the pineal gland and extracerebral bv (Figs. 1, 2) that resulted in overall increases in NGF protein expression (Fig. 3). For example, the 34kDa species in the pineal gland and the 32kDa species in the extracerebral blood vessels, both proA forms, were significantly increased by 274% and 168% respectively following a 3 week sympathetic denervation (Figs. 1, 2). The 75kda NGF species also was significantly increased in these two tissues by 148% and 222% respectively (Figs. 1, 2). In the external carotid artery, there was little change in NGF following sympathetic denervation with a significant increase in the 150 kDa species (Figs. 1 and 2).
Figure 2.
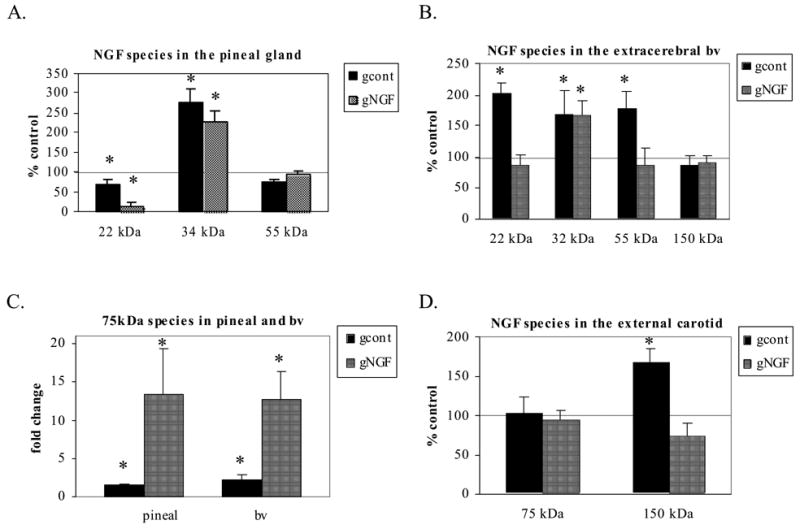
Semi-quantitative analysis showed specific changes in NGF species. The 34 kDa species in the pineal gland (A.) and the 32 kDa (B.) in the extracerebral bv, both proA forms, were significantly increased by 274% and 168% respectively. The 75 kDa NGF species (C.) also was significantly increased in these two tissues by 148% and 222% respectively. A 2 week administration of exogenous NGF following the denervation procedure (gNGF) revealed similar changes. One notable difference was the 75 kDa was dramatically increased both in the pineal gland and extracerebral bv. (*p<0.05 compared with control)
Figure 3.
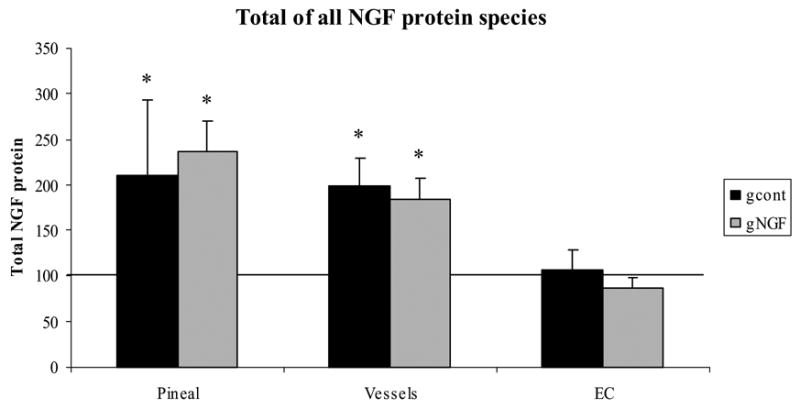
The overall NGF protein levels in each case were determined by calculating the net change in NGF protein, taking into account the changes in each species. The total amount of NGF protein for each tissue was calculated as described in the Experimental Procedures. A net increase in NGF protein was observed in both pineal gland and extracerebral bv, with no change in the external carotid artery.
A 2 week administration of NGF following the denervation procedure (gNGF; quantitative analysis shown in Fig. 2) generally did not affect the changes in NGF species observed following denervation only, although the 22–24 and 55kDa species in the extracerebral blood vessels returned to control values in this treatment group (Fig. 2). As observed in the denervation only cases, the 34kDa species in the pineal gland and the 32kDa species in the extracerebral blood vessels both were increased in the gNGF treatment. One notable change following the administration of NGF was a dramatic increase in the 75kDa NGF species in both the pineal gland and extracerebral blood vessels when compared to controls as well as when compared to the cases receiving denervation only (Fig. 2C). In contrast to the pineal gland and extracerebral bv, in the external carotid artery, the 75 kDa species was unaffected by exogenous NGF and the 150 kDa form was similar to controls, resulting in no significant changes (compared with controls) when NGF administration followed the denervation procedure (Fig. 2D).
NGF protein was examined in the trigeminal ganglion in order to determine whether increased NGF protein at the denervated targets might result in changes in the intact sensory neurons in the trigeminal ganglion. Following sympathetic denervation, levels of the 22–24 kDa species of NGF significantly increased and levels of this species were dramatically increased when NGF adminstration followed the denervation procedure (Figs. 6A and 6B).
Figure 6.
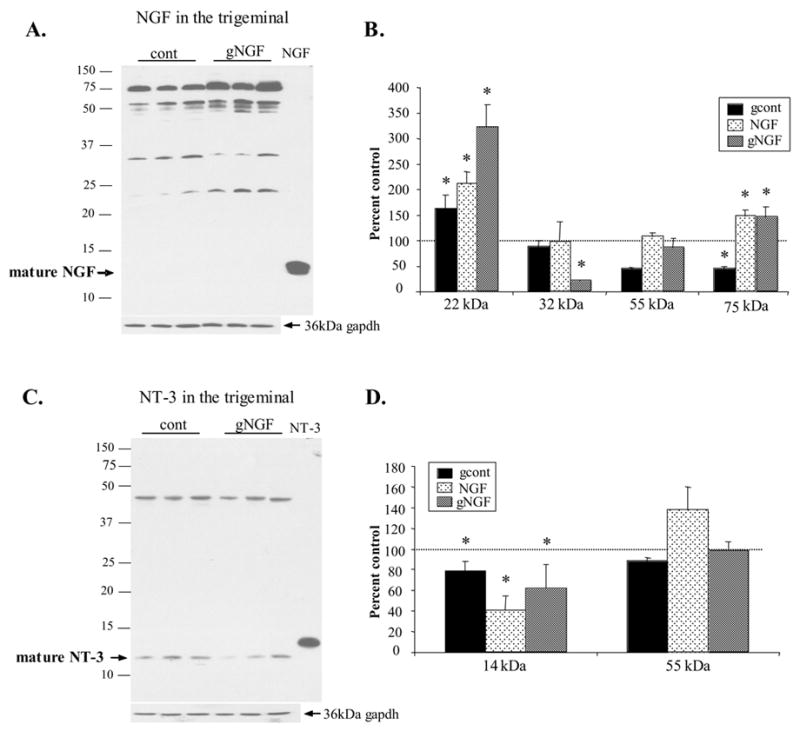
NGF and NT-3 species in the trigeminal ganglion following sympathetic denervation (gcont), 2 week NGF administration (NGF), and denervation followed by 2 week NGF administration (gNGF). A. Representative NGF western blot of trigeminal ganglion from gNGF animals (gNGF) and control animals (cont) revealed the presence of the 22, 32, 55, and 75kDa NGF proteins. B. Semi-quantitative analysis revealed a significant increase in the 22kDa NGF species in all three treatment groups. The 75kDa species was decreased following denervation but NGF administration resulted in an increase in this species. 20μg protein loaded per lane. Each lane represents a different animal. C. Representative blot reveals the presence of the mature NT-3 and a 50 kDa NT-3 species. The mature NT-3 form was decreased when sympathetic denervation was followed by NGF administration (gNGF) when compared with controls. B. Semi-quantitative analysis reveals a significant decrease in ‘mature’ NT-3 in all three treatment groups, suggesting that the reduction of NT-3 at the targets was not a result of increased transport to the trigeminal ganglion. The 50 kDa species was decreased in gcont treatment only. 20μg protein loaded per lane. Each lane represents a different animal. (*p<0.05 compared with control)
Calculation of overall changes in NGF protein in peripheral targets
Because there were increases and decreases in individual NGF species following denervation and also when exogenous NGF followed the denervation procedure, the overall NGF protein levels in each case were determined by calculating the net change in NGF protein, taking into account the changes in each species. As shown in Figure 3, a net increase in NGF protein was observed in both the pineal gland and extracerebral bv in both the gcont and gNGF treatments. No net change in total NGF protein was observed in the external carotid artery.
NT-3 protein expression in control animals and effects of denervation
NT-3 western analysis revealed an abundance of mature NT-3 at 14 kDa (Fig. 4). In addition, a 50kDa NT-3 species was consistently detected in the pineal gland and extracerebral bv. Because the detection of both NT-3 species was completely blocked following adsorption of the NT-3 antibody with excess NT-3 blocking peptide (Fig. 4D), we concluded that they were specific NT-3 protein forms. Others have described a 35kDa NT-3 species in neuronal tissues and peripheral targets (Huff et al. 1997; Loudes et al. 1999; Reinshagen et al. 2002), but this form was not detected in the current study using the NT-3 antibody from Santa Cruz.
Figure 4.

NT-3 western blot analysis of the pineal gland (A.), the extracerebral blood vessels (B.) and the external carotid artery (C.) reveals the presence of mature NT-3 and a 50 kDa precursor. No staining was observed when antibody was incubated overnight with excess NT-3 peptide (D.). NT-3 = human recombinant NT-3 (Santa Cruz Biotechnology). Blots are representative of 4 different animals.
Following a 3-week sympathetic denervation, the mature NT-3 species was significantly decreased both in the pineal gland and extracerebral blood vessels (Figs. 4, 5). This is in contrast to an increase in NGF protein in tissues from the same animals. In addition, though NGF was unchanged in the external carotid artery, a significant increase in mature NT-3 was observed following the removal of sympathetic input (Figs. 4 and 5). No significant changes following denervation were observed in the 50kDa species, when present, in any of the tissues examined.
Figure 5.
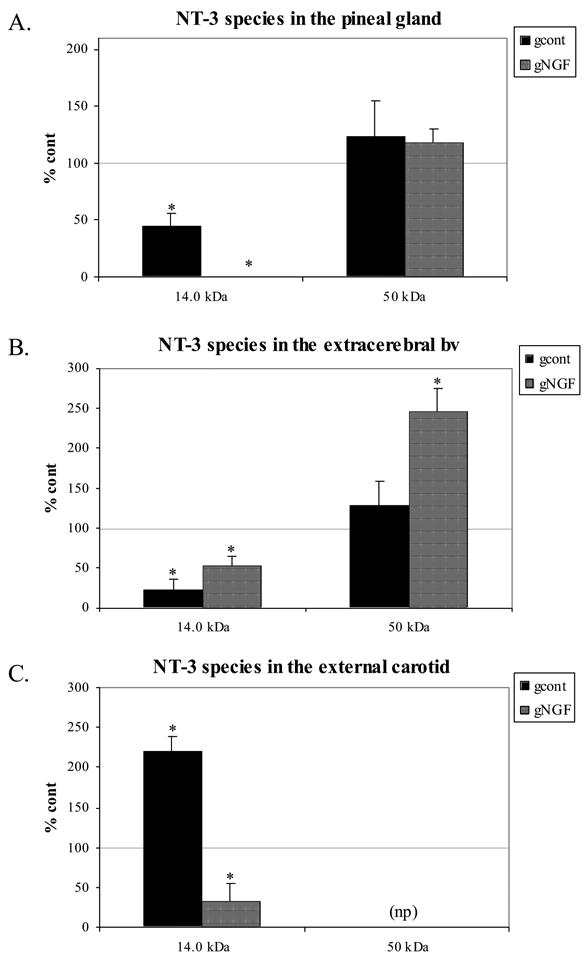
Semi-quantitative analysis of NT-3 protein shows a significant decrease in mature NT-3 in the pineal gland and extracerebral bv, and a significant increase in the external carotid. No significant change in the 50 kDa precursor was noted following denervation only. When NGF followed the sympathectomy, NT-3 levels remained depressed in the pineal gland and extracerebral bv, and also were significantly decreased in the external carotid artery. Note the lack of the 50 kDa precursor in the external carotid. 15 μg total protein loaded. NT-3 = human recombinant NT-3 (Santa Cruz Biotechnology). (*p<0.05 compared with control)
The decrease in mature NT-3 in the pineal gland following denervation only was enhanced by NGF administration (quantitative analysis shown in Fig. 5; p<0.05, gcont vs. gNGF). In the extracerebral blood vessels, NGF administration following denervation produced results similar to denervation alone (Fig. 5), with a significant decrease in mature NT-3 when compared to controls. In the external carotid artery, NGF administration following the removal of sympathetic input also resulted in a significant decrease in mature NT-3 (when compared both with control and gcont cases).
In order to test whether the decrease in NT-3 at the peripheral targets might be the result of enhanced uptake by intact sensory neurons following sympathetic removal, NT-3 protein in the trigeminal ganglion was examined following sympathetic denervation with and without NGF administration. Mature NT-3 was significantly decreased in the trigeminal ganglion following the removal of the SCG with and without subsequent NGF administration. In addition, NT-3 was reduced to only 40% of the control value following a 2 week NGF administration without prior removal of the SCG (Fig. 6).
3. Discussion
NGF and NT-3 protein in peripheral tissues following denervation
The present findings, in agreement with previous results from our laboratory using Fischer rats (Bierl et al. 2005), show that the mature form of NGF (13.5 kDa) is a minor species in the peripheral tissues of young adult Sprague Dawley rats and that higher molecular weight (MW) species are predominant. Furthermore, we provide documentation of changes in individual high MW NGF species, but not in the mature NGF form, in several peripheral tissues following sympathetic denervation. This is the first report of changes in specific NGF species in peripheral tissues following the loss of sympathetic input. These results extend previous studies that used NGF ELISA to show a significant increase in the submandibular gland following sympathetic denervation (Korsching and Thoenen 1985; Ekstrom and Reinhold 2004). Our current findings provide evidence that large MW NGF forms, particularly the proNGF-A (34kDa and 32kDa) as well as the 75 kDa species, are increased following the removal of sympathetic innervation. Furthermore, mature NGF, a minor species in the peripheral tissues examined, does not appear to change when sympathetic input is removed.
To our knowledge, there has been no western blot analysis of NT-3 in peripheral tissues following loss of sympathetic innervation. Our results show that, in contrast to NGF, where the mature NGF species is rare, the mature NT-3 form is abundant in control tissues and is dramatically affected by the loss of sympathetic input.
It is well established that target-derived neurotrophins such as NGF (Thoenen and Barde 1980; Schwab et al. 1982) and NT-3 (Zhou and Rush 1996; Zhou et al. 1997; Kuruvilla et al. 2004) are utilized by peripheral sympathetic neurons and that their ability to promote survival is dependent upon retrograde transport to the cell body from their site of synthesis in the target tissues. Therefore, it is possible that the increased NGF observed in the targets following denervation may result from a simple buildup of neurotrophin due to loss of sympathetic input (Korsching and Thoenen 1985). However, loss of any regulatory influences provided by norepinephrine (NE), the neurotransmitter released by sympathetic nerve terminals, also may contribute to the present findings. NE reduced NGF content in cardiac myocytes (Qin et al. 2002), suggesting that a loss of NE supplied to peripheral targets may result in increased NGF protein.
Because various NGF species reportedly are secreted by sympathetic neurons (Hasan et al. 2003), it is possible that a loss of these secretory products could impact NGF protein in target tissues. To address this possibility, we examined the effects of in vivo NGF administration on NGF and NT-3 protein following the loss of sympathetic input. Compared to denervation only, NGF administration following the denervation procedure (gNGF) did not alter the overall abundance of NGF species in the peripheral tissues examined, as the increase in total NGF was similar in the two treatment groups. However, NGF administration did alter some of the changes observed following denervation only. For example, in the pineal gland, the 22 and 55kDa species were decreased following denervation only when compared to controls. Yet, the 55kDa species returned to control levels in the gNGF treatment. In addition, the 22 and 55kDa NGF species in the extracerebral bv, which were increased following denervation, were similar to controls in the gNGF cases. Because exogenous NGF affected the distribution pattern of NGF species in the target, it may be that NGF species released by the sympathetic neuron, and which is lost following removal of sympathetic input, can influence NGF biosynthesis and/or expression in peripheral targets. The 75kDa species was dramatically increased and accounts for much of the net increase in NGF protein levels observed in the gNGF cases. Whether this increase results from the loss of secreted NGF by innervating neurons, is the result of NGF adminstration, and/or has biological significance remains to be determined.
Two different large MW NGF species (22 kDa and 75 kDa) were increased in the trigeminal ganglion, which contains cell bodies of neurons providing sensory input to the same targets, following sympathectomy and NGF administration as well as in the NGF only treatment. This result suggests that these species may be retrogradely transported from target sites, a possibility that is currently under investigation. For the purpose of this study, these increases indicate that increased NGF at the target level results in increases in the ganglion. The fact that NT-3 was not increased in the trigeminal in these treatments (see below) provides evidence that the decrease observed in the pineal gland and the extracerebral bv was not the result of enhanced NT-3 transport.
NT-3 protein expression was dramatically influenced by the loss of sympathetic input. Though there are no studies on the role of NE in the regulation of NT-3 by target tissues, it is possible that, in addition to regulating NGF production, NE influences the biosynthesis and/or release of NT-3 by target tissues. Alternatively, the decrease in mature NT-3 may be the direct result of increased NGF protein observed in the same tissues. This possibility is supported by decreased NT-3 observed following NGF administration. A two week administration of NGF following denervation resulted in decreased mature NT-3 levels in the pineal gland and extracerebral blood vessels. In the pineal gland, the decrease in mature NT-3 was more pronounced (compared to denervation only) when NGF administration followed the denervation procedure. The decrease in NT-3 in the extracerebral blood vessels following denervation was not enhanced by exogenous NGF, but NT-3 levels remained depressed, supporting the idea that increased NGF depresses NT-3 protein levels. Further, the external carotid showed increased NT-3 following denervation, which was reversed with subsequent NGF administration and a decrease was observed.
There is previous evidence that NGF influences NT-3 expression. NGF levels were increased (Heumann et al. 1987) and NT-3 levels were decreased (Cai et al. 1998) following sciatic crush injury. In addition, total neurotrophic support appears to be more important in the survival and maintenance of peripheral neurons than the influence of any one neurotrophin (Zhou et al. 1999). Thus, if loss of sympathetic innervation results in increased NGF protein, then decreased NT-3 may indirectly result from denervation.
As stated above, it appears that the decrease in NT-3 at the targets following denervation was not the result of enhanced NT-3 transport from the target sites. Although NGF protein was significantly increased in the trigeminal, there was no increase in NT-3. In fact, mature NT-3 was significantly decreased following sympathectomy, with and without subsequent NGF administration. Interestingly, NT-3 levels were dramatically reduced in ‘NGF only’ cases, providing evidence that increased NGF depressed NT-3 levels.
Variability in neurotrophin expression and responses across targets
The external carotid artery showed a very different pattern of NGF protein expression when compared with the pineal gland and extracerebral blood vessels. In this tissue, 75 kDa and 150 kDa NGF species were predominant and there was little protein present in the 32 kDa or 22–24 kDa range. The 75 kDa NGF form also was the predominant NGF form in the hair follicle (Yardley et al. 2000), and there is evidence that this NGF species may influence hair development (Peters et al. 2006). Interestingly, the 150 kDa species also was present in the extracerebral blood vessels, but not the pineal gland, and it may be that this NGF species is typically associated with the adult vasculature. The 150 kDa species also is abundant in the SCG, where the sympathetic cell bodies are housed, and this form is significantly decreased when the cell bodies are disconnected from their targets. Thus, the 150 kDa species (along with the 22 kDa form that also is decreased following axotomy) could represent a target-derived NGF species that is produced by vascular targets and then retrogradely transported to cell bodies in the SCG. Though the 150 kDa NGF form was unchanged in the extracerebral blood vessels following sympathetic denervation, this species was significantly increased in the external carotid. This increase in the 150 kDa species, however, was not sufficient to produce a net increase in total NGF protein in the following denervation.
The NT-3 response to denervation also was very different in the external carotid compared with the pineal gland and extracerebral blood vessels. Following the loss of sympathetic innervation, mature NT-3 was significantly increased in the external carotid artery in contrast to significant decreases observed in other target tissues.
Our findings suggest that each peripheral target has a characteristic neurotrophin expression pattern. It has been suggested recently that peripheral targets have variable NGF requirements for sympathetic innervation (Glebova and Ginty 2004) and our findings support this idea as the external carotid showed a very different NGF expression pattern and response to sympathetic denervation compared with the pineal gland and extracerebral blood vessels. In addition, it appears that proximal and distal targets serve different roles in their support of axonal development, where NT-3 in the proximal targets serves to mediate axon extension only and NGF in more distal targets stimulates branching as well as extension (Glebova and Ginty 2005). The results of the present study suggest that proximal and distal vascular targets may serve different functions in supporting the maintenance of adult sympathetic neurons.
Function of NGF and NT-3 protein species
While the male mouse submandibular gland is a rich source for mature 2.5S NGF (Mobley et al. 1976; Lakshmanan et al. 1989), most central and peripheral tissues appear to contain relatively small amounts of mature (13.5 or 16kDa) NGF protein (Fahnestock et al. 2001; Bierl and Isaacson 2005) and higher MW NGF precursor species predominate. The high MW species have been shown to be predominant in non-neuronal tissues such as rat round spermatids (Chen et al. 1997), human calf skin (Yiangou et al. 2002), and neuronal tissues such as the dorsal root ganglion and spinal cord (Reinshagen et al. 2000), the cortex and hippocampus (Fahnestock et al. 2001), the SCG (Hasan et al. 2003; Bierl et al. 2005) and the trigeminal ganglion (Bierl and Isaacson 2005). Taken together, these findings suggest that high MW NGF species are abundant in the nervous system and may have their own biological activity. Some have suggested an apoptotic role for proNGF (Lee et al. 2001; Beattie et al. 2002; Harrington et al. 2004; Pedraza et al. 2005), though proNGF also has been shown to have neurotrophic activities (Dicou et al. 1997; Reinshagen et al. 2000; Fahnestock et al. 2004).
Understanding the biological activities of the specific neurotrophin protein species has clinical importance since there appear to be alterations in NGF species in disease states as well as in aging. Indeed, subjects with Mild Cognitive Impairment (Peng et al., 2004) and Alzheimer’s disease (Fahnestock et al. 2001) have shown an increase in the 32kDa NGF precursor species in brain. Specific NGF species are altered in the development of early human diabetic neuropathy (Yiangou et al. 2002) and tissues that show an age-related loss of sympathetic innervation also show a dramatic increase in the 25kDa proNGF-B precursor (Bierl and Isaacson 2005).
4. Experimental procedure
Animals and tissue processing
Young adult (3 months of age) female Sprague Dawley (Harlan Labs, Indianapolis, IN) rats were housed in the Miami University Animal Facilities in a 12:12 light:dark environment at regulated temperature. Three treatment groups were used: 1) cont: animals received no treatment; 2) gcont: animals received a three-week bilateral ganglionectomy (removal of superior cervical ganglion); 3) gNGF: animals received a one-week bilateral ganglionectomy followed by a two-week infusion of NGF. Eight to ten animals per treatment were utilized. For direct comparison, NGF and NT-3 western analysis were conducted using tissues from the same animals. Three peripheral targets were examined: pineal gland, extracerebral blood vessels from the Circle of Willis at the base of the brain inside the cranial vault, and the external carotid artery just distal to the bifurcation of the carotid artery. All methods were approved by the Miami University Institutional Animal Care and Use Committee.
Bilateral ganglionectomy
Animals were anesthetized using an intramuscular injection of a Ketamine (80 mg/kg): Rompun (14 mg/kg) cocktail. A ventral midline incision, approximately 3.5 cm in length, was made in the neck region and blunt dissection of the musculature exposed the SCG at the bifurcation of the common carotid arteries. The post-ganglionic trunk of the SCG was gently separated from the carotid artery, the pre- and post-ganglionic nerves were severed with microdissecting scissors, and the SCG was removed. The remaining distal trunk was folded under to prevent any reinnervation by the severed axons. The incision was closed with tissue glue (Nexaband, Phoenix, AZ) and the animal was allowed to recover for three weeks. Following the 3 week survival period, sympathectomized animals were sacrificed via decapitation and tissue was collected. Some animals received a two week administration of NGF into the lateral ventricle following the one week bilateral ganglionectomy.
Intracerebroventricular infusion of NGF
The protocol for NGF infusion was based on methods first described by Williams et al. (1987), and modified for use in our laboratory (Isaacson et al. 1995; Isaacson and Billieu 1996; Isaacson and Crutcher 1998; Shoemaker and Isaacson 2002). Animals were anesthetized and a small hole was drilled at a location 1 mm lateral to Bregma (Paxinos and Watson 1986). The dura mater was removed and a 27 gauge cannula attached to an Alzet #2002 osmotic minipump reservoir (Durect) was inserted 4.4mm ventral from the brain surface (Paxinos and Watson 1986) and secured using dental acrylic. The pump reservoir, which contained approximately 220 μl of mouse NGF (100 μg/ml; Harlan Labs), was placed under the skin of the back. The fluorescent marker bisbenzimide (Sigma) was added to the infusate to monitor cannula placement (Isaacson et al. 1995; Isaacson and Billieu 1996).
Western analysis for NGF and NT-3 protein
Animals were sacrificed using a Harvard guillotine apparatus, and tissue was removed, snap-frozen in liquid nitrogen and stored at −80°C until further processing. The extracerebral bv were pooled to include the anterior cerebral, internal carotid, posterior cerebral and middle cerebral arteries. All other tissues were processed as individual samples. Total protein from each sample was collected by sonication in 0.01 M Tris-HCl buffer (pH 7.4) containing 1% SDS and 1% protease inhibitor cocktail (Sigma). Following centrifugation, protein concentrations in the supernatant were determined using a BCA protein assay (Pierce). Samples were prepared as described by Laemmli (1970).
Tissues (20 μg), 2.5S NGF peptide (10ng; Harlan; positive control for NGF blots) or human recombinant NT-3 (10ng; Chemicon; positive control for NT-3 blots) were run on a 5% SDS-polyacrylamide (PAGE) stacking gel and a 12% SDS-PAGE resolving gel. Protein isolated from the male mouse submandibular gland (smg) frequently was loaded as a positive control for mature NGF protein (See Figure 1). For determination of MW, Precision Plus protein unstained standard (Bio-Rad Labs) was loaded on the outside lane of the gel. Protein was transferred overnight at a total of 2,300–2,500mAmps to PVDF membrane in transfer buffer (25 mM Tris, 192 mM glycine, 10% (v/v) methanol) at 4°C. Following transfer, the membrane was placed in 100% methanol, allowed to dry, and the lanes containing standard were trimmed and processed separately. All membranes were rehydrated in 100% methanol. Membranes containing tissue protein were incubated in 8% non-fat dry milk diluted in Tris buffered saline containing Tween-20 (TBST) for 4h at room temperature and incubated overnight at 4°C in rabbit anti-NGF (H-20, 1:1,000; Santa Cruz Biotechnology) or rabbit anti-NT-3 (N-20, 1:1000; Santa Cruz Biotechnology). Membranes then were rinsed in TBST, and incubated in goat anti-rabbit HRP IgG (1:10,000 for NGF; 1:100,000 for NT-3; Chemicon) for 2 hours. The membrane containing the standard was incubated separately in 8% non-fat dry milk diluted in TBST for 4h at room temperature and overnight at 4°C in TBST, then rinsed in TBST, and incubated in Precision Protein StrepTactin HRP (1:500,000; Bio-Rad Labs) for 2 hours. All membranes were rinsed and covered with SuperSignal West Pico Chemiluminescent Substrate (Pierce) for 5 min, and placed in an autoradiography cassette for development. Membranes were then stripped and reprobed for either mouse anti-actin (1:150,000; Chemicon) or mouse anti-gapdh (1:400,000; RDI Inc.) followed by a two hour incubation in goat anti-mouse HRP IgG (1:80,000; Chemicon). Antibody specificity was determined by omission of primary antibody from Western blot protocol. As an additional control for specificity, membranes were incubated overnight at 4°C in a solution containing either NGF or NT-3 antibody with 10-fold excess of appropriate NGF or NT-3 blocking peptide (Santa Cruz Biotechnology).
Films were scanned into ImageQuant 5.2 for analysis. For determination of individual band differences, the intensity of each band was calculated from ImageQuant and a ratio of NGF or NT-3 to either actin or gapdh was generated. The mean ratio from the controls was set at 100% and treatments were expressed as percent of control. A Mann-Whitney test was performed to determine any significant differences. Significance was reported at p<0.05. Figures presented here are representative of 3–5 different animals for each tissue. To determine net change in NGF protein, the sum of the densitometry readings for all NGF species present in individual samples was calculated. For each sample, the total NGF was expressed as a ratio to the internal loading control (actin or gapdh). The mean ratio for the gcont or gNGF treatments was compared to the mean ratio generated from the control group.
Acknowledgments
Thanks for Ryan Walker and Alec Lawrence for their assistance with this project. This work was supported by NIH NS-051206 awarded to LGI.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Beattie MS, Harrington AW, Lee R, Kim JY, Boyce SL, Longo FM, Bresnahan JC, Hempstead BL, Yoon SO. ProNGF induces p75-mediated death of oligodendrocytes following spinal cord injury. Neuron. 2002;36:375–386. doi: 10.1016/s0896-6273(02)01005-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierl MA, Jones EE, Crutcher KA, Isaacson LG. ‘Mature’ nerve growth factor is a minor species in most peripheral tissues. Neurosci Lett. 2005;380:133–137. doi: 10.1016/j.neulet.2005.01.029. [DOI] [PubMed] [Google Scholar]
- Bierl MA, Isaacson LG. Increased NGF proforms in aged sympathetic neurons and their targets. Neurobiol Aging. 2006 Dec 22; doi: 10.1016/j.neurobiolaging.2005.11.008. In press. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Cai F, Tomlinson D, Fernyhough P. Effect of sciatic nerve crush on local and target tissue production of neurotrophin-3 transcripts in rats. Neuroscience Lett. 1998;252(1):45–48. doi: 10.1016/s0304-3940(98)00543-6. [DOI] [PubMed] [Google Scholar]
- Chen Y, Dicou E, Djakiew D. Characterization of nerve growth factor precursor protein expression in rat round spermatids and the trophic effects of nerve growth factor in the maintenance of sertoli cell viability. Mol Cell Endo. 1997;127:129–136. doi: 10.1016/s0303-7207(96)04001-4. [DOI] [PubMed] [Google Scholar]
- Crowley C, Spencer SD, Nishimura MC, Chen KS, Pitts-Meek S, Armanini MP, Ling LH, MacMahon SB, Shelton DL, Levinson AD. Mice lacking nerve growth factor display perinatal loss of sensory and sympathetic neurons yet develop basal forebrain cholinergic neurons. Cell. 1994;76(6):101–111. doi: 10.1016/0092-8674(94)90378-6. [DOI] [PubMed] [Google Scholar]
- Dicou E, Pflug B, Magazin M, Lehy T, Djakiew D, Ferrara P. Two peptides derived from the nerve growth factor precursor are biologically active. J Cell Bio. 1997;136(2):389–398. doi: 10.1083/jcb.136.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrom J, Reinhold AC. Increases in nerve growth factor immunoreactivity in the submandibular gland, but not the parotid gland, of the rat following sympathetic denervation. Arch Oral Biol. 2004;49:3–9. doi: 10.1016/s0003-9969(03)00181-x. [DOI] [PubMed] [Google Scholar]
- Fahnestock M, Michalski B, Xu B, Coughlin MD. The precursor pro-nerve growth factor is the predominant form of nerve growth factor in brain and is increased in Alzheimer’s disease. Mol Cell Neurosci. 2001;18:210–220. doi: 10.1006/mcne.2001.1016. [DOI] [PubMed] [Google Scholar]
- Fahnestock M, Yu G, Michalski B, Mathew S, Colquhoun A, Ross GM, Coughlin MD. The nerve growth factor precursor proNGF exhibits neurotrophic activity but is less active than mature nerve growth factor. J Neurochem. 2004;89:581–592. doi: 10.1111/j.1471-4159.2004.02360.x. [DOI] [PubMed] [Google Scholar]
- Farinas I, Jones KR, Backus C, Wang XY, Reichardt LF. Severe sensory and sympathetic deficits in mice lacking neurotrophin-3. Nature. 1994;369(6482):658–661. doi: 10.1038/369658a0. [DOI] [PubMed] [Google Scholar]
- Francis N, Farinas I, Brennan C, Rivas-Plata K, Backus C, Reichardt L, Landis S. NT-3, like NGF, is required for survival of sympathetic neurons, but not their precursors. Dev Biol. 1999;210:411–427. doi: 10.1006/dbio.1999.9269. [DOI] [PubMed] [Google Scholar]
- Ghasemlou N, Krol KM, MacDonald DR, Kawaja MD. Comparison of target innervation by sympathetic axons in adult wild type and heterozygous mice for nerve growth factor or its receptor trkA. J Pineal Res. 2004;37:230–240. doi: 10.1111/j.1600-079X.2004.00160.x. [DOI] [PubMed] [Google Scholar]
- Glebova NO, Ginty DD. Heterogeneous requirement of NGF for sympathetic target innervation in vivo. J Neurosci. 2004;24(2):743–751. doi: 10.1523/JNEUROSCI.4523-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glebova NO, Ginty DD. Growth and survival signals controlling sympathetic nervous system development. Ann Rev Neurosci. 2005;28:191–222. doi: 10.1146/annurev.neuro.28.061604.135659. [DOI] [PubMed] [Google Scholar]
- Gorin PD, Johnson EM., Jr Effects of exposure to nerve growth factor antibodies on the developing nervous system of the rat, an experimental autoimmune approach. Dev Bio. 1980;80(2):313–323. doi: 10.1016/0012-1606(80)90407-8. [DOI] [PubMed] [Google Scholar]
- Harrington AW, Leiner B, Blechschmitt C, Arevalo JC, Lee R, Mörl K, Meyer M, Hempstead BL, Yoon SO, Giehl KM. Secreted proNGF is a pathophysiological death-inducing ligand after adult CNS injury. PNAS. 2004;101(16):6226–6230. doi: 10.1073/pnas.0305755101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan W, Pedchenko T, Krizsan-Agbas D, Baum L, Smith PG. Sympathetic neurons synthesize and secrete pro-nerve growth factor protein. J Neurobiol. 2003;57:38–53. doi: 10.1002/neu.10250. [DOI] [PubMed] [Google Scholar]
- Heumann R, Korsching S, Bandtlow CE, Thoenen H. Changes of nerve growth factor synthesis in non-neuronal cells in response to sciatic nerve transection. J Cell Biol. 1987;104:1623–1631. doi: 10.1083/jcb.104.6.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huff K, Reinshagen M, Geerling I, Soinila S. Localization of neurotrophin-3 prohormone isoforms in developing and adult rat hippocampus and cerebellum. Ann Neurol. 1997;42(3 Pt 1) [Google Scholar]
- Humpel C, Lindqvist E, Olson L. Detection of nerve growth factor mRNA in rodent salivary glands with digoxigenin- and 33P-labeled oligonucleotides, effects of castration and sympathectomy. J Histochem Cytochem. 1993;41(5):703–708. doi: 10.1177/41.5.8468451. [DOI] [PubMed] [Google Scholar]
- Isaacson LG, Ondris D, Crutcher KA. Plasticity of mature sensory cerebrovascular axons following intracranial infusion of nerve growth factor. J Comp Neurol. 1995;361(3):451–460. doi: 10.1002/cne.903610309. [DOI] [PubMed] [Google Scholar]
- Isaacson LG, Billieu SC. Increased perivascular norepinephrine following intracerebroventricular infusion of NGF into adult rats. Exp Neurol. 1996;139(1):54–60. doi: 10.1006/exnr.1996.0080. [DOI] [PubMed] [Google Scholar]
- Isaacson LG, Crutcher KA. Uninjured aged sympathetic neurons sprout in response to exogenous NGF in vivo. Neurobiol Aging. 1998;19(4):333–339. doi: 10.1016/s0197-4580(98)00073-6. [DOI] [PubMed] [Google Scholar]
- Korsching S, Thoenen H. Treatment with 6-hydroxydopamine and colchicine decreases nerve growth factor levels in sympathetic ganglia and increases them in the corresponding target tissues. J Neurosci. 1985;5(4):1058–1061. doi: 10.1523/JNEUROSCI.05-04-01058.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuruvilla R, Zweifel L, Glebova N, Lonze B, Valdez G, Ye H. A neurotrophin signaling cascade coordinates sympathetic neuron development through differential control of TrkA trafficking and retrograde signaling. Cell. 2004;118(2):243–255. doi: 10.1016/j.cell.2004.06.021. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lakshmanan J, Beattie GM, Hayek A, Burns C, Fisher DA. Biological actions of 53 kDa nerve growth factor as studied by a blot and culture technique. Neurosci Lett. 1989;99:263–267. doi: 10.1016/0304-3940(89)90457-6. [DOI] [PubMed] [Google Scholar]
- Lee R, Kermani P, Teng KK, Hempstead BL. Regulation of cell survival by secreted proneurotrophins. Science. 2001;294:1945–1948. doi: 10.1126/science.1065057. [DOI] [PubMed] [Google Scholar]
- Loudes C, Petit F, Kordon C, Faivre-Bauman A. Distinct populations of hypothalamic dopaminergic neurons exhibit differential responses to brain-derived neurotrophic factor (BDNF) and neurotrophin-3 (NT-3) Eur J Neurosci. 1999;11:617–624. doi: 10.1046/j.1460-9568.1999.00463.x. [DOI] [PubMed] [Google Scholar]
- Mobley WC, Schenker A, Shooter EM. Characterization and isolation of proteolytically modified nerve growth factor. Biochem. 1976;25(25):5543–5552. doi: 10.1021/bi00670a019. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. San Diego: Academic; 1986. [DOI] [PubMed] [Google Scholar]
- Pedraza CE, Podlesniy P, Vidal N, Arévalo JC, Lee R, Hempstead BL, Ferrer I, Iglesias M, Espinet C. ProNGF isolated from the human brain affected by Alzheimer’s disease induces neuronal apoptosis mediated by p75NTR. Amer J Path. 2005;166(2):533–543. doi: 10.1016/S0002-9440(10)62275-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng S, Wuu J, Mufson EJ, Fahnestock M. Increased proNGF levels in subjects with mild cognitive impariment and mild Alzheimer Disease. J Neuropath Exp Neurol. 2004;63(6):641–649. doi: 10.1093/jnen/63.6.641. [DOI] [PubMed] [Google Scholar]
- Peters EMJ, Hendrix S, Golz G, Klapp BF, Arck PC, Paus R. Nerve growth factor and its precursor differentially regulate hair cycle progression in mice. J HistochemCytochem. 2006;54:275–288. doi: 10.1369/jhc.4A6585.2005. [DOI] [PubMed] [Google Scholar]
- Qin F, Vulapalli R, Stevens S, Liang C. Loss of cardiac sympathetic neurotransmitters in heart failure and NE infusion is associated with reduced NGF. Amer J Phys. 2002;282(1):H363–71. doi: 10.1152/ajpheart.00319.2001. [DOI] [PubMed] [Google Scholar]
- Reinshagen M, Geerling I, Huff KR. Localization of NT-3 prohormone in adult rat dorsal root ganglion (DRG), superior cervical ganglion (SCG), and hippocampus. Soc Neurosci. 2002 Abs Viewer. No. 732.3. [Google Scholar]
- Ruit K, Osborne P, Schmidt R, Johnson EMJ, Snider WD. Nerve growth factor regulates sympathetic ganglion cell morphology and survival in the adult mouse. J Neurosci. 1990;10(7):2412–2419. doi: 10.1523/JNEUROSCI.10-07-02412.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush RA, Chie E, Liu D, Tafreshi A, Zettler C, Zhou XF. Neurotrophic factors are required by mature sympathetic neurons for survival, transmission and connectivity. Clin Exp Pharmacol. 1997;24(8):549–555. doi: 10.1111/j.1440-1681.1997.tb02089.x. [DOI] [PubMed] [Google Scholar]
- Schwab M, Heumann R, Thoenen H. Communication between target organs and nerve cells, retrograde axonal transport and site of action of nerve growth factors. Cold Spring H Symp. 1982;46( Pt 1):125–134. doi: 10.1101/sqb.1982.046.01.016. [DOI] [PubMed] [Google Scholar]
- Shoemaker S, Kudwa A, Isaacson LG. Sympathetic ingrowth to the trigeminal ganglion following intracerebroventricular infusion of nerve growth factor. Brain Res. 2002;956(1):136–148. doi: 10.1016/s0006-8993(02)03490-x. [DOI] [PubMed] [Google Scholar]
- Tafreshi AP, Zhou XF, Rush RA. Endogenous nerve growth factor and neurotrophin-3 act simultaneously to ensure the survival of postnatal sympathetic neurons in vivo. Neurosci. 1998;83(2):373–380. doi: 10.1016/s0306-4522(97)00385-0. [DOI] [PubMed] [Google Scholar]
- Thoenen H, Barde A. Physiology of nerve growth factor. Phys Rev. 1980;60(4):1284–1335. doi: 10.1152/physrev.1980.60.4.1284. [DOI] [PubMed] [Google Scholar]
- Williams LR, Vahlsing HL, Linwood T, Varon S, Gage FH, Manthorpe M. A small-gauge cannula device for continuous infusion of exogenous agents into the brain. Exp Neurol. 1987;95:743–754. doi: 10.1016/0014-4886(87)90313-x. [DOI] [PubMed] [Google Scholar]
- Yardley G, Relf B, Lakshmanan J, Reinshagen M, Moore GPM. Expression of nerve growth factor mRNA and its translation products in the anagen hair follicle. Exp Dermatol. 2000;9:283–289. doi: 10.1034/j.1600-0625.2000.009004283.x. [DOI] [PubMed] [Google Scholar]
- Yiangou Y, Facer P, Sinicropi DV, Boucher TJ, Bennett DL, McMahon SB, Anand P. Molecular forms of NGF in human and rat neuropathic tissues, decreased NGF precursor-like immunoreactivity in human diabetic skin. J Periph Nerv Syst. 2002;7:190–197. doi: 10.1046/j.1529-8027.2002.02024.x. [DOI] [PubMed] [Google Scholar]
- Zhou XF, Rush RA. Sympathetic neurons in neonatal rats require endogenous neurotrophin-3 for survival. J Neurosci. 1995;15(10):6521–6530. doi: 10.1523/JNEUROSCI.15-10-06521.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou XF, Rush RA. Functional roles of neurotrophin 3 in the developing and mature sympathetic nervous system. Mol Neurobiol. 1996;13(3):185–197. doi: 10.1007/BF02740622. [DOI] [PubMed] [Google Scholar]
- Zhou X, Chie E, Deng Y, Rush RA. Rat mature sympathetic neurones derive neurotrophin 3 from peripheral effector tissues. Eur J Neurosci. 1997;9(12):2753–2764. doi: 10.1111/j.1460-9568.1997.tb01704.x. [DOI] [PubMed] [Google Scholar]
- Zhou X, Deng Y, Chie E, Xue Q, Zhong J, McLachlan EM. Satellite-cell-derived nerve growth factor and neurotrophin-3 are involved in noradrenergic sprouting in the dorsal root ganglia following peripheral nerve injury in the rat. Eur J Neurosci. 1999;11(5):1711–1722. doi: 10.1046/j.1460-9568.1999.00589.x. [DOI] [PubMed] [Google Scholar]


