Abstract
We designed this retrospective study to evaluate the effectiveness of percutaneous approaches for repair of paraanastomotic aneurysms that develop after surgical aortic reconstruction.
The catheterization records of patients who had undergone percutaneous repair of para-anastomotic aneurysms from January 2001 through December 2005 were reviewed, and data regarding preoperative aneurysm size, risk factors, intraoperative techniques, morbidity, and death were recorded.
Eight patients had undergone exclusion of a total of 10 paraanastomotic aneurysms. The average age of the prosthetic graft at diagnosis was 11.7 years. Four of the patients were symptomatic; none of these had a ruptured aneurysm. All patients received commercially available devices. Technical success was achieved in all patients. Conscious sedation alone was administered to 7 patients. There were no in-hospital deaths, and morbidity was minimal.
We conclude that endovascular exclusion of paraanastomotic aneurysms after aortic reconstruction is a viable alternative to open surgical repair and greatly reduces the risk of morbidity and death.
Key words: Anastomosis, surgical/adverse effects; aneurysm, false/etiology; aneurysm, para-anastomotic/therapy; aortic aneurysm, abdominal/diagnosis/etiology/therapy; blood vessel prosthesis/adverse effects; endovascular repair; graft occlusion, vascular/etiology/therapy; postoperative complications; time factors
Paraanastomotic aneurysms of the abdominal aorta are an under-recognized complication of aortic reconstructive surgery. Historically, surgical management of these aneurysms has been fraught with risk of complications and death. However, as endovascular techniques have advanced, new methods of excluding paraanastomotic aneurysms have been developed. We describe the characteristics, treatment, and outcomes of 8 patients who underwent successful percutaneous treatment at our institution for paraanastomotic aneurysms. In addition, we review the English-language medical literature concerning paraanastomotic aneurysms and their treatment.
Methods
The peripheral catheterization records for our hospital were reviewed retrospectively for cases of paraanastomotic aneurysms repaired percutaneously from January 2001 through December 2005. Procedures to repair true aneurysms, pseudoaneurysms, or both were included. Patients' records were reviewed to obtain information on the preoperative characteristics of the aneurysm, risk factors, and intraoperative proceedings. In-hospital morbidity and death were noted. Follow-up information, when available, was recorded.
Results
During the 5-year period that we studied, 8 patients underwent percutaneous exclusion of a total of 10 paraanastomotic aneurysms. The patients' mean age was 66.5 years (range, 50–75 yr) (Table I). Six patients had undergone initial surgical repair for abdominal aortic aneurysms (AAAs) and 2 for vascular stenoses (Table II). The average age of the grafts at the time of diagnosis was 11.7 years. The initial surgical procedures included 5 aortobiiliac bypasses and 3 aortobifemoral bypasses. Half of the patients had coronary artery disease, and all were judged to be at high risk.
TABLE I. Baseline Characteristics of 8 Patients Who Underwent Endovascular Repair of Paraanastomotic Aneurysms
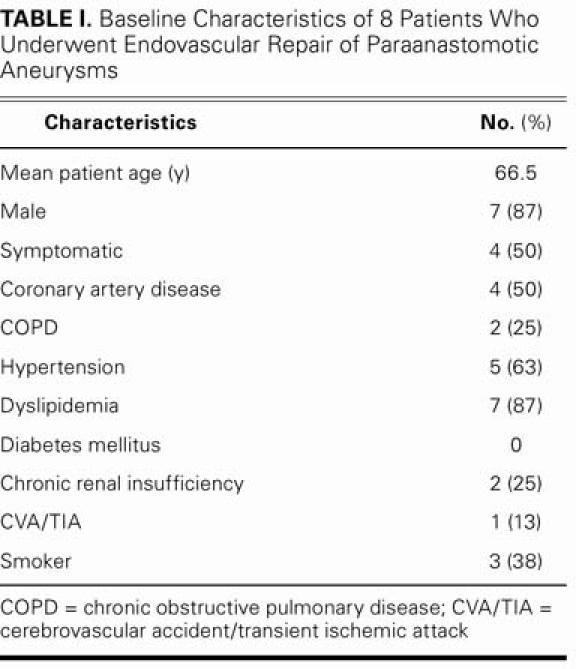
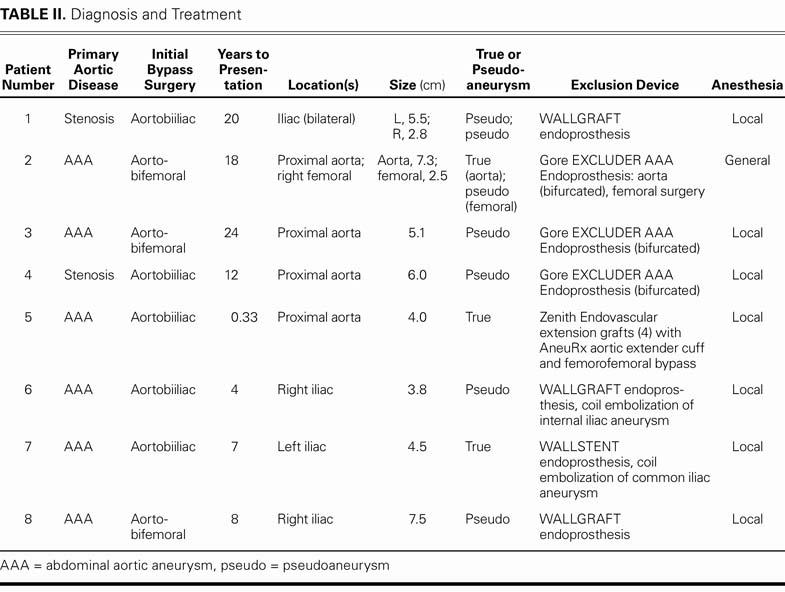
TABLE II. Diagnosis and Treatment
At the time of diagnosis, 4 of 8 patients reported symptoms. Of these, the 1st patient presented with ureteral obstruction and secondary renal failure, the 2nd with atheroemboli to the feet, the 3rd with radiating back pain, and the 4th with a nonhealing amputation, along with chills and fever. In the 4 asymptomatic patients, aneurysms were discovered incidentally on routine screening examination. Computed tomography, ultrasonography, or both revealed no active rupture of the aneurysms in any of the patients.
A total of 10 aneurysms were treated: 7 pseudoaneurysms and 3 true aneurysms. Two patients had 2 aneu-rysms: 1 patient who had previously undergone aorto-bifemoral bypass had a true aneurysm at the proximal anastomosis and a pseudoaneurysm at the femoral anastomosis. Another patient had bilateral iliac pseudoaneurysms at the site of anastomosis.
Procedure Characteristics
All 8 patients underwent aneurysm exclusion with commercial devices. Three patients received a bifurcated stent graft (Excluder® AAA Endoprosthesis, W.L. Gore & Associates, Inc.; Flagstaff, Ariz). One received 4 serial Zenith® aorto-mono-iliac tube extension grafts (Cook Medical, Inc.; Bloomington, Ind) with an AneuRx® aortic extender cuff (Medtronic, Inc, Minneapolis, Minn). Three patients were treated with Wallgraft® endoprostheses, and 1 patient was treated with a Wallstent® endoprosthesis (Boston Scientific Corporation; Natick, Mass). Seven of the patients were under conscious sedation alone during the procedure. General anesthesia was required in the patient who had both proximal and distal aneurysms. After percutaneous exclusion of the proximal site, the patient underwent surgical closure of the femoral aneurysm and of the opposite access site.
Angiographically documented aneurysmal exclusion was achieved in all patients. No death or residual endoleak was reported. Minimal complications included an access-site hematoma in 1 patient and the requirement of a 3-unit transfusion of packed red blood cells for the patient who underwent open femoral repair.
Representative Case
A 50-year-old man presented at his local hospital because of abdominal pain that radiated to his back. Testing revealed a ruptured AAA (Table II, Patient 5). Emergency resection of the aneurysm and an aortobiiliac bypass were performed. Two days postoperatively, the patient developed pain in his left leg, with isch-emic changes. At surgical re-exploration, acute thrombosis of the left aortoiliac bypass graft was discovered. The patient underwent above-the-knee amputation of the left leg because of surgical complications. His right iliac conduit remained intact.
Three months postoperatively, the patient continued to have episodes of fever, chills, and night sweats. The amputation stump had failed to heal completely, and residual ulceration was present. After presenting at our institution for evaluation of the nonhealing ulcer, the patient underwent computed tomographic (CT) angiography, which revealed distal occlusion of the native aorta and a patent unilateral aortic-to-right-iliac bypass graft. At the site of the proximal anastomosis, there was residual aneurysmal dilation of 4 cm. The left iliac artery bypass graft was occluded, although some flow reconstituted at the internal–external bifurcation via contralateral collateral vessels (Fig. 1).
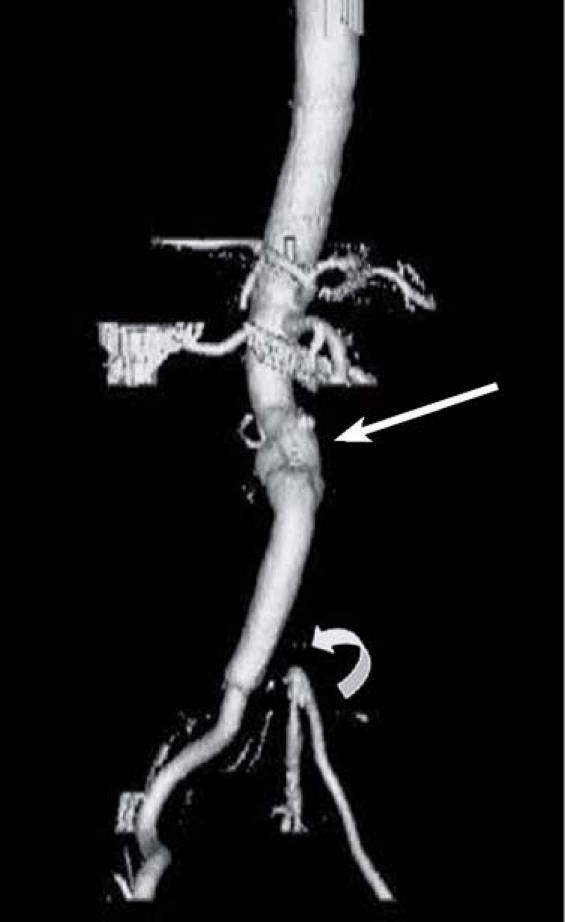
Fig. 1 Patient 5. Initial computed tomographic angiogram reveals distal occlusion of the native aorta and a patent unilateral aortic-to-right-iliac bypass graft. At the proximal anastomosis, there is residual aneurysmal dilation of 4 cm (arrow). Thrombus is present in the left bypass graft, which is not visible (curved arrow).
At the cardiac catheterization laboratory, an initial angiogram revealed the paraanastomotic aneurysm and occlusion of the left aortoiliac bypass graft (Fig. 2). With the patient under local anesthesia and conscious sedation, the following Zenith (Cook) exclusion devices were placed proximally to distally: a 24 × 55-mm iliac leg extension, a 22 × 55-mm iliac leg extension, a 22 × 36-mm main body extension, and a 24 × 36-mm main body extension. After balloon dilation, a type III endoleak was found between the 1st and 2nd exclusion devices. The endoleak was successfully sealed by placement of a 24 × 37.5-mm AneuRx extender cuff (Medtronic) and repeat balloon dilation (Fig. 3). A sub-sequent femorofemoral bypass was performed to pro-vide flow to the nonhealing stump. The patient's sub-sequent hospital course was uncomplicated.
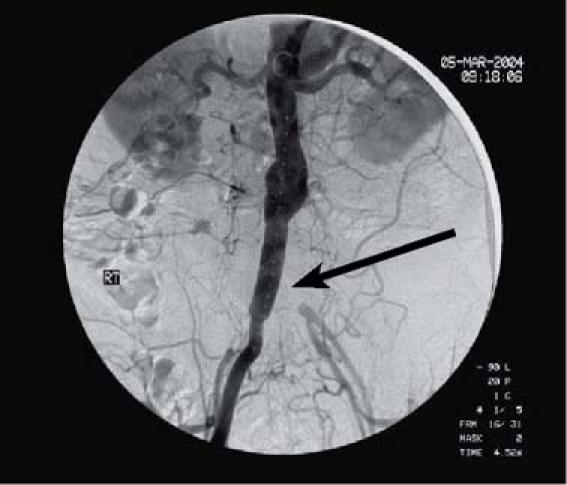
Fig. 2 Patient 5. Initial angiogram shows para-anastomotic aneurysmal dilation, with occlusion of the left aortoiliac bypass graft (arrow).
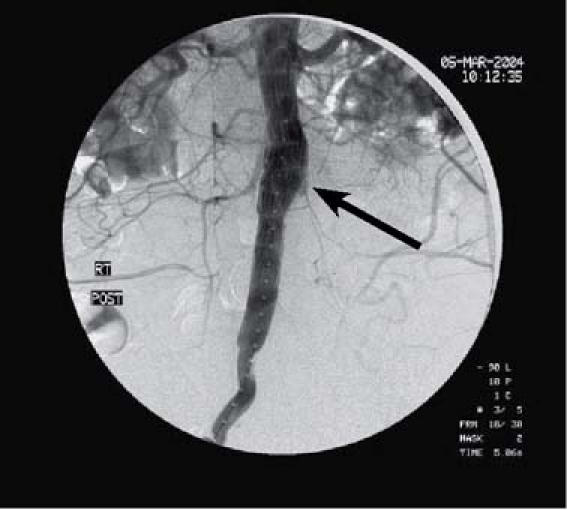
Fig. 3 Patient 5. Angiogram reveals exclusion of the para-anas-tomotic aneurysm after deployment of 4 Zenith (Cook) aortic extension grafts. An AneuRx cuff (Medtronic) was placed to seal a residual type III endoleak (arrow).
At 3-month follow-up, the patient reported no further fever or chills, and the amputation stump had healed. Repeat CT angiography revealed a patent femorofemoral bypass graft, no residual endoleak, and no re-formation of the paraanastomotic aneurysm (Fig. 4).
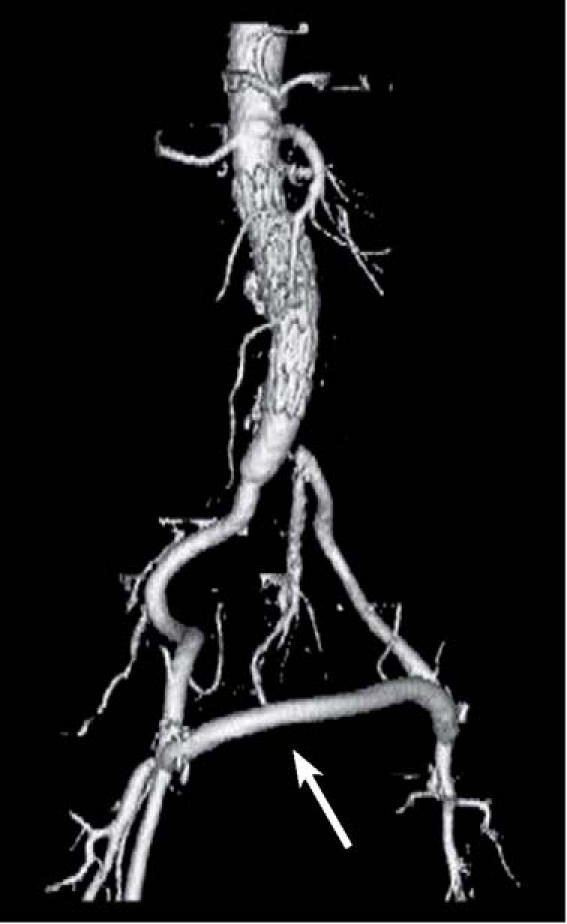
Fig. 4 Patient 5. Computed tomographic angiogram at 3-month follow-up shows a patent femorofemoral bypass graft (arrow) and no recurrence of aneurysm or endoleak.
Discussion
Paraanastomotic aneurysms of the abdominal aorta after aortic reconstructive surgery are under-recognized complications that can lead to morbidity and death. Such aneurysms can be true or false. True aneurysms, which have a residual aortic wall, most often occur after inadequate resection of an AAA; the remnant of the diseased vessel may continue to enlarge over time.1–3 Pseudoaneurysms, on the other hand, result from disruption of the graft-to-vessel anastomosis, and the actual defect is found within the arterial wall.2,4–8 Historically, pseudoaneurysms were believed to be related to the use of silk suture material; however, it is now known that the use of any suture material can lead to their development.4
The reported prevalence of all paraanastomotic aneurysms ranges from 2% to 29%.6,8–11 A study by Edwards and colleagues11 reported a 5% prevalence at 8-year follow-up and 30% at 15 years. False aneurysms are believed to be 2 to 3 times more common than true aneurysms.
Paraanastomotic aneurysms are associated with high rupture and mortality rates. Without surgery, the reported rupture rates range from 15% to 55%.5,12–14 Intraoperative mortality rates vary widely, depending on whether the procedure is elective or emergent. Elective repairs have mortality rates ranging from 4.5% to 17%, whereas emergency procedures can have mortality rates from 24% to 100%.1,5,7,8,11,13–15 Because the average time to diagnosis of an aneurysm is 8 years8,15 (11.7 years in our study), patients who are already at high operative risk have a long time for their underlying cardiac, respiratory, or renal disease to progress. Mulder and co-authors15 reported a postoperative morbidity rate of 53% (82/156 patients).
Because of the high mortality rates associated with open surgery, endovascular repair can be a safer option. Previous studies have found low perioperative mortality rates with primary endovascular aortic exclusion of AAAs.16,17 In the medical literature, there are few retrospective reports concerning endovascular repair of paraanastomotic aneurysms that form after aortic reconstruction. Two of the largest studies, performed by Yuan and associates18 and van Herwaarden and coworkers,19 involved a total of 24 patients with true or false paraanastomotic aortic or iliac aneurysms that had developed after aortic reconstruction. All patients were successfully treated by endograft exclusion: the 10 patients in the Yuan study12 were treated with homemade devices, and the 14 patients in van Herwaarden's study19 received commercially available prostheses. For these 24 patients, the mean operative time was 147 minutes, and the mean estimated blood loss was 350 mL. No perioperative deaths occurred. Three of the 24 patients had postoperative morbidity from hematoma, myocardial infarction, or pneumonia. During a combined average follow-up period of 13.4 months, there were 4 documented deaths, but only 1 was related to aneurysm exclusion. The patient who died had a type I endoleak that resulted in conversion to open repair.19 The surgery was complicated by perioperative mesenteric ischemia that ultimately led to death. Another patient in that series had an endoleak that necessitated conversion to open repair, but that procedure was successful. Both patients received tube-graft endoprostheses. All 10 grafts used by Yuan and colleagues were tube grafts, and no endoleaks were reported.18 Patients in the van Herwaarden study who received bifurcated stent grafts (11/14) had no episodes of migration.19
In our experience, results of endovascular repair have been excellent. Although we cannot comment on treatment in patients with pending rupture (radiographically or angiographically documented), we note that there were no deaths among our patients. Morbidity was similarly low: 1 patient required transfusions, and a 2nd patient had a postprocedural hematoma.
It is likely that our aggressive use of local anesthesia and the application of percutaneous access and closure procedures have contributed greatly to the low morbidity and mortality rates. Since May of 2002, 482 patients have undergone primary percutaneous AAA repair under local anesthesia at our institution, with a technical success rate of 100%. One procedure-related death occurred secondary to respiratory failure. The benefits of avoiding general anesthesia and open repair in patients with multiple comorbidities have included an average of 1 hour for patients to return to a normal diet and an average of 8 ± 3 hours for them to begin walking.*
When compared with prior endovascular aortic exclusion procedures using general anesthesia and surgical cutdown (339 procedures performed at our institution**), the use of local anesthesia and percutaneous repair has reduced total procedure time from 237 to 142 minutes, decreased hematocrit loss from 8.7% to 4.1%, and shortened the length of hospital stay from 2.0 to 1.1 days. In addition, given results from prior studies16,20 involving open surgical repair of primary AAAs, the advantages of the use of local anesthesia, endovascular repair, and percutaneous closure are clear: return to a normal diet, 4.9 days; time to ambulation, 3.6 days20; average blood loss, 1.6 L; and mean length of hospital stay, 13 days.16
Advances in commercially available devices for primary endovascular aneurysm repair are making the exclusion of paraanastomotic aneurysms much easier, and access-site morbidity rates are decreasing. Whereas previous homemade devices required large sheath sizes and surgical cutdown for delivery, the newest generation of devices can be delivered percutaneously and closed with little need for operative repair.20,21
Conclusion
Our experience and that of others has shown that endovascular exclusion of paraanastomotic aneurysms is a viable alternative to open surgical repair. The procedure has several advantages, including the avoidance of reoperation on a surgically “hostile” abdomen, avoidance of general anesthesia, and reduced intraoperative blood loss. Most important, these experiences suggest that the endovascular approach dramatically reduces perioperative mortality rates. We conclude that endovascular exclusion of paraanastomotic aneurysms after aortic reconstruction should be considered a 1st-line treatment option by cardiologists and vascular surgeons.
Footnotes
*Unpublished data from our institution; May 2002–present
**Unpublished data from our institution; November 1998–May 2002
Address for reprints: Zvonimir Krajcer, MD, 6624 Fannin, #2780, Houston, TX 77030. E-mail: ZvonkoMD@aol.com
References
- 1.Curl GR, Faggioli GL, Ricotta JJ. Proximal para-anastomotic aortic aneurysm. In: Veith FJ, editor. Current critical problems in vascular surgery. Vol 5. St. Louis: Quality Medical Publishing; 1993. p. 240–6.
- 2.Allen RC, Schneider J, Longenecker L, Smith RB 3rd, Lumsden AB. Paraanastomotic aneurysms of the abdominal aorta. J Vasc Surg 1993;18:424–32. [PubMed]
- 3.Sonesson B, Resch T, Lanne T, Ivancev K. The fate of the infrarenal aortic neck after open aneurysm surgery. J Vasc Surg 1998;28:889–94. [DOI] [PubMed]
- 4.Starr DS, Weatherford SC, Lawrie GM, Morris GC Jr. Suture material as a factor in the occurrence of anastomotic false aneurysms. An analysis of 26 cases. Arch Surg 1979;114:412–5. [DOI] [PubMed]
- 5.Curl GR, Faggioli GL, Stella A, D'Addato M, Ricotta JJ. Aneurysmal change at or above the proximal anastomosis after infrarenal aortic grafting. J Vasc Surg 1992;16:855–60. [PubMed]
- 6.van den Akker PJ, Brand R, van Schilfgaarde R, van Bockel JH, Terpstra JL. False aneurysms after prosthetic reconstructions for aortoiliac obstructive disease. Ann Surg 1989;210: 658–66. [DOI] [PMC free article] [PubMed]
- 7.Szilagyi DE, Elliott JP Jr, Smith RF, Reddy DJ, McPharlin M. A thirty-year survey of the reconstructive surgical treatment of aortoiliac occlusive disease. J Vasc Surg 1986;3:421–36. [DOI] [PubMed]
- 8.Locati P, Socrate AM, Costantini E. Paraanastomotic aneurysms of the abdominal aorta: a 15-year experience review. Cardiovasc Surg 2000;8:274–9. [DOI] [PubMed]
- 9.den Hoed PT, Veen HF. The late complications of aorto-ilio-femoral Dacron prostheses: dilatation and anastomotic aneurysm formation. Eur J Vasc Surg 1992;6:282–7. [DOI] [PubMed]
- 10.Sieswerda C, Skotnicki SH, Barentsz JO, Heystraten FM. Anastomotic aneurysms–an underdiagnosed complication after aorto-iliac reconstructions. Eur J Vasc Surg 1989;3:233–8. [DOI] [PubMed]
- 11.Edwards JM, Teefey SA, Zierler RE, Kohler TR. Intraabdominal paraanastomotic aneurysms after aortic bypass grafting. J Vasc Surg 1992;15:344–53. [PubMed]
- 12.Crawford ES, Beckett WC, Greer MS. Juxtarenal infrarenal abdominal aortic aneurysm. Special diagnostic and therapeutic considerations. Ann Surg 1986;203:661–70. [DOI] [PMC free article] [PubMed]
- 13.Plate G, Hollier LA, O'Brien P, Pairolero PC, Cherry KJ, Kazmier FJ. Recurrent aneurysms and late vascular complications following repair of abdominal aortic aneurysms. Arch Surg 1985;120:590–4. [DOI] [PubMed]
- 14.Treiman GS, Weaver FA, Cossman DV, Foran RF, Cohen JL, Levin PM, Treiman RL. Anastomotic false aneurysms of the abdominal aorta and the iliac arteries. J Vasc Surg 1988; 8:268–73. [PubMed]
- 15.Mulder EJ, van Bockel JH, Maas J, van den Akker PJ, Hermans J. Morbidity and mortality of reconstructive surgery of noninfected false aneurysms detected long after aortic prosthetic reconstruction. Arch Surg 1998;133:45–9. [DOI] [PubMed]
- 16.Prinssen M, Verhoeven EL, Buth J, Cuypers PW, van Sambeek MR, Balm R, et al. A randomized trial comparing conventional and endovascular repair of abdominal aortic aneurysms. N Engl J Med 2004;351:1607–18. [DOI] [PubMed]
- 17.Howell MH, Strickman N, Mortazavi A, Hallman CH, Krajcer Z. Preliminary results of endovascular abdominal aortic aneurysm exclusion with the AneuRx stent-graft. J Am Coll Cardiol 2001;38:1040–6. [DOI] [PubMed]
- 18.Yuan JG, Marin ML, Veith FJ, Ohki T, Sanchez LA, Suggs WD, et al. Endovascular grafts for noninfected aortoiliac anastomotic aneurysms. J Vasc Surg 1997;26:210–21. [DOI] [PubMed]
- 19.van Herwaarden JA, Waasdorp EJ, Bendermacher BL, van den Berg JC, Teijink JA, Moll FL. Endovascular repair of paraanastomotic aneurysms after previous open aortic prosthetic reconstruction. Ann Vasc Surg 2004;18:280–6. [DOI] [PubMed]
- 20.Zarins CK, White RA, Schwarten D, Kinney E, Diethrich EB, Hodgson KJ, Fogarty TJ. AneuRx stent graft versus open surgical repair of abdominal aortic aneurysms: multicenter prospective clinical trial. J Vasc Surg 1999;29:292–308. [DOI] [PubMed]
- 21.Howell M, Villareal R, Krajcer Z. Percutaneous access and closure of femoral artery access sites associated with endoluminal repair of abdominal aortic aneurysms. J Endovasc Ther 2001;8:68–74. [DOI] [PubMed]


