Summary
Objective
Growth factor therapy may be useful for stimulation of cartilage matrix synthesis and repair. Thus, the purpose of our study was to further understand the effect of combined insulin-like growth factor 1 (IGF-1) and osteogenic protein 1 (OP-1) treatment on the matrix synthesized by human adult normal and osteoarthritic (OA) chondrocytes.
Design
Chondrocytes were isolated post-mortem from articular cartilage from tali of normal human donors and femoral condyles of OA patients undergoing knee replacement surgery. Cells were cultured in alginate beads for 21 days in four experimental groups: 1) “mini-ITS”-control; 2) 100ng/ml IGF-1; 3) 100ng/ml OP-1; 4) IGF-1 + OP-1, each at 100 ng/ml. Beads were processed for histological (Safranin O and fast green), morphometrical and immunohistochemical (aggrecan, decorin, type I, II, VI, and X collagens, and fibronectin accumulation) analyses.
Results
Histology showed that IGF-1 alone did not induce substantial matrix production. OP-1 alone caused a considerable matrix formation, but the highest matrix accumulation by normal and OA chondrocytes was found when OP-1 and IGF-1 were added together. Morphometrical analysis indicated larger matrices produced by OA chondrocytes than by normal cells under the combined treatment. All tested matrix proteins were more abundant in the combination group. Type X collagen was detected only under the combined OP-1 and IGF-1 treatment and was present at very low levels. Type I collagen was found only in OA chondrocytes.
Conclusions
The results obtained in the current study suggest that combined therapy with IGF-1 and OP-1 may have a greater potential in treating cartilage defects seen in OA than use of either growth factor alone.
Keywords: human normal chondrocytes, human OA chondrocytes, growth factors, extracellular matrix
Introduction
Cartilage repair is a major challenge faced by clinicians and scientists in the field of orthopedics, where numerous efforts are being made to “re-establish a structurally and functionally competent repair tissue” 1. In this light, growth factor therapy has received considerable attention due to the ability of different anabolic mediators to induce and facilitate cartilage matrix synthesis and repair.
In adult articular cartilage, chondrocytes occupy less than 5% of the total tissue volume, yet they are responsible for the extracellular matrix (ECM) synthesis, assembly, regulation, and maintenance. Chondrocytes maintain normal cartilage homeostasis through the balance between anabolic and catabolic activities. The growth factors that are the focus for the current study, insulin-like growth factor-1 (IGF-1) and osteogenic protein-1 (OP-1) (also known as bone morphogenetic protein-7 [BMP-7]), have been identified endogenously in adult articular cartilage 2-3 and shown to stimulate significant anabolic activities of human, bovine, monkey, goat, and sheep chondrocytes 4-11. OP-1 induced the synthesis of major ECM components, aggrecan, collagen type II, and hyaluronan (HA) 7-8, 12 with continued expression of the differentiated chondrocyte phenotype (no type I or type X collagen synthesis) 7,13 and no induction of chondrocyte proliferation 14. Furthermore, OP-1 stimulated the synthesis of the HA receptor, CD44, and HA synthase-2 thus promoting the formation and retention of the ECM 8,15. Importantly, it counteracted catabolic events, such as interleukin-1β, fibronectin fragment and HA hexasaccharide-induced cartilage degeneration 12,16,17.
In cartilage, IGF-1 could function in a paracrine and autocrine manner to stimulate matrix synthesis 18-21 and inhibit matrix degradation by down-regulating matrix metalloproteinases and inflammatory cytokines 22-24. IGF-1 also promotes chondrocyte survival 25. The overexpression of human IGF-1 induced new tissue formation in an ex vivo model of articular cartilage transplantation 20. However, there is a limited amount of data pertaining to human cartilage showing changes in response to IGF-1 especially in early osteoarthritic (OA) cartilage. Doré et al 26 demonstrated a lack of response to IGF-1 by OA chondrocytes in comparison to normal ones, where the cells studied were from cartilage obtained at the time of joint replacement surgery, which likely represents end-stage OA. It is not known if a reduced response to IGF-1 contributes to earlier phases of the disease. Studies by Loeser et al 11,25 support the notion of a reduced IGF-1 response in chondrocytes from cartilage with OA changes. However, the effect of OA score in those studies was more variable than the effect of age and after adjustment for age, only proline incorporation and not sulfate incorporation was significantly reduced in OA cells 11. It appears that the response to IGF-1 may be influenced by age and by disease severity and that the mechanisms responsible for the reduced responsiveness may differ between early and late-stage OA chondrocytes.
Although IGF-1 has been shown to be the major stimulator of proteoglycan (PG) synthesis present in synovial fluid and fetal calf serum, Schneiderman et al 2 have suggested that the amount of IGF-1 present in normal adult articular cartilage (about 10 ng/g tissue) is not sufficient to make it a major stimulator of PG synthesis in situ, which implies a possible role of other anabolic factors as was hypothesized by Trippel 27. This is supported by study of Fan et al 28, which showed that OA chondrocytes express statistically higher levels of anabolic genes, aggrecan and collagen type II, than normal chondrocytes in response to stimulation by OP-1.
Only limited studies have addressed questions of cartilage metabolism and combined growth factor therapy. It has been shown that either epidermal growth factor (EGF) 29, fibroblast growth factor (FGF) 6, 29-30, or transforming growth factor-β (TGF-β) 6,31-33 can modulate the response of chondrocytes to IGF-1. Previously, we showed that addition of OP-1 to IGF-1 induces an additive effect of both factors on PG synthesis and a synergistic effect on cell proliferation 25. Similar effects on cell differentiation and proliferation by these two growth factors have been also demonstrated in rat osteoblastic cells 34. Furthermore, autocrine IGF-1 production could be up-regulated by OP-1 at both transcriptional and posttranscriptional level in osteoblastic cells 35-36 and in adult articular chondrocytes 24. In bone cells, OP-1 modulated expression of the components of the IGF-1 regulatory system 37.
The objective of this continuing study was to further understand the effect of combined treatment and to identify and visualize which matrix constituents are synthesized in response to the combination of IGF-1 and OP-1 by human adult normal and OA chondrocytes cultured for long-term in alginate beads.
Materials and Methods
Materials
Dulbecco’s Modified Eagle’s Medium (DMEM), Ham’s F12, fetal bovine serum (FBS), and Gentamycin were purchased from Invitrogen (Carlsbad, CA). Mini-insulin, transferrin, and selenous acid medium (mini-ITS) was produced as previously described 25. Low-viscosity alginate (Keltone, LV) was obtained from Kelco (Chicago, IL). “Live/Dead Viability/Cytotoxicity Kit” was obtained from Molecular Probes (Eugene, OR). Safranin O and fast green dyes were purchased from Fisher Scientific (Pittsburgh, PA). Anti-fibronectin monoclonal antibody was purchased from Chemicon International Inc (Temecula, CA); anti-aggrecan, anti-decorin, anti-type II collagen and anti-type VI collagen monoclonal antibodies were purchased from the Developmental Studies Hybridoma Bank, University of Iowa (Iowa City, IA). Anti-type I collagen polyclonal antibody 38 and monoclonal antibody to type X collagen 39 were provided by Dr. Aigner. Recombinant human OP-1 (rhOP-1) was kindly provided by Stryker Biotech (Hopkinton, MA). Recombinant human IGF-1 was a gift from Chiron Corp. (Emeryville, CA). Peroxidase labeled goat anti-mouse IgG was purchased from Organon Technika Corp. (Durham, NC). All other used chemicals were molecular biology grade and purchased either from Sigma (St. Louis, MO) or Fischer Scientific (Pittsburgh, PA). Pronase and Collagenase P (Clostridium histolyticum) were purchased from Calbiochem (San Diego, CA) and Boehringer Mannheim (Indianapolis, IN) respectively.
Chondrocyte isolation and culture
Normal cartilage was obtained from the ankle joints of 7 tissue donors within 24 hours of death through collaboration with the Gift of Hope Organ & Tissue Donor Network (Elmhurst, IL) with Institutional Review Board Approval and appropriate consent. The donors had no documented history of joint disease. Each joint was graded with a modified Collins scale for morphological appearance as described 40. For this study only normal joints of grade 0–1 were used. The mean ± SD age of the donors was 45 ± 8 years (range 20–76). OA cartilage was obtained from patients undergoing total knee joint arthroplasty (n = 6) at the Department of Orthopaedic Surgery at Rush University Medical Center within 3 hours of surgery, with Institutional Review Board Approval and appropriate consent. The average age of OA patients was 66 ± 11 years (range 51–78).
Cartilage was dissected from the joints, with care taken to avoid underlying bone and osteophytes. The tissue was digested in DMEM/Ham’s F-12 medium (1:1) containing 0.2% pronase in an incubator with continuous agitation for 1 hour, and then overnight with 0.025% collagenase P in DMEM/Ham’s F-12 supplemented with 5% FBS. After isolation, the cells were counted. The initial viability prior to culture was assessed using trypan blue dye exclusion assay and was determined to be >90%. The cells were resuspended at 2 × 106/ml in sodium alginate. Alginate beads were produced as previously described 11, resulting in ~20,000 cells per bead.
Alginate beads were cultured at 8 beads per well in 24-well plates, in serum-free DMEM/Ham’s F-12 (1:1; 1 ml/well). All media were supplemented with 1% mini-ITS+, which contains 5 nM insulin (“mini”-dose insulin so that the IGF-1 receptor is not stimulated), 2 μg/ml transferrin, 2 ng/ml selenous acid, 25 μg/ml ascorbic acid, and bovine serum albumin/linoleic acid at 420/2.1 μg/ml 11. Cultures were maintained for 21 days. The beads were divided into 4 experimental groups: media control; beads treated with IGF-1 (100 ng/ml); beads treated with OP-1 (100 ng/ml); and beads treated with the combination of IGF-1 (100 ng/ml) and OP-1 (100 ng/ml). Medium was changed every 48 hours, with the addition of fresh growth factors to the treated wells.
Histology and immunohistochemistry on alginate beads
To visualize the matrix deposited by chondrocytes, alginate beads were fixed in 4% paraformaldehyde in 0.1 M cacodylate buffer (pH 7.4) containing 10 mM CaCl2 for 4 hours at 20°C, washed overnight at 4°C in 0.1 M cacodylate buffer (pH 7.4) containing 50 mM BaCl2, dehydrated through serial alcohols and xylene, embedded in paraffin, sectioned at 6 μm and processed for histology and immunohistochemistry as described 41. For histology, the beads were stained with Safranin O and fast green 42.
For immunohistochemistry, before incubation with the primary antibodies, bead sections were digested with keratanase [Pseudomonas sp; EC 3.2.1.103 (0.01 U/ml)], keratanase II [Bacillus sp. KS 36 (0.0001 U/ml)], and chondroitinase ABC [Proteus vulgaris; EC 4.2.2.2 (0.01 U/ml)] in 100 mM Tris/50 mM Na-acetate buffer (pH 6.5) at 37°C for 90 min to increase the penetration of antibodies into the matrix. All three proteinases were obtained from Seikagaku (Tokyo, Japan). For negative controls, the primary antibodies were replaced with either normal serum or secondary antibody alone. Anti-type I and anti-type X collagen antibodies were applied at 1:500 and 1:1000 dilutions; all other primary antibodies were applied at a 1:100 dilution. To detect aggrecan, decorin, and collagen type II, ImmunoPure ABC Alkaline Phosphatase mouse IgG staining kit for immunohistochemistry was used (Pierce Biotechnology, Rockford, IL). To localize fibronectin and type VI collagen, horseradish peroxidase conjugated mouse IgG secondary antibody was used; the sections were counterstained with hematoxylin. Type I and X collagens were detected according to the protocol described by Aigner et al 43, in which cartilage sections were enzymatically pretreated with hyaluronidase (2 mg/ml in phosphate buffer saline (PBS), pH 5.0, 60 minutes at 37°C) and protease XXIV (0.02 mg/ml in PBS, pH 7.3, 60 minutes at 37°C), incubated overnight with corresponding antibodies at 4°C, and visualized with a streptavidin-biotin complex kit (Super Sensitive Immunodetection System for mouse primary antibodies; Biogenex, San Ramon, CA). The red stain was fast red, a substrate for the alkaline phosphatase used in the detection kit; counterstaining was done with hematoxylin to stain the nuclei of the cells. Evaluation and documentation of the results was done with a Nikon Eclipse 600 microscope (Tokyo, Japan) with a Spot 2 camera; the pictures were taken with the MetaMorph software program (Universal Imaging Corporation, PA, USA).
Morphometrical analysis
Safranin O stained sections underwent morphometrical analysis under 400x magnification to quantify the size of the newly deposited matrix using a Nikon Eclipse E600 microscope connected to a PC running MetaMorph Imaging series 6.1. Using this software, the area occupied by each chondrocyte and its newly synthesized matrix was determined by selecting the area of the “cavity” left in the alginate bead, forming a better representation of the actual size of the newly synthesized matrix before shrinkage artifact from histological preparation (Fig. 2A). The control and all experimental groups were subjected to the morphometrical analysis. The data were collected for 17–38 cells/cell clones with their surrounding matrix for each group in a blind manner and normalized to the number of cells within selected area. The size of the cell per se was not subtracted from the size of the matrix since it was difficult to distinguish between cell-associated matrix and cell itself. The statistical significance of results was determined by analysis of variance, using Prizm software and one-way ANOVA. Comparison of the different growth factor treatments was done with two-tailed student’s t-test.
Figure 2.
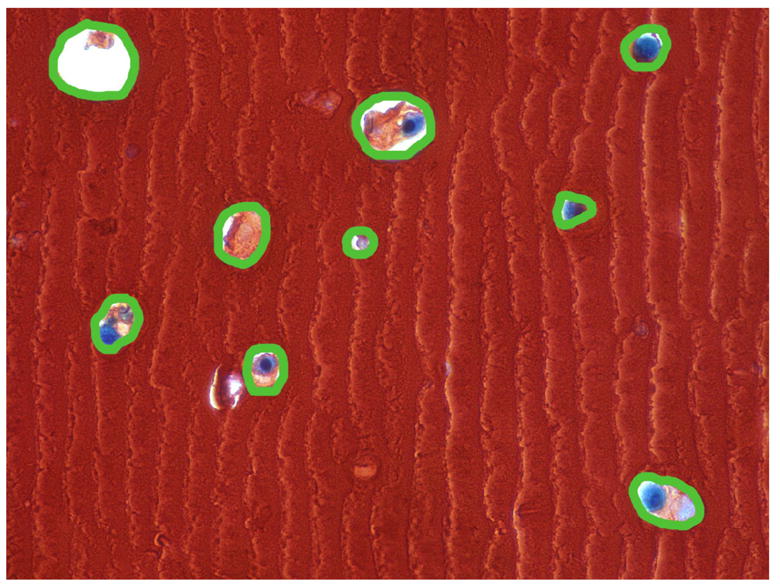
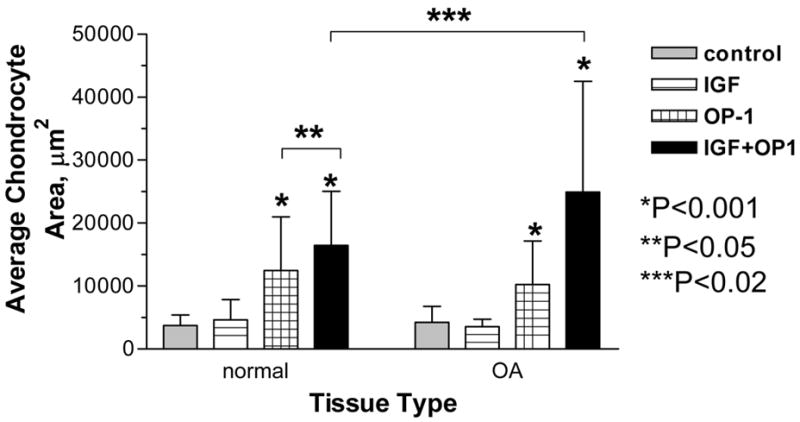
Morphometrical analysis of the matrix deposited by normal and OA chondrocytes cultured in alginate. A, representative picture with the outline of the cell with surrounding extracellular matrix deposited within alginate; B, average size of the matrix produced by chondrocytes; comparison is made between treatment groups separately for cells isolated from each tissue type (normal or OA). For normal chondrocytes the differences were statistically significant between the following groups: OP-1 vs Control or vs IGF-1, P<0.001; OP-1+IGF-1 vs Control or vs IGF-1, P<0.001; OP-1+IGF-1 vs OP-1, P<0.05. For OA chondrocytes, OP-1 vs Control or vs IGF-1, P<0.001; OP-1+IGF-1 vs control or vs IGF-1 or vs OP-1, P<0.001. Comparison was also made between the tissue types and statistically significant differences (P<0.02) were found between normal and OA chondrocytes treated with OP-1+IGF-1.
Results
Histology
Staining of alginate beads with Safranin O and fast green allowed visualization of newly synthesized matrix. Nonspecific binding of Safranin O dye to the negatively charged alginate matrix produced intense red stain, while matrix synthesized by chondrocytes appeared with a lighter intensity of red (Fig. 1). In the ITS control group (Fig. 1A&E) barely visible matrix was seen around chondrocytes. The histological appearance of alginate beads cultured in the presence of growth factors indicated the highest matrix formation under the treatment with combined IGF-1 and OP-1 (Fig. 1D&H). In the IGF-1 alone group (Fig. 1B&F) no significant matrix deposition was noticed, especially by OA chondrocytes. OP-1 alone (Fig. 1C&G) caused a considerable matrix formation by both normal and OA chondrocytes, but less than in the combination group. Of note, no visible differences were observed in the amount of matrix deposited within the alginate by normal and OA chondrocytes whether in the presence or absence of growth factors. The apparent shrinkage of the matrix within the areas occupied by chondrocytes is believed to be due to the artifacts produced by fixation and processing procedures.
Figure 1.
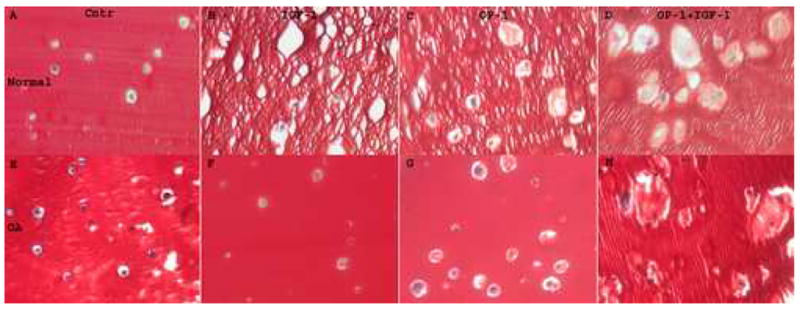
Safranin O staining of normal (A–D) and OA (E–H) chondrocytes cultured in alginate beads. A & E, Cells cultured in the presence of mini-ITS; B & F, Cells cultured in the presence of IGF-1 (100 ng/ml); C & G, Cells cultured in the presence of OP-1 (100 ng/ml); D & H, Cells cultured in the presence of combined OP-1 and IGF-1. Original magnification 400x.
Morphometry
To quantify the amount of matrix deposited by chondrocytes in alginate, we applied morphometrical analysis, where the size of the matrix surrounding each cell or clone of cells in square μm was divided by the number of chondrocytes residing within this area (example is shown in Fig. 2A). IGF-1 alone did not induce significant matrix accumulation in comparison to control (Fig. 2B), while OP-1 was able to stimulate matrix synthesis by both normal and OA chondrocytes. The size of the matrix produced by OP-1 was significantly different in comparison to IGF-1 treatment or ITS control (2.6 fold as compared to IGF-1 treatment and 3.3-fold in comparison to ITS control for normal chondrocytes; 2.9-fold and 2.4-fold correspondingly for OA chondrocytes; P<0.001). The largest matrix was synthesized by chondrocytes stimulated by the combination of growth factors (Fig. 2B). The effect was statistically significant in comparison to the control (4.4-fold increase for normal chondrocytes and 5.9-fold increase for OA chondrocytes; P<0.001), IGF-1 (3.5- and 7-fold correspondingly; P<0.001), or OP-1 alone treatments (1.3-fold, P<0.05 for normal chondrocytes and 2.4-fold, P<0.001 for OA cells). Noteworthy, although combined treatment induced chondrocyte proliferation, as shown here and in a previous study 25, matrix production per cell was still greatest for the combination of both growth factors. We also compared the response of normal and OA chondrocytes to the stimulation with growth factors and found no statistical differences in the size of the matrix synthesized in the control, IGF-1 alone, or OP-1 alone groups. Under combined treatment, the size of newly synthesized matrix was statistically larger in OA chondrocytes than in normal chondrocytes (1.5-fold difference; P<0.02; Fig. 2B).
Immunohistochemistry
Type II collagen staining
Immunostaining with anti-type II collagen antibody showed that both normal and OA chondrocytes cultured in alginate beads were able to synthesize and deposit type II collagen under stimulation with growth factors. In the control groups, treatment with ITS media did not appear to increase levels of type II collagen in the matrix (Fig. 3A&E). Low levels of type II collagen protein were observed in the presence of IGF-1 (Fig. 3B&F). Type II collagen was primarily localized in the interterritorial, further-removed matrix. In the OP-1 treated beads, the pattern of type II collagen distribution and localization was similar for normal and OA chondrocytes. In both cell types collagen type II was mainly detected in the interterritorial matrix at the edges of the alginate matrix (Fig. 3C&G), although it appears that less type II collagen was detected in OA chondrocytes than in normal cells. The highest levels of type II collagen were identified under combined treatment with growth factors (Fig. 3D&H), where type II collagen protein was localized in all matrix compartments: pericellular, territorial, and interterritorial. The intensity of stain was similar for both normal and OA cells.
Figure 3.
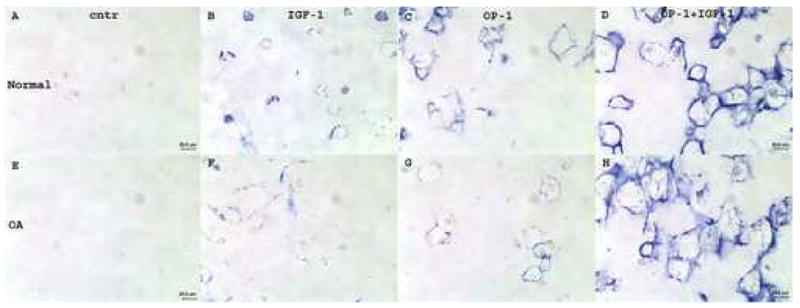
Immunostaining of chondrocytes in alginate beads with anti-type II collagen antibody. A–D, chondrocytes isolated from normal cartilage; E–H, chondrocytes isolated from OA cartilage. A&E, cells cultured in media with mini-ITS; B&F, cells treated with IGF-1; C&G, cells treated with OP-1; D&H, cells treated with combined OP-1 and IGF-1. Original magnification 400x.
Aggrecan immunostaining
In contrast to collagen type II, some aggrecan synthesis was detected in the beads from the mini-ITS control groups of normal (Fig. 4a, A) and OA chondrocytes (Fig. 4b, A). Stimulation with IGF-1 alone induced some additional aggrecan accumulation in the interterritorial matrix by normal cells (Fig. 4a, B), but no such response was evident for OA cells (Fig. 4b, B). The effect of OP-1 treatment was much more pronounced. In beads with normal cells, OP-1 stimulated aggrecan accumulation primarily in the interterritorial matrix with some aggrecan presence in the pericellular and territorial matrices (Fig. 4a, C). In beads with OA cells, OP-1 stimulated a very strong staining for aggrecan which was found mainly in the pericellular and territorial matrices with only weak stain in the interterritorial areas (Fig. 4b, C). Combined treatment induced more synthesis of the aggrecan core protein in either cell type than each growth factor separate. The distribution and localization of aggrecan was similar for both normal (Fig. 4a, D) and OA chondrocytes (Fig. 4b, D) and it was detected in the territorial and interterritorial matrices.
Figure 4.
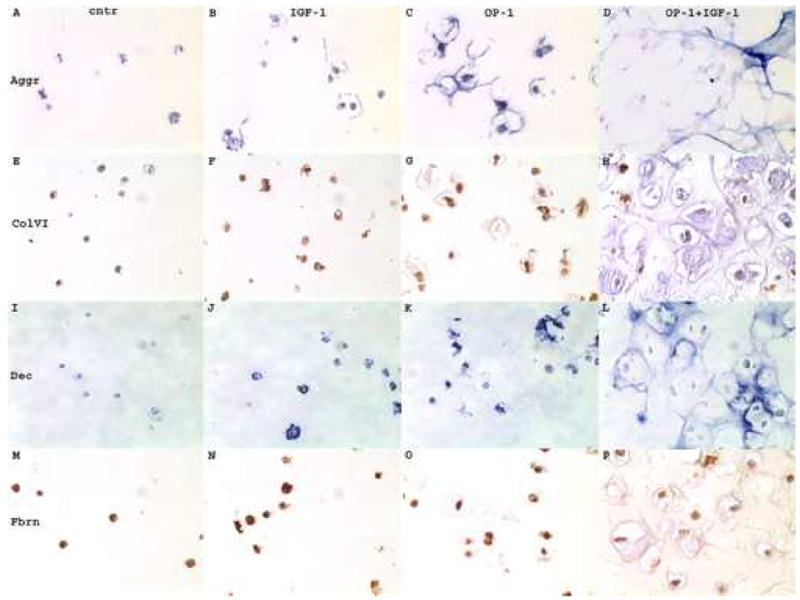
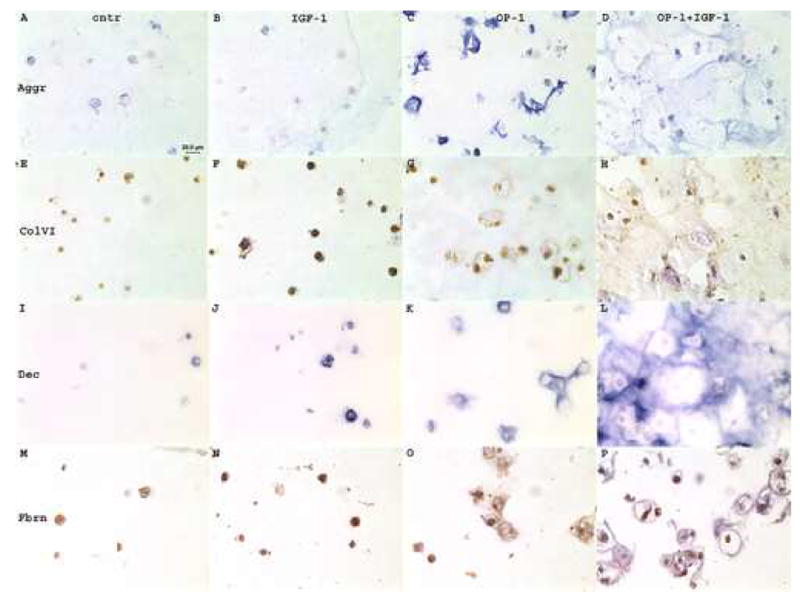
Immunohistochemistry of chondrocytes cultured in alginate beads and treated with growth factors. 4a, normal chondrocytes; 4b, OA chondrocytes. A–D, chondrocytes stained with anti-aggrecan antibody (Aggr); E–H, chondrocytes stained with anti-type VI collagen antibody (ColVI); I–L, chondrocytes stained with anti-decorin antibody (Dec); M–P chondrocytes stained with anti-fibronectin antibody (Fbrn). A,E,I,M, mini-ITS control; B,F,J,N, cells treated with IGF-1; C,G,K,O, cells treated with OP-1; D,H,L,P, cells treated with combined OP-1 + IGF-1. Aggrecan and decorin immunostaining was developed with ImmunoPure ABC Alkaline Phosphatase staining kits that produced blue color; type VI collagen and fibronectin stains were developed by the application of horseradish-peroxidase conjugated secondary antibodies that produced brown color. Original magnification 400x.
Type VI collagen
Type VI collagen was detected in all experimental groups including mini-ITS controls (Fig. 4a&b, E). In normal chondrocytes (Fig. 4a) no apparent differences in the amount of type VI collagen and patterns of its distribution were found between treatment groups (Fig. 4a, E–H). In all groups type VI collagen was primarily localized in the pericellular matrix. In OA chondrocytes (Fig. 4b) in the group of combined OP-1 and IGF-1 (Fig. 4b, H), in addition to the pericellular localization type VI collagen was also largely present in the interterritorial matrix. Surprisingly, the highest intensity of cellular type VI collagen staining in OA chondrocytes was found under the treatment with IGF-1 alone (Fig. 4b, F).
Decorin
Decorin was barely detected on the background levels in the mini-ITS control beads containing either OA (Fig. 4b, I) or normal chondrocytes (Fig. 4a, I). IGF-1 treatment induced very low accumulation of decorin by both cell types and it was localized in all three matrix compartments (Fig. 4a&b, J). The OP-1 effect was more pronounced than that of IGF-1 on decorin synthesis and the amount of decorin deposited by OA chondrocytes (Fig. 4b, K) was more abundant than by normal cells (Fig. 4a, K). Combination of the two growth factors stimulated the highest decorin synthesis in normal (Fig. 4a, L) and OA chondrocytes (Fig. 4b, L). In both cell types decorin was primarily localized in the interterritorial matrix; however in normal chondrocytes decorin matrix appeared of more native structural organization and resembled a honeycomb structure (Fig. 4a, L). OA chondrocytes did not reveal such distinct pattern (Fig. 4b, L).
Fibronectin
Fibronectin protein was detected in all experimental groups in both normal (Fig. 4a, M–P) and OA chondrocytes (Fig. 4b, M–P). Overall, immunostaining with fibronectin antibody appeared stronger for normal cells than for OA cells. In the mini-ITS control group (Fig. 4a&b, M) and in the IGF-1-treated (Fig. 4a&b, N) chondrocytes fibronectin was primarily localized in the pericellular matrix; however in the presence of OP-1 (Fig. 4a&b, O) or combined OP-1 and IGF-1 (Fig. 4a&b, P) some fibronectin was also detected in the interterritorial matrix at the edges of the lacunas.
Immunostaining with anti-type I and anti-type X collagen antibodies
To verify the phenotype of chondrocytes cultured in alginate beads for 21 days we stained the beads with antibodies against type I and type X collagens. We found low to moderate levels of type I collagen primarily present in OA chondrocytes (Fig. 5C&D) and more in cells treated with combined OP-1 and IGF-1 (Fig. 5D). In other experimental groups (for example, mini-ITS control, Fig. 5C) and in normal chondrocytes type I collagen was barely detectable or was below the detection limit (Fig. 5A&B). Staining of normal (Fig. 5E&F) and OA chondrocytes (Fig. 5G&H) with anti-type X collagen antibody showed no detectable type X collagen in cells treated with ITS control (Fig. 5E&G) or in all other experimental groups (not shown) except for chondrocytes cultured under combined treatment (Fig. 5F&H). In this group in both types of cells (normal and OA) collagen type X was present at very low, almost negligible levels.
Figure 5.
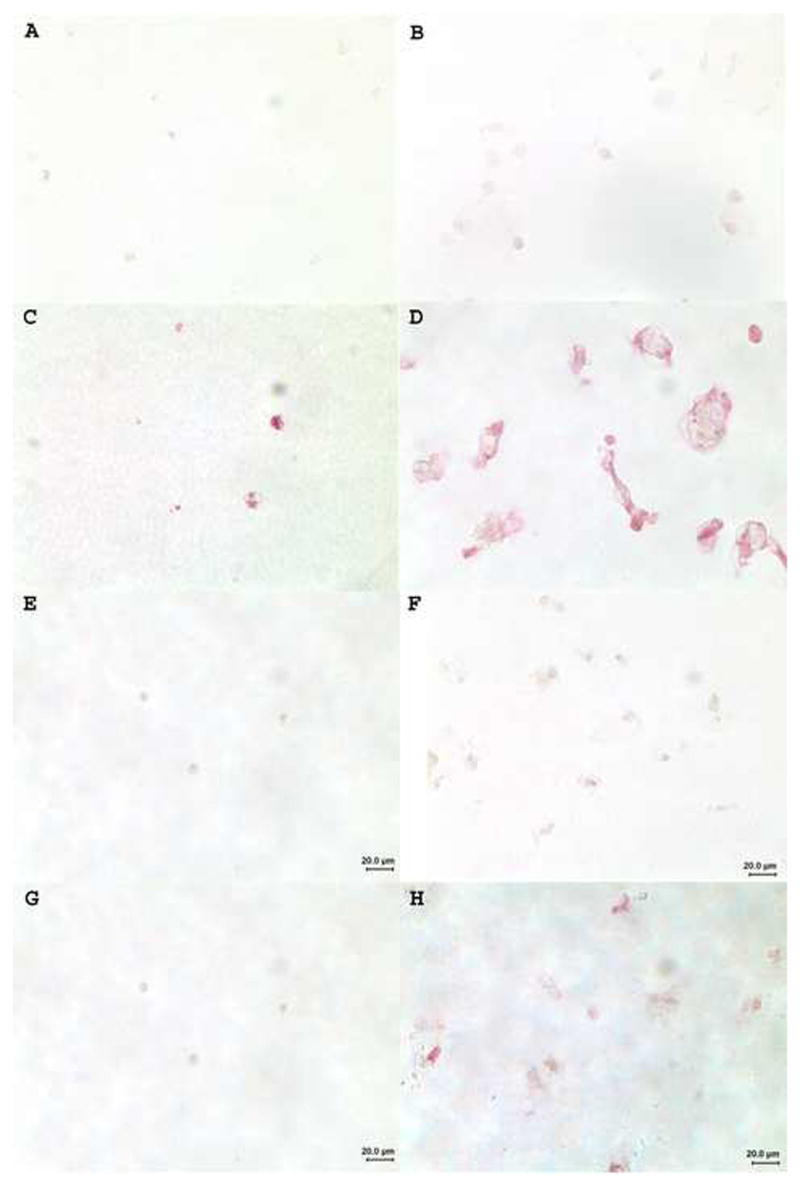
Immunostaining of normal (A&B; E&F) and OA (C&D; G&H) chondrocytes with anti-type I (A–D) and anti-type X (E–H) collagen antibodies.. A, C, E & G, cells cultured in the presence of mini-ITS control; B, D, F & H, cells treated with combined OP-1 + IGF-1. Original magnification 400x.
Discussion
This is a continuing study of Loeser et al 25 that directly compares the ability of IGF-1, OP-1, or their combination to stimulate matrix production by normal and OA chondrocytes. In this study, we found that OA chondrocytes are metabolically active and that they could respond anabolically to the treatment with growth factors by producing cartilage-specific proteins. Similar findings were reported by Fan et al 28, in which OA chondrocytes showed a higher expression of anabolic genes, collagen type II and aggrecan, when stimulated by OP-1. Here we also found that normal and OA chondrocytes isolated from their native matrix and cultured under conditions defined in this study respond better to OP-1 than to IGF-1, but only with regard to the synthesis of certain major cartilage matrix molecules: collagen type II, aggrecan, and decorin. Clearly, the combination of IGF-1 and OP-1 showed a synergistic effect on the synthesis of these cartilage matrix constituents by both normal and OA chondrocytes. Collagen type II, aggrecan and decorin were more abundant under the combined treatment than under the treatment with each growth factor separately. Moreover, as shown previously 25, the combined treatment caused new effects including increased proliferation and cell survival that were not seen under the treatment with individual growth factors.
To our surprise, we found by morphometrical analysis that OA chondrocytes deposited significantly more extracellular matrix than normal cells under the combined treatment with the two growth factors. Previously 25, no difference was detected in proteoglycan production by normal and OA chondrocytes under combined treatment when using the DMMB assay. However, morphometrical analysis that measures the net result of total matrix deposited by chondrocytes suggests that matrix proteins in addition to the proteoglycans are significantly stimulated by IGF-1 plus OP-1 treatment of OA cells. Among candidates responsible for such difference could be perhaps type I collagen (in addition to type II collagen and aggrecan) 28, which was found in our study mainly in OA chondrocytes treated with combined OP-1 and IGF-1. We also detected more of collagen type VI in OA chondrocytes under the combined treatment than in any other group, which also could contribute to the differences in the amount of deposited matrix by normal and OA chondrocytes stimulated by the combination of the two growth factors. Interestingly, only in the beads with OA chondrocytes treated with OP-1 + IGF-1 most of type VI collagen was detected in the interterritorial matrix rather than being restricted to the pericellular matrix as has been known for normal articular cartilage 44-46. A similar increase in type VI collagen in the ECM of OA cartilage has been reported 47-48. However, it is still unclear whether type VI collagen is a native component of the OA ECM or represents remnants of the degradation products which diffuse in this matrix. Apparently, type VI collagen was the only matrix protein that was affected by the treatment with IGF-1 when used alone in our study. This may provide additional supportive evidence for an association between IGF-1 signaling and type VI collagen in chondrocytes reported previously by Loeser 49. It has been shown that stimulation of chondrocytes with IGF-1 induces cell surface α1β1 integrin which is a type VI collagen receptor 50 and thus mediates chondrocyte adhesion to type VI collagen. Strong accumulation of type VI collagen in the pericellular matrix of IGF-1 treated cultures found in our study may be due to increased binding of type VI collagen to the cell resulting from the concurrent stimulation of α1β1 integrin levels.
We were not particularly surprised to find minimal levels of type X collagen under the combined treatment. Since only combined treatment stimulated significant cell proliferation (unlike individual growth factors) 25, it is possible that proliferating chondrocytes may continue their programmed developmental pathway and undergo hypertrophy, where type X collagen expression is one of the characteristic changes 51. Low levels of type X suggest, however, that only a small subpopulation of cells may be hypertrophic.
Collectively, we found that combined treatment with IGF-1 and OP-1 induced more accumulation of matrix proteins in the ECM than with each growth factor separately. The larger matrix found in the combined group could be explained by a couple of reasons. First, the increased number of metabolically active cells 25 overall could produce more matrix, although in this and previous studies 25, 51 all quantitative data were normalized to the cell number. Secondly, we think that the most real scenario is that each growth factor stimulates and promotes the signaling of a second factor and together they may stimulate pathways that are not affected by individual growth factors. Some evidence for this hypothesis was provided in previous work 24, where OP-1 was shown to stimulate autocrine expression of IGF-1 and its receptor. We also anticipate that OP-1 may improve or restore the responsiveness of OA and old chondrocytes to the treatment with IGF-1. Studies have shown that growth factors (EGF, FGF, TGF-β) may in fact modulate the response of chondrocytes to other growth factors 30-33. But the mechanism of this effect remains to be verified. With regard to the role of OP-1 in IGF-1 signaling, besides our work the combination of OP-1 and IGF-1 was extensively studied only in rat calvaria cells in the laboratory of Dr. Lee 34-36, where a synergistic effect on cell differentiation and proliferation was found for the two growth factors.
In conclusion, the results obtained in the current study suggest that combined therapy with IGF-1 and OP-1 may be more effective in treating cartilage defects seen in OA. The understanding of the mechanisms of the synergistic response may provide important information needed to design effective growth factor therapy for arthritis.
Acknowledgments
This work was supported by National Institutes of Health Grants AG16697 (RL) and AR47654 (SC), Stryker Biotech grant SC-001 (SC) and the German Ministry of Research and Technology grant 01GG9824 (TA). We gratefully acknowledge the Gift of Hope Organ and Tissue Donor Network and the donor families for providing tissue and the assistance of Dr. Arkady Margulis for collecting donor tissues.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hunziker EB. Articular cartilage repair: basic science and clinical progress. A review of the current status and prospects. Osteoarthritis Cartilage. 2002;10(6):432–63. doi: 10.1053/joca.2002.0801. [DOI] [PubMed] [Google Scholar]
- 2.Schneiderman R, Rosenberg N, Hiss J, Lee P, Liu F, Hintz RL, et al. Concentration and size distribution of insulin-like growth factor-1 in human normal and osteoarthritic synovial fluid and cartilage. Arch Biochem Biophys. 1995;324:173–88. doi: 10.1006/abbi.1995.9913. [DOI] [PubMed] [Google Scholar]
- 3.Chubinskaya S, Merrihew C, Cs-Szabo G, Mollenhauer J, McCartney J, Rueger DL, et al. Human articular chondrocytes express osteogenic protein-1. J Histochem Cytochem. 2000;48(2):239–50. doi: 10.1177/002215540004800209. [DOI] [PubMed] [Google Scholar]
- 4.van Osch GJ, van den Berg WB, Hunziker EB, Hauselmann HJ. Differential effects of IGF-1 and TGF beta-2 on the assembly of proteoglycans in pericellular and territorial matrix by cultured bovine articular chondrocytes. Osteoarthritis & Cart. 1998;6(3):187–95. doi: 10.1053/joca.1998.0111. [DOI] [PubMed] [Google Scholar]
- 5.Hui W, Cawston T, Rowan AD. Transforming growth factor β1 and insulin-like growth factor 1 block collagen degradation induced by oncostatin M in combination with tumor necrosis factor α from bovine cartilage. Ann Rheum Dis. 2003;62:172–4. doi: 10.1136/ard.62.2.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Richmond JD, Sage AB, Shelton E, Schumacher B, Sah RL, Watson D. Effect of growth factors on cell proliferation, matrix deposition, and morphology of human nasal septal chondrocytes cultured in monolayers. Laryngoscope. 2005;115:1553–60. doi: 10.1097/01.MLG.0000175541.31131.A5. [DOI] [PubMed] [Google Scholar]
- 7.Flechtenmacher J, Huch K, Thonar EJ-MA, Mollenhauer JA, Davies SR, Schmid TM, et al. Recombinant human osteogenic protein 1 is a potent stimulator of the synthesis of cartilage proteoglycans and collagens by human articular chondrocytes. Arthritis Rheum. 1996;39:1896–904. doi: 10.1002/art.1780391117. [DOI] [PubMed] [Google Scholar]
- 8.Nishida Y, Knudson CB, Eger W, Kuettner KE, Knudson W. Osteogenic protein-1 stimulates cell-associated matrix assembly by normal human articular chondrocytes: upregulation of hyaluronan synthase, CD 44 and aggrecan. Arthritis Rheum. 2000;43:206–14. doi: 10.1002/1529-0131(200001)43:1<206::AID-ANR25>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 9.Jelic M, Pecina M, Haspl M, Kos J, Taylor K, Maticic D, et al. Regeneration of articular cartilage chondral defects by osteogenic protein-1 (bone morphogenetic protein-7) in sheep. Growth Factors. 2001;19(2):101–13. doi: 10.3109/08977190109001079. [DOI] [PubMed] [Google Scholar]
- 10.Hurtig MB, Chubinskaya S. The protective effect of OP-1 in early traumatic osteoarthritis-animal studies. Trans. 5th Combined meeting of the ORS of Canada, USA, Japan, and Europe; Banff, Alberta, Canada. October 10–13, 2004; p. 70. [Google Scholar]
- 11.Loeser RF, Shanker G, Carlson CS, Gardin JF, Shelton BJ, Sonntag WE. Reduction in the chondrocyte response to insulin-like growth factor 1 in aging and osteoarthritis: studies in a non-human primate model of naturally occurring disease. Arthritis Rheum. 2000;43(9):2110–20. doi: 10.1002/1529-0131(200009)43:9<2110::AID-ANR23>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 12.Huch K, Wilbrink B, Flechtenmacher J, Koepp HE, Aydelotte MB, Sampath TK, et al. Effects of recombinant human osteogenic protein 1 on the production of proteoglycan, prostaglandin E2, and interleukin-1 receptor antagonist by human articular chondrocytes cultured in the presence of interleukin-1β. Arthritis Rheum. 1997;40:2157–61. doi: 10.1002/art.1780401209. [DOI] [PubMed] [Google Scholar]
- 13.Chen P, Vukicevic S, Sampath TK, Luyten FP. Bovine articular chondrocytes do not undergo hypertrophy when cultured in the presence of serum and osteogenic protein-1. Biochem Biophys Res Comm. 1993;197:1253–9. doi: 10.1006/bbrc.1993.2612. [DOI] [PubMed] [Google Scholar]
- 14.Chubinskaya S, Rueger DC, Berger RA, Kuettner KE. Osteogenic protein-1 and its receptors in human articular cartilage. In: Hascall VC, Kuettner KE, editors. The Many faces of osteoarthritis. Birkhauser Verlag Basel; Switzerland: 2002. pp. 81–9. [Google Scholar]
- 15.Nishida Y, Knudson CB, Kuettner KE, Knudson W. Osteogenic protein-1 promotes the synthesis and retention of extracellular matrix within bovine articular cartilage and chondrocyte cultures. Osteoarthritis & Cart. 2000;8:127–36. doi: 10.1053/joca.1999.0281. [DOI] [PubMed] [Google Scholar]
- 16.Koepp HE, Sampath TK, Kuettner KE, Homandberg GA. Osteogenic protein-1 (OP-1) blocks cartilage damage caused by fibronectin fragments and promotes repair by enhancing proteoglycan synthesis. Inflamm Res. 1999;47:1–6. doi: 10.1007/s000110050446. [DOI] [PubMed] [Google Scholar]
- 17.Nishida Y, Knudson CB, Knudson W. Osteogenic Protein-1 inhibits matrix depletion in a hyaluronan hexasaccharide-induced model of osteoarthritis. Osteoarthritis & Cart. 2004;12:374–82. doi: 10.1016/j.joca.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 18.Tyler JA. Insulin-like growth factor 1 can decrease degradation and promote synthesis of proteoglycans in cartilage exposed to cytokines. Biochem J. 1989;260:543–8. doi: 10.1042/bj2600543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morales TI. Polypeptide regulators in matrix homeostasis in articular cartilage. In: Kuettner KE, Peyron JG, Schleyerbach R, Hascall VC, editors. Articular cartilage and osteoarthritis. New York: Raven Press; 1992. pp. 265–79. [Google Scholar]
- 20.Madry H, Zurakowski D, Trippel SB. Overexpression of human insulin-like growth factor-I promotes new tissue formation in an ex vivo model of articular chondrocyte transplantation. Gene Ther. 2001;8:1443–9. doi: 10.1038/sj.gt.3301535. [DOI] [PubMed] [Google Scholar]
- 21.Fortier LA, Mohammed HO, Lus G, Nixon AJ. Insulin-like growth factor-I enhances cell-based repair of articular cartilage. J Bone Joint Surg Br. 2002;84:276–88. doi: 10.1302/0301-620x.84b2.11167. [DOI] [PubMed] [Google Scholar]
- 22.Delany AM, Rydziel S, Canalis E. Autocrine down-regulation of collagenase-3 in rat bone cell cultures by insulin-like growth factors. Endocrinology. 1996;137:4665–70. doi: 10.1210/endo.137.11.8895331. [DOI] [PubMed] [Google Scholar]
- 23.Hui W, Rowan AD, Cawston T. Insulin-like growth factor 1 blocks collagen release and down regulates matrix metalloproteinase-1, -3, -8, and -13 mRNA expression in bovine nasal cartilage stimulated with oncostatin M in combination with interleukin1 alpha. Ann Rheum Dis. 2001;60:254–61. doi: 10.1136/ard.60.3.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Im H-J, Pacione C, Chubinskaya S, Sun Y, van Wijnen AJ, Loeser RF. Inhibitory effect of insulin-like growth factor-1 and osteogenic protein-1 on fibronectin fragment- or interleukin-1β-stimulated MMP-13 expression by human chondrocytes. J Bio Chem. 2003;278(28):25386–94. doi: 10.1074/jbc.M302048200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Loeser RF, Pacione CA, Chubinskaya S. The combination of insulin-like growth factor-1 and osteogenic protein-1 promotes increased survival of and matrix synthesis by normal and osteoarthritic human articular chondrocytes. Arthritis & Rheum. 2003;48(8):2188–96. doi: 10.1002/art.11209. [DOI] [PubMed] [Google Scholar]
- 26.Doré S, Pelletier J-P, DiBattista JA, Tardif G, Brazeau P, Martel-Pelletier J. Human osteoarthritic chondrocytes possess an increased number of insulin-like growth factor 1 binding sites but are unresponsive to its stimulation: possible role of IGF-1binding proteins. Arthritis Rheum. 1994;37:253–63. doi: 10.1002/art.1780370215. [DOI] [PubMed] [Google Scholar]
- 27.Trippel SB. Growth factor actions on articular cartilage. J Rheumatol Suppl. 1995;43:129–32. [PubMed] [Google Scholar]
- 28.Fan Z, Chubinskaya S, Rueger DC, Bau B, Haag J, Aigner T. Regulation of anabolic and catabolic gene expression in normal and osteoarthritic adult human articular chondrocytes by osteogenic protein-1. Clinical and Exper Rheum. 2004;22:103–6. [PubMed] [Google Scholar]
- 29.Osborn KD, Trippel SB, Mankin HJ. Growth factor stimulation of adult articular cartilage. J Orthop Res. 1989;7(1):35–42. doi: 10.1002/jor.1100070106. [DOI] [PubMed] [Google Scholar]
- 30.Sah RL, Chen AC, Grodzinsky AJ, Trippel SB. Differential effects of bFGF and IGF-1 on matrix metabolism in calf and adult bovine cartilage explants. Arch Biochem Biophys. 1994;308(1):137–47. doi: 10.1006/abbi.1994.1020. [DOI] [PubMed] [Google Scholar]
- 31.Yaeger PC, Masi TL, de Ortiz JL, Binette F, Tubo R, McPherson JM. Synergistic action of transforming growth factor-beta and insulin-like growth factor-I induces expression of type II collagen and aggrecan genes in adult human articular chondrocytes. Exp Cell Res. 1997;237(2):318–25. doi: 10.1006/excr.1997.3781. [DOI] [PubMed] [Google Scholar]
- 32.Sviri GE, Blumenfeld II, Livne E. Differential metabolic responses to local administration of TGF-beta and IGF-1 in temporomandibular joint cartilage of aged mice. Arch Gerontol Geriatr. 2000;31(2):159–76. doi: 10.1016/s0167-4943(00)00079-0. [DOI] [PubMed] [Google Scholar]
- 33.Tsukazaki T, Usa T, Matsumoto T, Enomoto H, Ohtsuru A, Namba H, et al. Effect of transforming growth factor-beta on the insulin-like growth factor-I autocrine/paracrine axis in cultured rat articular chondrocytes. Exp Cell Res. 1994;215(1):9–16. doi: 10.1006/excr.1994.1307. [DOI] [PubMed] [Google Scholar]
- 34.Yeh L-CC, Adamo ML, Olson MS, Lee JC. Osteogenic protein-1 and insulin-like growth factor I synergistically stimulate rat osteoblastic cell differentiation and proliferation. Endocrinology. 1997;138:4181–90. doi: 10.1210/endo.138.10.5465. [DOI] [PubMed] [Google Scholar]
- 35.Yeh L-CC, Adamo ML, Kitten AM, Olson MS, Lee JC. Osteogenic protein-1-mediated insulin-like growth factor gene expression in primary cultures of rat osteoblastic cells. Endocrinology. 1996;137:1921–31. doi: 10.1210/endo.137.5.8612532. [DOI] [PubMed] [Google Scholar]
- 36.Yeh L-C, Adamo ML, Duan C, Lee JC. Osteogenic protein-1 regulates insulin-like growth factor-1 (IGF-1), IGF-2, and IGF-binding protein-5 (IGFBP-5) gene expression in fetal rat calvaria cells by different mechanisms. J Cell Physiol. 1998;175:78–88. doi: 10.1002/(SICI)1097-4652(199804)175:1<78::AID-JCP9>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 37.Knutsen R, Honda Y, Strong DD, Sampath TK, Baylink DJ, Mohan S. Regulation of insulin-like growth factor system components by osteogenic protein-1 in human bone cells. Endocrinology. 1995;136:857–65. doi: 10.1210/endo.136.3.7532581. [DOI] [PubMed] [Google Scholar]
- 38.Roach HI, Erenpreisa J, Aigner T. Osteogenic differentiation of hypertrophic chondrocytes involves asymmetric cell divisions and apoptosis. J Cell Biol. 1995;131(2):483–94. doi: 10.1083/jcb.131.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Girkontaité I, Frischholz S, Lammi P, Wagner K, Swoboda B, Aigner T, et al. Immunolocalization of type X collagen in normal fetal and adult osteoarthritic cartilage with monoclonal antibodies. Matrix Biol. 1996;15:231–8. doi: 10.1016/s0945-053x(96)90114-6. [DOI] [PubMed] [Google Scholar]
- 40.Muehleman C, Bareither D, Huch K, Cole A, Kuettner KE. Prevalence of degenerative morphological changes in the joints of the lower extremity. Osteoarthritis & Cart. 1997;5:23–37. doi: 10.1016/s1063-4584(97)80029-5. [DOI] [PubMed] [Google Scholar]
- 41.Chubinskaya S, Huch K, Otten L, Aydellotte MB, Cole AA. Gene expression by human articular chondrocytes cultured in alginate beads . J Histochem & Cytochem. 2001;49(10):1211–9. doi: 10.1177/002215540104901003. [DOI] [PubMed] [Google Scholar]
- 42.Rosenberg L. Chemical basis for the histological use of safranin O in the study of articular cartilage. J Bone Joint Surg Am. 1971;53(1):69–82. [PubMed] [Google Scholar]
- 43.Aigner T, Loos S, Inwards C, Perris R, Perissinotto D, Unni KK, et al. Chondroblastoma is an osteoid-forming, but not cartilage-forming neoplasm. J Pathol. 1999;189:463–9. doi: 10.1002/(SICI)1096-9896(199912)189:4<463::AID-PATH476>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 44.Poole CA, Ayad S, Schofield JR. Chondrons from articular cartilage. I. Immunolocalization of type VI collagen in the pericellular capsule of isolated canine tibial chondrons. J Cell Sci. 1988;90:635–43. doi: 10.1242/jcs.90.4.635. [DOI] [PubMed] [Google Scholar]
- 45.Poole CA, Ayad S, Gilbert RT. Chondrons from articular cartilage. V. Immunohistochemical evaluation of type VI collagen organization in isolated chondrons by light, confocal and electron microscopy. J Cell Sci. 1992;103:1101–10. doi: 10.1242/jcs.103.4.1101. [DOI] [PubMed] [Google Scholar]
- 46.Ayad S, Evans H, Weiss JB, Holt L. Type VI collagen but not type V collagen is present in cartilage. Collagen Relat Res. 1984;4:165–8. doi: 10.1016/s0174-173x(84)80023-0. [DOI] [PubMed] [Google Scholar]
- 47.Hambach L, Neureuter D, Zeiler G, Kirchner T, Aigner T. Severe disturbance of the distribution and expression of type VI collagen chains in osteoarthritic articular cartilage. Arthritis Rheum. 1998;41:986–97. doi: 10.1002/1529-0131(199806)41:6<986::AID-ART5>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 48.Soder S, Hambach L, Lissner R, Kirchner T, Aigner T. Ultrastructural localization of type VI collagen in normal adult and osteoarthritic human articular cartilage. Osteoarthritis & Cart. 2002;10(6):464–70. doi: 10.1053/joca.2002.0512. [DOI] [PubMed] [Google Scholar]
- 49.Loeser RF. Growth factor regulation of chondrocyte integrins. Differential effects of insulin-like growth factor 1 and transforming growth factor beta on alpha 1 beta 1 integrin expression and chondrocyte adhesion to type VI collagen. Arthritis & Rheum. 1997;40(2):270–6. doi: 10.1002/art.1780400211. [DOI] [PubMed] [Google Scholar]
- 50.Loeser RF, Sadiev S, Tan L, Goldring MB. Integrin expression by primary and immortalized human chondrocytes: evidence of a differential role for alpha1beta1 and alpha2beta1 integrins in mediating chondrocyte adhesion to types II and VI collagen. Osteoarthritis & Cart. 2000;8:96–105. doi: 10.1053/joca.1999.0277. [DOI] [PubMed] [Google Scholar]
- 51.Schmid TM, Bonen DK, Luchene L, Linsenmayer TF. Late events in chondrocytes differentiation: hypertrophy, type X collagen synthesis and matrix calcification. In Vivo. 1991;5(5):533–40. [PubMed] [Google Scholar]
- 52.Loeser RF, Chubinskaya S, Pacione C, Im HJ. Basic fibroblast growth factor inhibits the anabolic activity of insulin-like growth factor 1 and osteogenic protein 1 in adult human articular chondrocytes. Arthritis Rheum. 2005;52(12):3910–7. doi: 10.1002/art.21472. [DOI] [PMC free article] [PubMed] [Google Scholar]


