Abstract
The fine-tuning of network activity provides a modulating influence on how information is processed and interpreted in the brain. Here, we use brain slices of rat prefrontal cortex to study how recurrent network activity is affected by neuromodulators known to alter normal cortical function. We previously determined that glutamate spillover and stimulation of extrasynaptic NMDA receptors are required to support hallucinogen-induced cortical network activity. Since microdialysis studies suggest that psychedelic hallucinogens and dopamine D1/D5 receptor agonists have opposite effects on extracellular glutamate in prefrontal cortex, we hypothesized that these two families of psychoactive drugs would have opposite effects on cortical network activity.
We found that network activity can be enhanced by DOI (a psychedelic hallucinogen that is a partial agonist of serotonin 5-HT2A/2C receptors) and suppressed by the selective D1/D5 agonist SKF 38393. This suppression could be mimicked by direct activation of adenylyl cyclase with forskolin or by addition of a cAMP analog. These findings are consistent with previous work showing that activation of adenylyl cyclase can upregulate neuronal glutamate transporters, thereby decreasing synaptic spillover of glutamate. Consistent with this hypothesis, a low concentration of the glutamate transporter inhibitor TBOA restored electrically-evoked recurrent activity in the presence of a selective D1/D5 agonist, whereas recurrent activity in the presence of a low level of the GABAA antagonist bicuculline was not resistant to suppression by the D1/D5 agonist. The tempering of network UP states by D1/D5 receptor activation may have implications for the proposed use of D1/D5 agonists in the treatment of schizophrenia.
Keywords: rat, 5-HT2A receptors, adenylyl cyclase, cAMP, neuronal glutamate transporter, EAAT3, EAAC1, caveolins
Introduction
In cortical neurons in vivo, recurrent network activity drives periods of depolarization (UP states) (Steriade et al., 1993; Cowan & Wilson, 1994; Lewis and O'Donnell, 2000; Petersen et al., 2003). These states appear to be mediated synaptically, driven by a mixture of excitatory and inhibitory inputs both in vivo (Haider et al., 2006) and in vitro (Shu et al., 2003). Network activity has been suggested to provide a “context” in which information is interpreted and decisions are made (McCormick, 2005). Bi-directional modulation of network activity may work to enhance cortical efficiency by increasing the signal-to-noise ratio (Winterer and Weinberger, 2004). It has been hypothesized that aberrations in the modulation of network activity may account for the perceptual and cognitive abnormalities in schizophrenia (Lewis et al., 2005).
Persistent network activity can be observed in brain slice of prefrontal cortex. Such network activity occurs spontaneously or can be evoked with a small electrical stimulus to the mid-layers of ferret cortex (McCormick et al., 2003; Shu et al., 2003) or multiple stimuli to the thalamus in mouse thalamcortical slice (Beierlein et al., 2002). Such network activity has been shown to rely on NMDA receptors (Shu et al., 2003) and increases the likelihood that a neuron will fire in response to an excitatory synaptic input (Shu et al., 2003; Haider et al., 2006). Similar states (originally termed “asynchronous, late excitatory postsynaptic currents (EPSCs)”) evoked by local electrical stimulation have also been described in rat prefrontal slice (Aghajanian and Marek, 1999). These states could be enhanced by psychedelic hallucingens (Aghajanian and Marek, 1999) and were also found to be NMDA dependent (Stutzmann et al., 2001). Here, we show that these hallucinogen-enhanced, late “EPSCs” involve both inhibitory as well as excitatory activity, similar to the in vitro and in vivo network activity recently described by McCormick and colleagues (Shu et al., 2003; Haider et al., 2006).
How neurotransmitters and drugs modulate recurrent network activity is not well understood. We previously found that glutamate spillover and stimulation of extrasynaptic NMDA receptors plays a key role in network activity in the presence of psychedelic hallucinogens (Lambe and Aghajanian, 2006). Since microdialysis has shown that these compounds increase extracellular glutamate in prefrontal cortex (Scruggs et al., 2003; Muschamp et al., 2004), we hypothesized that a modulator that decreases extracellular glutamate in prefrontal cortex would decrease recurrent network activity induced by hallucinogens. Dopamine D1/D5 receptor agonists meet this criteria because they have been found to decrease extracellular glutamate in prefrontal cortex in microdialysis experiments (Abekawa et al., 2000; Harte and O'Connor, 2004). Consistent with this hypothesis, dopamine D1/D5 receptors reduce the reliability of unitary excitatory transmission in ferret slice (Gao et al., 2001) and excite prefrontal interneurons in rat slice (Zhou et al., 1999; Seamans et al., 2001b; Gorelova et al., 2002; Trantham-Davidson et al., 2004). A recent study in prefrontal slice from nonhuman primate showed that D1/D5 receptors selectively excite fast-spiking, non-adapting interneurons (Kroner et al., 2006).
Consistant with the hypothesis that dopamine may reduce noise in prefrontal cortex, imaging studies in humans suggest that dopamine focuses the hemodynamic response of prefrontal cortex during tasks (reviewed in Winterer and Weinberger, 2004; Mattay et al., 1996, 2002, 2003). Prefrontal recordings in nonhuman primates performing working memory tasks have shown that a low and local blockade of D1/D5 receptors enhances task-related excitability (Williams and Goldman-Rakic, 1995). Conversely, the delay-period excitability of single prefrontal neurons could be reduced to background levels with local application of the D1/D5 receptor agonist SKF38393 (Williams and Goldman-Rakic, 1995). Recent work from Arnsten and colleagues shows that D1 agonists and cAMP-analogs, suppresses delay period firing of prefrontal neurons involved in a working memory task (Arnsten, 2006; Wang et al., 2006).
Yet, there is a paradox. A substantial literature supports an excitatory role for D1/D5 stimulation in prefrontal cortex. The D1/D5 receptors have been shown to enhance NMDA-mediated activity in vitro (Wang and O'Donnell, 2001; Seamans et al., 2001a). In the presence of excitatory agents, such as NMDA (6-8 μM) or high dose bicuculline (10 μM), high concentrations of D1/D5 agonists can induce avalanches of synaptic activity in acute slice (Beggs and Plenz, 2003) induce bistable membrane states (Tseng and O'Donnell, 2005), and enhance the spatiotemporal spread of cortical excitation (Bandyopadhyay et al., 2005). Interestingly, a recent study showing an enhancement of recurrent activity by D1/D5 receptors in the presence of bicuculline also showed a suppression of recurrent activity by dopamine in the absence of bicuculline (Onn et al., 2006); however, the dopamine receptor mediating this suppression was not identified. In vivo stimulation of the ventral tegmental area, whose neurons release dopamine and glutamate in cortex, can prolong plateau depolarizations in a D1-dependent manner (Lewis and O'Donnell, 2000). Yet these depolarizations are associated with reduced spiking (Lewis and O'Donnell, 2000), and thus may be different than network driven UP states which are associated with enhanced spiking in vivo (Haider et al., 2006) and in vitro (Shu et al., 2003).
In prefrontal cortical slice from rat, we found that low concentrations of agonists of D1/D5 receptors potently suppressed recurrent activity in prefrontal cortex. Their effects were opposite to the enhancement of network-driven UP states by psychedelic hallucinogens and could completely overwhelm both basal and DOI-enhanced network activity.
Experimental procedures
Coronal slices (400 μm thick) of the medial prefrontal cortex were prepared from adolescent/young adult Sprague–Dawley rats (35–60 days old), in accordance with protocols approved by the Yale University Animal Care Committee. The brain was cooled as rapidly as possible with ∼4°C oxygenated sucrose ACSF (254mM sucrose was substituted for NaCl). The slices were placed in a modified superfusion chamber (Warner Instruments, Hamden, CT) mounted on the stage of an Olympus BX50WI microscope (Olympus; Melville, NY). Great care was taken not to damage the cortical network during slice placement. Regular ACSF (128mM NaCl, 3mM KCl, 1.25mM NaH2PO4, 10mM D-glucose, 26mM NaHCO3, 2mM CaCl2 and 2mM MgSO4; pH 7.35) was bubbled with 95% oxygen and 5% carbon dioxide and flowed over slice at 3–4 ml/min. After 5 minutes of recovery in room temperature ACSF, the bath was warmed to 33°C. After 1-2 hours of recovery, the slice was warmed to 35-36°C for recording. The temperatures during brain removal, recovery, and recording were found to be critical for recurrent network activity. For the supplementary experiment, we used ACSF with more physiological concentrations of calcium and magnesium ions (1 mM each).
Whole-cell patch electrodes (4–6 MΩ) contained 120mM potassium gluconate, 5mM KCl, 2mM MgCl, 4mM K2- ATP, 0.4mM Na2-GTP, 10mM Na2-phosphocreatine, and 10mM HEPES (adjusted to pH 7.33 with KOH). Layer V pyramidal neurons were patched under visual control using IR-DIC microscopy. The mean membrane properties of pyramidal neurons were as follows: 88.1 ± 2.5 mV spike amplitude; 81.0 ± 9.7 MΩ input resistance, and −73.3 ± 1.1 mV resting potential. Neurons were recorded in current clamp or voltage-clamp modes. In current-clamp, neurons were recorded at their resting potentials. In voltage-clamp, neurons were held either near the reversal potential for chloride or at a more depolarized potential to examine inhibitory components. Currents were recorded using continuous single electrode voltage clamp mode with an Axoclamp 2A or 2B, lowpass filtered at 3 kHz, amplified 100x through Cyberamp and digitized at 15 kHz, and acquired using pClamp 9/ Digidata 1322A (Axon Instruments, Union City, CA).
Platinum–iridium bipolar stimulating electrodes with sharpened, 3 μm tips (FHC, Bowdoinham, ME) were placed midlayer in the medial prefrontal cortex and controlled via a Pulsemaster A300 interval generator (WPI, Sarasota, FL) and a stimulus isolation unit (Axon Instruments). Stimulating currents were 0.2 ms in duration and ranged from 5-30μA. During the baseline period in each experiment, stimuli were adjusted to produce a distinct fast EPSC but kept below the level that recruited late outward currents. Fast and late EPSC areas (nA*ms) were measured using Axograph software (Axon Instruments). We measured the absolute area under the curve from the point of return to baseline after the fast EPSC (or deviation from smooth decay phase in the case of a late EPSC that emerged without a return to baseline) to the point of return to baseline (or end of the trace) after the late EPSC.
Drugs were obtained from Sigma (St Louis, MO) or Tocris (Ellisville, MO) and applied in the fast-flowing bath (3-4 ml/min). Dopamine stock solution was prepared in 20 mM sodium meta-bisulfite to prevent oxidation. For each application of dopamine, new solution was prepared by adding an aliquot of stock solution (stored at −20°C) to pre-oxygenated ACSF containing 20 μM sodium meta-bisulfite. Room lights were kept off, and the dopamine solution was oxygenated during application.
Results
Recurrent network activity can be evoked electrically in brain slice of rat prefrontal cortex
Local cortical stimulation can evoke a mixture of excitatory and inhibitory recurrent activity in rat prefrontal slice, as shown in current clamp in Figure 1 and as previously described in ferret prefrontal slice (Shu et al., 2003). Voltage-clamping a single neuron does not change the activity of the network, and allows the isolation of either the excitatory or inhibitory component of the synaptic input (Figure 1A2). The electrical stimulus evokes both a fast EPSC and a fast IPSC, each of which can be seen at different membrane potentials. The fast IPSC is not seen when the neuron is held near the reversal potential for chloride (∼78mV). However, when the neuron is clamped to a depolarized potential, there is reduced driving force on sodium and potassium ions through the AMPA channels and enhanced driving force on the chloride ions through GABA-A channels, which makes the fast and network IPSCs appear more prominent. As suggested by previous work (Aghajanian and Marek, 1999) and as shown in Figure 1B, bath application of the psychedelic hallucinogen DOI increases the probability of recurrent network activity occurring after any given stimulus and increases the duration of this network activity.
Figure 1.

Recurrent network activity can be evoked electrically in brain slice of rat prefrontal cortex. (A) Current and voltage clamp recordings from a neuron under basal conditions. (1) A stimulus (10 μA; gray arrow) evokes network activity in the slice that depolarizes the neuron and increases its synaptic noise and spiking. (2) Voltage-clamping this neuron at different potentials allows the visualization of the inhibitory and excitatory aspects of the fast PSC and electrically-evoked network activity in the slice (VH = holding potential). No channel inhibitors are applied. (B) The oscillations of the basal and DOI-enhanced network activity show characteristic gamma wavelength activity (30 – 80 Hz). Single sweeps in voltage clamp (VH = −75 mV) show electrically-evoked (0.1 Hz) fast EPSCs and the excitatory component of the evoked network activity under basal conditions and following application of the psychedelic hallucinogen DOI (3 μM; 15 minutes).
Figure 2.
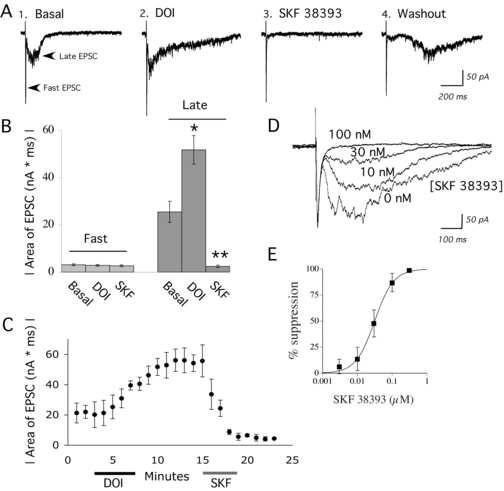
A psychedelic hallucinogen and a D1/D5 agonist have opposite effects on recurrent network activity in brain slice of rat prefrontal cortex. (A) Average of 10 sweeps (0.1 Hz) in voltage-clamp (VH = −78 mV) showing the electrically-evoked fast EPSC and the excitatory component of evoked network activity (late EPSCs): (1) under basal conditions, (2) after application of DOI (3 μM; 5 minutes), (3) during application of SKF 38393 (100 nM; 4 minutes), and (4) after 15 minutes washout from SKF 38393. Note the delay in evoked network activity during washout of SKF 38393. (B) Bar graph shows the areas (mean ± S.E.) of the electrically-evoked fast EPSC and the late EPSC or network activity in the above conditions (N = 10 neurons). DOI significantly enhanced the area of the late EPSC, and SKF 38393 significantly suppressed the late EPSC. * denotes P < 0.01; ** denotes P < 0.00001. (C) Graph shows the time course of network activity and the evolution of the drug effects on a representative neuron. Each circle is the average of the 6 electrically-evoked network events in voltage-clamp that are stimulated during each one minute period (0.1 Hz stimulus frequency). DOI (3 μM; 5 min) and SKF 38393 (100 nM, 4 min) were applied as indicated. It must be noted that the DOI-mediated enhancement of network activity is slow to wash out and remains at a peak for hours. (D) Superimposed averaged sweeps in voltage clamp during sequential application of increasing doses of SKF 38393 (0 nM to 300 nM; 4 minute applications) for one neuron. As shown, the fast EPSC has not changed, but the network activity is dose-dependently suppressed. (D) Dose response curve from 6 neurons for the SKF 38393 suppression of DOI-enhanced network activity.
Recurrent network activity is enhanced by 5-HT2A yet suppressed by D1/D5 stimulation
In order to quantify the selective effects of neuromodulators on the recurrent network activity underlying electrically-evoked UP states, we compared how treatments changed the areas of the evoked excitatory postsynaptic current (EPSC) and the excitatory component of the network activity (late EPSCs). Averaged traces in Figure 2 show the ability of the psychedelic hallucinogen DOI (3 μM; 5-15 minutes; N = 10) to extend the duration of recurrent network activity evoked with a small electrical stimulus. Each trace shown is the average of 10 responses in voltage clamp to the same electrical stimulation delivered at 0.1 Hz. Although averages are most reliable for detecting the effects of pharmacological manipulations, they tend to mask the gamma frequency (30 – 80 Hz) of the oscillations. These oscillations in both basal and DOI-enhanced network activity can be better seen in the individual traces shown in Figure 1.
A low concentration of the D1/D5 partial agonist SKF 38393 (100 nM; 4 minutes) almost completely suppressed both basal (N = 5) and DOI-enhanced network activity (N = 10). This suppression occurred without consistent effects on resting potential or area of the fast EPSC. The data for the effects of SKF 38393 on the fast EPSC and network activity are summarized in Figure 2. In Figure 2C, the time course of the effects of DOI and SKF 38393 on network activity are shown. It must be noted that the effects of DOI are very slow to washout and remain maximal for several hours (Lambe and Aghajanian, 2006). The dose response relationship for SKF 38393 suppression of DOI-enhanced network activity gave an IC50 of 31.2 nM. This suppression was similar to the SKF 38393 suppression of basal network activity in the absence of DOI (IC50 of 34.9; illustrated in Figure 2D, E). The IC50 figures are consistent with the efficacy and potency previously shown for SKF38393 in an adenylyl cyclase assay (Arnt et al., 1992). There was also no significant change in the fast EPSC for the group of neurons in Figure 1E (Area under basal conditions, 3.0 ± 0.7 nA*ms; area during maximum SKF, 2.7 ± 0.6; N = 6, Paired t test; P = NS).
Recurrent network activity occurs spontaneously with greater frequency in ACSF with lower concentrations of magnesium and calcium ions, as has been previously described in brain slice of ferret (Shu et al., 2003). In slices pretreated with DOI, spontaneous network activity was elicited by lowering the calcium and magnesium from 2 mM to 1 mM (Supplemental Data). Like electrically-evoked network activity, this spontaneous network activity could be completely suppressed with SKF 38393 (100 nM; 3-5 minutes; N=3).
Suppression of recurrent network activity appears specific to the D1/D5 dopamine receptors
Dopamine (1-10 μM) also suppressed recurrent network activity evoked electrically, as illustrated in Figure 3A and B. This suppression was unchanged by pre-treatment and co-application of raclopride (100 nM – 1 μM; N = 4), an antagonist of the D2 family of dopamine receptors. However, the suppression of network activity by dopamine was reduced dramatically by prior application of SCH 23390 (300 nM; N = 5), a dopamine D1/D5 family antagonist.
Figure 3.
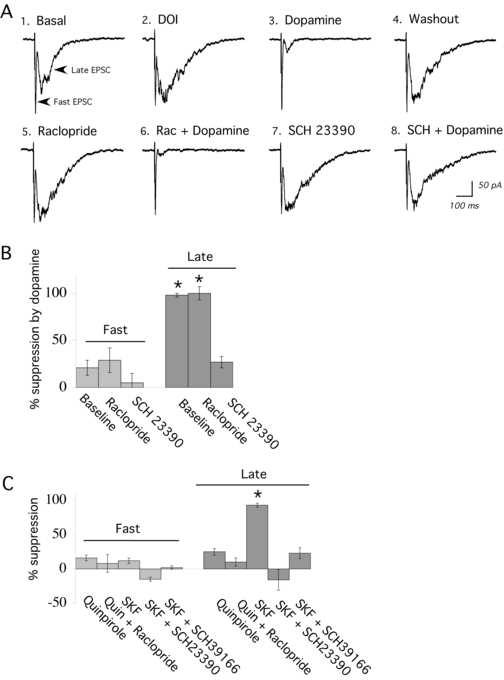
Evoked network activity is suppressed selectively through D1/D5 activation. (A) Averaged sweeps in voltage clamp showing electrically-evoked fast EPSC and the excitatory component of the evoked network activity (late EPSCs): (1) under basal conditions, (2) after application of DOI, (3) during application of dopamine (10 μM); (4) after washout of dopamine; (5) during pre-application of raclopride (300 nM; 10 minutes); (6) during co-application of raclopride (300 nM) and dopamine (10 μM); (7) after pre-application of SCH 23390 (300 nM; 15 minutes); and (8) during application of dopamine (10 μM). (B) Bar chart showing the percentage by which dopamine (3-10 μM) suppresses the fast and the late EPSCs under basal conditions and in the presence of the D2 antagonist, raclopride (300 nM – 1 μM; N = 4), or after application of the D1/D5 antagonist SCH23390 (300 nM; 10 minutes; N = 5). Dopamine significantly suppressed the late EPSC under basal conditions and in the presence of raclopride; * denotes P < 0.01. Except for illustration in part (A) of this figure, raclopride and SCH23390 were not used in the same slices. (C) Bar chart shows the percentage by which the respective D2 and D1/D5 agonists, quinpirole (quin; 1 μM; 10 minutes; N = 4) and SKF 38393 (SKF; 100 nM; 4 minutes; N = 10) suppress the fast and the late EPSCs under basal conditions and in the presence of antagonists. Only SKF 38393 suppressed evoked network activity or late EPSCs (* denotes P < 0.001). This suppression was blocked by pre-application of the selective D1/D5 receptor antagonists SCH 23390 (300 nM; 20 minutes; N = 6) or SCH 36199 (1 μM; 15-20 minutes; N = 5).
In agreement with this observation, the D2 family agonist quinpirole (1 μM; 10 minutes; N = 4) did not significantly suppress recurrent network activity (Figure 3C). By contrast, the SKF 38393 suppression of network activity could be completely blocked by the D1/D5 receptor antagonist SCH 23390 (300 nM; 20 minutes; N = 6). Since SCH 23390 can bind to 5-HT2A receptors, we also tested a different D1/D5 antagonist, SCH 39166 (1 μM; 15-20 minutes; N = 5). The latter antagonist is somewhat less potent but has low affinity for 5-HT2A receptors (Alburges et al., 1992). Like SCH 23390, it strongly blocks SKF 38393 suppression of network activity (Fig. 3C).
D1/D5 receptor suppression can be mimicked with 8-Br-cAMP or forskolin
Since it has been suggested that D1/D5 receptors may couple to more than one transduction pathway, we examined the signaling pathway involved in the suppression of recurrent network activity. As shown in Figure 4, directly activating adenylyl cyclase with forskolin (10 μM; 5 minutes; N= 4) mimicked D1/D5 activation in that it was able to suppress recurrent network activity completely. By contrast, 1, 9-dideoxyforskolin – a negative control that does not stimulate adenylyl cyclase – failed to suppress network activity (data not shown; N = 3). To test the effects of a second messenger further along the Gs pathway, we applied a phosphodiesterase-resistant cAMP analog, 8-Br-cAMP (0.5 mM; 5-10 minutes; N= 5). Like forskolin, 8-Br-cAMP potently suppressed network activity, as shown in Figure 4, without significantly altering the fast EPSC (area of fast EPSC before forskolin, 1.8 ± 0.2 nA*ms; after forskolin, 1.9 ± 0.1 nA*ms; paired t test, P = NS; N= 4. Area of fast EPSC before 8-Br-cAMP, 2.5 ± 0.3, after 8-Br-cAMP, 2.1 ± 0.1; paired t test, P = NS; N= 5;). Cyclic AMP can act through the protein kinase A pathway, but also has direct effects in cortex, including enhancement of hyperpolarization activated currents (Rosenkrantz and Johnston, 2006).
Figure 4.
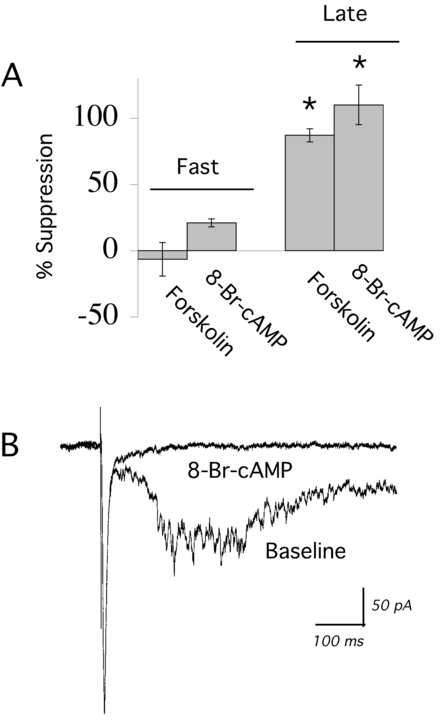
Second messengers in the Gs pathway suppress evoked network activity. (A) Bar chart shows the mean ± S.E. data for the effects of forskolin (direct activator of adenylyl cyclase; 10 μM; 4 minutes; N = 4) and 8-Br-cAMP (0.5 mM; 5-10 minutes; N = 4) on the fast EPSC and evoked network activity (late EPSCs). Both treatments selectively suppressed network activity (* denotes P < 0.01). (B) Averaged sweeps in voltage clamp showing that 8-Br-cAMP (0.5 mM; 5 minutes) suppresses the evoked network activity without changing the fast EPSC.
Mechanism of D1/D5 suppression of recurrent network activity
Since many studies have shown D1 excitation of cortical GABAergic activity (Zhou et al., 1999; Seamans et al., 2001b; Gorelova et al., 2002; Trantham-Davidson et al., 2004; Kroner et al., 2006), we examined whether network activity enhanced by a low dose of bicuculline methiodide would persist in the presence of SKF 38393. These experiments confirmed earlier work showing that a low concentration of bicuculline (1 μM) itself enhances electrically-evoked network activity (Luhmann and Prince, 1990) without triggering epileptiform activity (Aghajanian and Marek, 1999). However, Figure 5 shows that network activity in the presence of bicuculline was readily suppressed by same low concentration of SKF 38393 that suppresses basal or DOI enhanced network activity (100 nM; N = 6).
Figure 5.
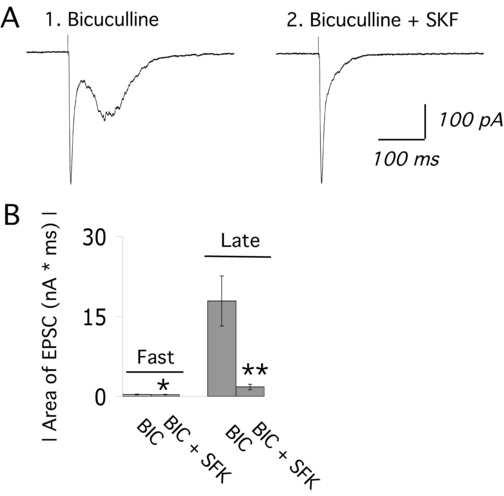
SKF 38393 suppresses network activity in the presence of a low dose of the GABA-A receptor antagonist, bicuculline methiodide. (A1) Averaged traces show the electrically-evoked fast EPSC and network activity in the presence of a low dose of bicuculline (BIC; 1 μM; 3 minutes). (2) In continued presence of bicuculline (1 μM), SKF 38393 (100 nM; 5 min) suppresses network activity but does not alter the fast EPSC in this neuron. (B) The bar chart shows the mean ± S.E. for the area of the fast EPSC and the excitatory component of evoked network activity (late EPSCs) during application of bicuculline alone (1 μM; 3-5 min) and then bicuculline (1μM) co-applied with SKF 38393 (100 nM; 3-5 min). SKF 38393 dramatically reduced network activity (N = 6; paired t test; ** denotes P < 0.01). The fast EPSC was also slightly suppressed by SKF 38393 (N = 6; paired t test; * denotes P = 0.04).
Earlier work from our laboratory has shown that glutamate spillover is critical for recurrent network activity (Lambe and Aghajanian, 2006). Neuronal glutamate transporters have been suggested to be critically important in preventing glutamate from escaping from synapses (Takayasu et al., 2005). Interestingly, surface expression of the neuronal glutamate transporter present in cerebral cortex, EAAT3, is upregulated rapidly through the Gs pathway (Pisano et al. 1996; Guillet et al., 2001). To address the hypothesis that D1/D5 receptors suppress network activity by upregulating glutamate transport and decreasing glutamate spillover, we added a low level of the relatively nonspecific glutamate transporter inhibitor TBOA (6 μM; 5-10 minutes; N=5) to a concentration of D1/D5 agonist able to maximally suppress recurrent network activity (100 nM SKF 38393). In the presence of TBOA, network activity reappeared, despite the continued application of SKF 38393 (Figure 6A). Prior application of TBOA (3-6 μM; 10 minutes), shifted the dose response curve for SKF 38393 to the right by almost two orders of magnitude. TBOA has high affinity for both the neuronal (EAAT3; Ki = 6 μM; Jabaudon et al., 1999) and glial (EAAT2; Ki = 6 μM; Jabaudon et al., 1999) glutamate transporters. By contrast, an inhibitor of glial glutmate transporters, trans-PDC (6-30μM; 10-15 minutes; N = 6) – which has similar affinity for EAAT2 as TBOA (Ki = 16 μM; Koch et al., 1999) but very low affinity for EAAT3 (Ki > 300 μM; Koch et al., 1999) – did not alter the SKF38393 suppression of network activity, as shown in Figure 6. While these results are suggestive that D1 dopamine stimulation may alter glutamate uptake, further investigation is needed to prove this relationship.
Figure 6.
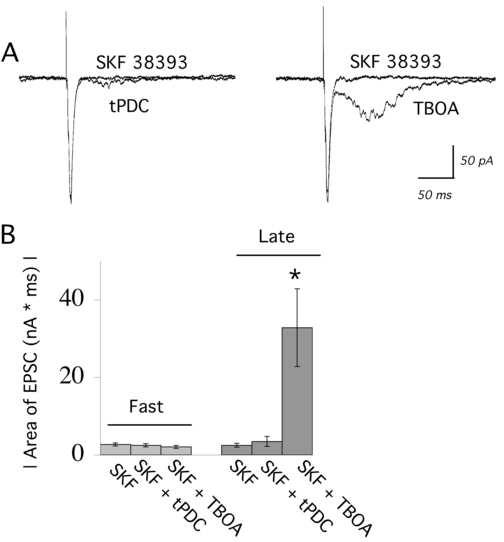
Blocking the neuronal, but not the glial, glutamate transporter can restore evoked network activity in the presence of the D1/D5 stimulation. (A) The superimposed traces on the left show that the glial glutamate transporter inhibitor trans-PDC (6 μM; 10 minutes) cannot restore evoked network activity in the presence of SKF 38393 (100 nM). The traces on the right show that TBOA (6 μM; 5 minutes), which also blocks the neuronal glutamate transporter, can restore evoked network activity in the presence of a maximally-suppressing level of SKF 38393. (B) Bar chart shows the mean ± S.E. for the area of the fast EPSC and the excitatory component of evoked network activity (late EPSCs) during application of SKF 38393 (100 nM) in the presence of trans-PDC (6-30 μM; 10 minutes; N = 6) or TBOA (3-6 μM; 3-10 minutes; N = 5). * denotes P < 0.01.
Discussion
D1/D5 dopamine receptors were found to be remarkably potent modulators of network activity in brain slice of rat prefrontal cortex. While the psychedelic hallucinogen DOI could intensify electrically-evoked recurrent network activity in frequency and duration, DOI-enhanced network activity was still susceptible to D1/D5 suppression. Consistent with the known coupling of D1/D5 receptors through the cyclic AMP pathway, the suppression of recurrent network activity by SKF 38393 was mimicked by forskolin, a direct activator of adenylyl cyclase, and by 8-Br-cAMP, a phosphodiesterase-resistant of cAMP. These findings were surprising since D1/D5 receptors have been suggested to enhance network activity in slice (Beggs and Plenz, 2003; Tseng and O'Donnell, 2005; Bandyopadhyay et al., 2005; Onn et al., 2006). However, these studies show this D1/D5 enhancement in the presence of excitatory agents (such as NMDA or high doses of bicuculline) that override endogenous mechanisms of glutamate uptake. In Tseng and O'Donnell (2005), exogenous NMDA is shown to put the neurons into a constant state of depolarization and spiking (essentially a continuous UP state) and the co-application of SKF 38393 brings in the hyperpolarized, nonresponsive periods (Tseng and O'Donnell, 2005). To our knowledge, this is the first study in vitro to examine the effects of selective D1/D5 receptor activation on recurrent network activity under basal conditions and in the presence of a psychedelic hallucinogen.
Network activity depends on glutamate spillover (Lambe and Aghajanian, 2006), and activation of adenylyl cyclase can decrease synaptic glutamate spillover by rapidly increasing surface expression the neuronal glutamate transporter EAAT3 (Pisano et al., 1996; Guillet et al., 2005). Consistent with this idea, we found that inhibiting neuronal glutamate transporters overcame D1/D5 suppression of network activity in prefrontal cortical slice. This experiment is similar in some ways to the studies that examined D1 effects on network activity after manipulations with high NMDA or bicuculline that override endogenous mechanisms of glutamate uptake (see above). The neuronal glutamate transporter EAAT3 is abundantly present in rat prefrontal cortex (Shashidharan et al., 1997), a region with high levels of 5-HT2A receptors (Willins et al., 1997; Miner et al., 2003) and D1/D5 dopamine receptors (Ciliax et al., 2000; Khan et al., 2000). Recent work has shown that all three of these proteins bind to caveolins (Bhatnagar et al., 2004; Yu et al., 2004; Gonzalez et al., SFN abstract, 2005). Although there is limited evidence that caveolins are present in cortical pyramidal cells, the closely-related flotillins are found in prefrontal cortex (Kokubo et al., 2000). These associations suggest that a macromolecular signaling complex could be involved in the modulation of recurrent network activity.
Several candidate mechanisms
D1/D5 receptors have been shown to have myriad, often contradictory, effects on cortical neurons. Recent work suggests that D1/D5 activation in cerebral cortex can suppress certain aspects of pyramidal neuron excitability by increasing hyperpolarization-activated currents (Rosenkranz and Johnston, 2006) or through decreasing transient sodium currents (Maurice et al., 2001; Peterson et al., 2006). These mechanisms may be more critical for the modulation of network activity than previously suspected. These findings are also consistent with the D1/D5 reduction of efficacy of unitary excitatory neurotransmission shown with dual whole cell recordings (Gao et al., 2001). In addition, D1/D5 dopamine receptors excite prefrontal interneurons in rat (Zhou et al., 1999; Seamans et al., 2001b; Gorelova et al., 2002; Trantham-Davidson et al., 2004) and selectively activate fast-spiking interneurons in monkey (Kroner et al., 2006). Recent work has demonstrated the critical role of interneuron synchrony in generating recurrent activity (Hasenstaub et al., 2005). This work suggests that changing the balance of excitatory and inhibitory synaptic activity would alter network activity. Since glutamate is a precursor for GABA synthesis, another aspect of the explanation could lie in the presence of EAAT3 in interneurons (Sepkuty et al., 2002; Weller et al., SFN abstract, 2005). If EAAT3 upregulation through the G(s) pathway were sufficient to increase GABA production, greater tonic release of GABA by a subpopulation of interneurons could be involved in suppressing network activity. Overall, multiple mechanisms may contribute to the D1/D5 suppression of basal and DOI-enhanced cortical recurrent activity. While our results suggest that D1 dopamine stimulation may alter glutamate uptake, further, further studies are necessary to examine this potential mechanism directly.
Clinical implications
The potency of the dopamine D1/D5 suppression of network activity raises the question of its role at the peak of the inverted “U” relationship – where too little dopamine and too much dopamine each decrease prefrontal cognitive function (Arnsten et al., 1994). Our results suggest that dopamine would modulate the timing and duration of recurrent network activity, perhaps keeping them within a range of normal by balancing other influences that would prolong them (such as 5-HT2A stimulation). This view of dopamine is consistent with the recent proposal that D1 receptor stimulation promotes efficient cortical activation (Winterer and Weinberger, 2004; Mattay et al., 2002), as well as with clinical literature showing that D1/D5 antagonists exacerbate psychosis in people with schizophrenia (see Abi-Dhargham and Laurelle, 2005). Promoting D1/D5 receptor activation in prefrontal cortex has been suggested as a potential adjunct in treatment of schizophrenia (Abi-Dhargham and Laurelle, 2005, Marcus et al., 2005). However, these relationships are far from straightforward. In the rat, infusion of low levels of SKF 38393 and other D1/D5 agonists into medial prefrontal cortex of rat disrupts performance on certain prefrontal tasks (Zahrt et al., 1997) and enhances others (Granon et al., 2000). Understanding the mechanism through which D1/D5 receptors affect signal and noise aspects of network activity will help fine-tune a strategy to improve cognitive deficits in schizophrenia without worsening psychosis.
Supplementary Material
D1/D5 activation can suppress spontaneous network activity in addition to electrically-evoked network activity. In ACSF containing 1 mM Ca2+ and 1 mM Mg2+, spontaneously-occurring network activity can be observed. A continuous voltage clamp recording shows the occurrence of such recurrent activity. SKF 38393 (100 nM; 2 minutes) completely suppresses this spontaneously-occurring network activity. Two sections of the trace are shown at higher magnification.
Acknowledgements
This work was supported by the Kevin Hines NARSAD Young Investigator Award (EKL) and NIMH 17871 (GKA).
List of abbreviations
- 5-HT
5-hydroxytryptamine (serotonin)
- DOI
2,5-dimethoxy-4-iodoamphetamine
- NMDA
N-methyl-D-aspartic acid
- EPSCs
excitatory postsynaptic currents
- IPSCs
inhibitory postsynaptic currents
- ACSF
artificial cerebral spinal fluid
- IR-DIC
infrared differential interference contrast
- EAAT
excitatory amino acid transporter
- TBOA
threo-ß-benzoylaspartic acid
- trans-PDC
trans-pyrrolidine-2,4-dicarboxylate
- BIC
bicuculline methiodide
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abekawa T, Ohmori T, Ito K, Koyama T. D1 dopamine receptor activation reduces extracellular glutamate and GABA concentrations in the medial prefrontal cortex. Brain Res. 2000;867:250–4. doi: 10.1016/s0006-8993(00)02298-8. [DOI] [PubMed] [Google Scholar]
- Abi-Dargham A, Laruelle M. Mechanisms of action of second generation antipsychotic drugs in schizophrenia: insights from brain imaging studies. Eur Psychiatry. 2005;20:15–27. doi: 10.1016/j.eurpsy.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Aghajanian GK, Marek GJ. Serotonin and hallucinogens. Neuropsychopharmacology. 1999;21:16S–23S. doi: 10.1016/S0893-133X(98)00135-3. [DOI] [PubMed] [Google Scholar]
- Alburges ME, Hunt ME, McQuade RD, Wamsley JK. D1-receptor antagonists: comparison of [3H] SCH39166 to [3H]SCH23390. J Chem Neuroanat. 1992;5:357–66. doi: 10.1016/0891-0618(92)90051-q. [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Cai JX, Murphy BL, Goldman-Rakic PS. Dopamine D1 receptor mechanisms in the cognitive performance of young adult and aged monkeys. Psychopharmacology (Berl) 1994;116:143–51. doi: 10.1007/BF02245056. [DOI] [PubMed] [Google Scholar]
- Arnsten AF. Second messenger mechanisms contributing to stress-induced prefrontal cortical dysfunction. Neuropsychopharm (Annual Meeting Supplement) 2006:S53–S54. [Google Scholar]
- Arnt J, Hyttel J, Sánchez C. Partial and full dopamine D1 receptor agonists in mice and rats: relation between behavioural effects and stimulation of adenylate cyclase activity in vitro. Eur J Pharmacol. 1992;213:259–67. doi: 10.1016/0014-2999(92)90690-6. [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay S, Gonzalez-Islas C, Hablitz JJ. Dopamine enhances spatiotemporal spread of activity in rat prefrontal cortex. J Neurophysiol. 2005;93:864–72. doi: 10.1152/jn.00922.2004. [DOI] [PubMed] [Google Scholar]
- Beggs JM, Plenz D. Neuronal avalanches in neocortical circuits. J Neurosci. 2003;23:11167–77. doi: 10.1523/JNEUROSCI.23-35-11167.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beierlein M, Fall CP, Rinzel J, Yuste R. Thalamocortical bursts trigger recurrent activity in neocortical networks: layer 4 as a frequency-dependent gate. J Neurosci. 2002;22:9885–94. doi: 10.1523/JNEUROSCI.22-22-09885.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatnagar A, Sheffler DJ, Kroeze WK, Compton-Toth B, Roth BL. Caveolin-1 interacts with 5-HT2A serotonin receptors and profoundly modulates the signaling of selected Galphaq-coupled protein receptors. J Biol Chem. 2004;279:34614–23. doi: 10.1074/jbc.M404673200. [DOI] [PubMed] [Google Scholar]
- Ciliax BJ, Nash N, Heilman C, Sunahara R, Hartney A, Tiberi M, Rye DB, Caron MG, Niznik HB, Levey AI. Dopamine D(5) receptor immunolocalization in rat and monkey brain. Synapse. 2000;37:125–45. doi: 10.1002/1098-2396(200008)37:2<125::AID-SYN7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Cowan RL, Wilson CJ. Spontaneous firing patterns and axonal projections of single corticostriatal neurons in the rat medial agranular cortex. J Neurophysiol. 1994;71:17–32. doi: 10.1152/jn.1994.71.1.17. [DOI] [PubMed] [Google Scholar]
- Gao WJ, Krimer LS, Goldman-Rakic PS. Presynaptic regulation of recurrent excitation by D1 receptors in prefrontal circuits. Proc Natl Acad Sci U S A. 2001;98:295–300. doi: 10.1073/pnas.011524298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez MI, Krizman-Genda EN, Bergman WK, Fournier KM, Robinson MB. Involvement of caveolin-1 in the regulation of the glutamate transporter EAAC1/EAAT3. SFN. 2005 [Google Scholar]
- Gonzalez-Islas C, Hablitz JJ. Dopamine enhances EPSCs in layer II-III pyramidal neurons in rat prefrontal cortex. J Neurosci. 2003;23:867–75. doi: 10.1523/JNEUROSCI.23-03-00867.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelova N, Seamans JK, Yang CR. Mechanisms of dopamine activation of fast-spiking interneurons that exert inhibition in rat prefrontal cortex. J Neurophysiol. 2002;88:3150–66. doi: 10.1152/jn.00335.2002. [DOI] [PubMed] [Google Scholar]
- Granon S, Passetti F, Thomas KL, Dalley JW, Everitt BJ, Robbins TW. Enhanced and impaired attentional performance after infusion of D1 dopaminergic receptor agents into rat prefrontal cortex. J Neurosci. 2000;20:1208–15. doi: 10.1523/JNEUROSCI.20-03-01208.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillet BA, Velly LJ, Canolle B, Masmejean FM, Nieoullon AL, Pisano P. Differential regulation by protein kinases of activity and cell surface expression of glutamate transporters in neuron-enriched cultures. Neurochem Int. 2005;46:337–46. doi: 10.1016/j.neuint.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Haider B, Duque A, Hasenstaub AR, McCormick DA. Neocortical network activity in vivo is generated through a dynamic balance of excitation and inhibition. J Neurosci. 2006;26:4535–45. doi: 10.1523/JNEUROSCI.5297-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harte M, O'Connor WT. Evidence for a differential medial prefrontal dopamine D1 and D2 receptor regulation of local and ventral tegmental glutamate and GABA release: a dual probe microdialysis study in the awake rat. Brain Res. 2004;1017:120–9. doi: 10.1016/j.brainres.2004.05.027. [DOI] [PubMed] [Google Scholar]
- Hasenstaub A, Shu Y, Haider B, Kraushaar U, Duque A, McCormick DA. Inhibitory postsynaptic potentials carry synchronized frequency information in active cortical networks. Neuron. 2005;47:423–35. doi: 10.1016/j.neuron.2005.06.016. [DOI] [PubMed] [Google Scholar]
- Jabaudon D, Shimamoto K, Yasuda-Kamatani Y, Scanziani M, Gähwiler BH, Gerber U. Inhibition of uptake unmasks rapid extracellular turnover of glutamate of nonvesicular origin. Proc Natl Acad Sci U S A. 1999;96:8733–8. doi: 10.1073/pnas.96.15.8733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan ZU, Gutiérrez A, Martín R, Peñafiel A, Rivera A, de la Calle A. Dopamine D5 receptors of rat and human brain. Neuroscience. 2000;100:689–99. doi: 10.1016/s0306-4522(00)00274-8. [DOI] [PubMed] [Google Scholar]
- Koch HP, Kavanaugh MP, Esslinger CS, Zerangue N, Humphrey JM, Amara SG, Chamberlin AR, Bridges RJ. Differentiation of substrate and nonsubstrate inhibitors of the high-affinity, sodium-dependent glutamate transporters. Mol Pharmacol. 1999;56:1095–104. doi: 10.1124/mol.56.6.1095. [DOI] [PubMed] [Google Scholar]
- Kokubo H, Lemere CA, Yamaguchi H. Localization of flotillins in human brain and their accumulation with the progression of Alzheimer's disease pathology. Neurosci Lett. 2000;290:93–6. doi: 10.1016/s0304-3940(00)01334-3. [DOI] [PubMed] [Google Scholar]
- Kröner S, Krimer LS, Lewis DA, Barrionuevo G. Dopamine Increases Inhibition in the Monkey Dorsolateral Prefrontal Cortex through Cell Type-Specific Modulation of Interneurons. Cereb Cortex. 2006 doi: 10.1093/cercor/bhl012. [DOI] [PubMed] [Google Scholar]
- Lambe EK, Aghajanian GK. Hallucinogen-Induced UP States in the Brain Slice of Rat Prefrontal Cortex: Role of Glutamate Spillover and NR2B-NMDA Receptors. Neuropsychopharmacology. 2006;31:1682–9. doi: 10.1038/sj.npp.1300944. [DOI] [PubMed] [Google Scholar]
- Lavin A, Moore HM, Grace AA. Prenatal disruption of neocortical development alters prefrontal cortical neuron responses to dopamine in adult rats. Neuropsychopharmacology. 2005;30:1426–35. doi: 10.1038/sj.npp.1300696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis BL, O'Donnell P. Ventral tegmental area afferents to the prefrontal cortex maintain membrane potential ‘up’ states in pyramidal neurons via D(1) dopamine receptors. Cereb Cortex. 2000;10:1168–75. doi: 10.1093/cercor/10.12.1168. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–24. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- Luhmann HJ, Prince DA. Transient expression of polysynaptic NMDA receptor-mediated activity during neocortical development. Neurosci Lett. 1990;111:109–15. doi: 10.1016/0304-3940(90)90353-b. [DOI] [PubMed] [Google Scholar]
- Marcus MM, Jardemark KE, Wadenberg ML, Langlois X, Hertel P, Svensson TH. Combined alpha2 and D2/3 receptor blockade enhances cortical glutamatergic transmission and reverses cognitive impairment in the rat. Int J Neuropsychopharmacol. 2005;8:315–27. doi: 10.1017/S1461145705005328. [DOI] [PubMed] [Google Scholar]
- Maurice N, Tkatch T, Meisler M, Sprunger LK, Surmeier DJ. D1/D5 dopamine receptor activation differentially modulates rapidly inactivating and persistent sodium currents in prefrontal cortex pyramidal neurons. J Neurosci. 2001;21:2268–77. doi: 10.1523/JNEUROSCI.21-07-02268.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattay VS, Berman KF, Ostrem JL, Esposito G, Van Horn JD, Bigelow LB, Weinberger DR. Dextroamphetamine enhances “neural network-specific” physiological signals: a positron-emission tomography rCBF study. J Neurosci. 1996;16:4816–22. doi: 10.1523/JNEUROSCI.16-15-04816.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattay VS, Tessitore A, Callicott JH, Bertolino A, Goldberg TE, Chase TN, Hyde TM, Weinberger DR. Dopaminergic modulation of cortical function in patients with Parkinson's disease. Ann Neurol. 2002;51:156–64. doi: 10.1002/ana.10078. [DOI] [PubMed] [Google Scholar]
- Mattay VS, Goldberg TE, Fera F, Hariri AR, Tessitore A, Egan MF, Kolachana B, Callicott JH, Weinberger DR. Catechol O-methyltransferase val158-met genotype and individual variation in the brain response to amphetamine. Proc Natl Acad Sci U S A. 2003;100:6186–91. doi: 10.1073/pnas.0931309100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, Shu Y, Hasenstaub A, Sanchez-Vives M, Badoual M, Bal T. Persistent cortical activity: mechanisms of generation and effects on neuronal excitability. Cereb Cortex. 2003;13:1219–31. doi: 10.1093/cercor/bhg104. [DOI] [PubMed] [Google Scholar]
- McCormick DA. Neuronal networks: flip-flops in the brain. Curr Biol. 2005;15:R294–6. doi: 10.1016/j.cub.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Miner LA, Backstrom JR, Sanders-Bush E, Sesack SR. Ultrastructural localization of serotonin2A receptors in the middle layers of the rat prelimbic prefrontal cortex. Neuroscience. 2003;116:107–17. doi: 10.1016/s0306-4522(02)00580-8. [DOI] [PubMed] [Google Scholar]
- Muschamp JW, Regina MJ, Hull EM, Winter JC, Rabin RA. Lysergic acid diethylamide and [−]-2,5-dimethoxy-4-methylamphetamine increase extracellular glutamate in rat prefrontal cortex. Brain Res. 2004;1023:134–40. doi: 10.1016/j.brainres.2004.07.044. [DOI] [PubMed] [Google Scholar]
- Onn SP, Wang XB, Lin M, Grace AA. Dopamine D1 and D4 receptor subtypes differentially modulate recurrent excitatory synapses in prefrontal cortical pyramidal neurons. Neuropsychopharmacology. 2006;31:318–38. doi: 10.1038/sj.npp.1300829. [DOI] [PubMed] [Google Scholar]
- Petersen CC, Hahn TT, Mehta M, Grinvald A, Sakmann B. Interaction of sensory responses with spontaneous depolarization in layer 2/3 barrel cortex. Proc Natl Acad Sci U S A. 2003;100:13638–43. doi: 10.1073/pnas.2235811100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson JD, Wolf ME, White FJ. Repeated amphetamine administration decreases D1 dopamine receptor-mediated inhibition of voltage-gated sodium currents in the prefrontal cortex. J Neurosci. 2006;26:3164–8. doi: 10.1523/JNEUROSCI.2375-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisano P, Samuel D, Nieoullon A, Kerkerian-Le Goff L. Activation of the adenylate cyclase-dependent protein kinase pathway increases high affinity glutamate uptake into rat striatal synaptosomes. Neuropharmacology. 1996;35:541–7. doi: 10.1016/0028-3908(96)84624-7. [DOI] [PubMed] [Google Scholar]
- Rosenkranz JA, Johnston D. Dopaminergic regulation of neuronal excitability through modulation of Ih in layer V entorhinal cortex. J Neurosci. 2006;26:3229–44. doi: 10.1523/JNEUROSCI.4333-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scruggs JL, Schmidt D, Deutch AY. The hallucinogen 1-[2,5-dimethoxy-4-iodophenyl]-2-aminopropane (DOI) increases cortical extracellular glutamate levels in rats. Neurosci Lett. 2003;346:137–40. doi: 10.1016/s0304-3940(03)00547-0. [DOI] [PubMed] [Google Scholar]
- Seamans JK, Durstewitz D, Christie BR, Stevens CF, Sejnowski TJ. Dopamine D1/D5 receptor modulation of excitatory synaptic inputs to layer V prefrontal cortex neurons. Proc Natl Acad Sci U S A. 2001a;98:301–6. doi: 10.1073/pnas.011518798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seamans JK, Gorelova N, Durstewitz D, Yang CR. Bidirectional dopamine modulation of GABAergic inhibition in prefrontal cortical pyramidal neurons. J Neurosci. 2001b;21:3628–38. doi: 10.1523/JNEUROSCI.21-10-03628.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepkuty JP, Cohen AS, Eccles C, Rafiq A, Behar K, Ganel R, Coulter DA, Rothstein JD. A neuronal glutamate transporter contributes to neurotransmitter GABA synthesis and epilepsy. J Neurosci. 2002;22:6372–9. doi: 10.1523/JNEUROSCI.22-15-06372.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shashidharan P, Huntley GW, Murray JM, Buku A, Moran T, Walsh MJ, Morrison JH, Plaitakis A. Immunohistochemical localization of the neuron-specific glutamate transporter EAAC1 (EAAT3) in rat brain and spinal cord revealed by a novel monoclonal antibody. Brain Res. 1997;773:139–48. doi: 10.1016/s0006-8993(97)00921-9. [DOI] [PubMed] [Google Scholar]
- Shu Y, Hasenstaub A, McCormick DA. Turning on and off recurrent balanced cortical activity. Nature. 2003;423:288–93. doi: 10.1038/nature01616. [DOI] [PubMed] [Google Scholar]
- Steriade M, Nuñez A, Amzica F. A novel slow (< 1 Hz) oscillation of neocortical neurons in vivo: depolarizing and hyperpolarizing components. J Neurosci. 1993;13:3252–65. doi: 10.1523/JNEUROSCI.13-08-03252.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stutzmann GE, Marek GJ, Aghajanian GK. Adenosine preferentially suppresses serotonin2A receptor-enhanced excitatory postsynaptic currents in layer V neurons of the rat medial prefrontal cortex. Neuroscience. 2001;105:55–69. doi: 10.1016/s0306-4522(01)00170-1. [DOI] [PubMed] [Google Scholar]
- Takayasu Y, Iino M, Kakegawa W, Maeno H, Watase K, Wada K, Yanagihara D, Miyazaki T, Komine O, Watanabe M, Tanaka K, Ozawa S. Differential roles of glial and neuronal glutamate transporters in Purkinje cell synapses. J Neurosci. 2005;25:8788–93. doi: 10.1523/JNEUROSCI.1020-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trantham-Davidson H, Neely LC, Lavin A, Seamans JK. Mechanisms underlying differential D1 versus D2 dopamine receptor regulation of inhibition in prefrontal cortex. J Neurosci. 2004;24:10652–9. doi: 10.1523/JNEUROSCI.3179-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng KY, O'Donnell P. Post-pubertal emergence of prefrontal cortical up states induced by D1-NMDA co-activation. Cereb Cortex. 2005;15:49–57. doi: 10.1093/cercor/bhh107. [DOI] [PubMed] [Google Scholar]
- Wang J, O'Donnell P. D(1) dopamine receptors potentiate nmda-mediated excitability increase in layer V prefrontal cortical pyramidal neurons. Cereb Cortex. 2001;11:452–62. doi: 10.1093/cercor/11.5.452. [DOI] [PubMed] [Google Scholar]
- Wang M, Vijayraghavan S, McCormick D, Mazer J, Arnsten A. Alpha2Aadrenoreceptor stimulation strengthens working memory networks by inhibiting cAMP production and closing HCN channels in prefrontal cortex; SFN conference abstract; Atlanta GA. 2006. [Google Scholar]
- Weller ML, Stone IM, York AM, Wells SM, Rhoderick JF, Poulsen DJ. Modulation of GABA synthesis and inhibitory response by altered EAAT3 expression in GABAergic neurons. SFN. 2005 [Google Scholar]
- Williams GV, Goldman-Rakic PS. Modulation of memory fields by dopamine D1 receptors in prefrontal cortex. Nature. 1995;376:572–5. doi: 10.1038/376572a0. [DOI] [PubMed] [Google Scholar]
- Willins DL, Deutch AY, Roth BL. Serotonin 5-HT2A receptors are expressed on pyramidal cells and interneurons in the rat cortex. Synapse. 1997;27:79–82. doi: 10.1002/(SICI)1098-2396(199709)27:1<79::AID-SYN8>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Winterer G, Weinberger DR. Genes, dopamine and cortical signal-to-noise ratio in schizophrenia. Trends Neurosci. 2004;27:683–90. doi: 10.1016/j.tins.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Yu P, Yang Z, Jones JE, Wang Z, Owens SA, Mueller SC, Felder RA, Jose PA. D1 dopamine receptor signaling involves caveolin-2 in HEK-293 cells. Kidney Int. 2004;66:2167–80. doi: 10.1111/j.1523-1755.2004.66007.x. [DOI] [PubMed] [Google Scholar]
- Zahrt J, Taylor JR, Mathew RG, Arnsten AF. Supranormal stimulation of D1 dopamine receptors in the rodent prefrontal cortex impairs spatial working memory performance. J Neurosci. 1997;17:8528–35. doi: 10.1523/JNEUROSCI.17-21-08528.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou FM, Hablitz JJ. Dopamine modulation of membrane and synaptic properties of interneurons in rat cerebral cortex. J Neurophysiol. 1999;81:967–76. doi: 10.1152/jn.1999.81.3.967. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
D1/D5 activation can suppress spontaneous network activity in addition to electrically-evoked network activity. In ACSF containing 1 mM Ca2+ and 1 mM Mg2+, spontaneously-occurring network activity can be observed. A continuous voltage clamp recording shows the occurrence of such recurrent activity. SKF 38393 (100 nM; 2 minutes) completely suppresses this spontaneously-occurring network activity. Two sections of the trace are shown at higher magnification.


