Abstract
Aspirin has long been established as a useful analgesic and antipyretic. Even in ancient times, salicylate-containing plants such as the willow were commonly used to relieve pain and fever. In the 20th century, scientists discovered many details of aspirin's anti-inflammatory and analgesic properties, including its molecular mechanism of action. In addition, the latter half of the century brought reports that daily, low doses of aspirin could prevent myocardial infarction and stroke. This finding was first reported by Lawrence Craven, a suburban general practitioner in Glendale, California. Unfortunately, Craven's work went largely unnoticed, and decades passed before his observations were verified by clinical trial. We present Craven's story, which demonstrates the value of a single physician's commitment to lifelong learning. In addition, we summarize the work of the physicians and scientists who discovered the molecular mechanisms by which aspirin exerts its antiplatelet effects. Collectively, these discoveries exemplify the complementary roles of basic science and clinical observation in advancing medicine.
Key words: Aspirin/history/pharmacology/therapeutic use; bleeding time; blood coagulation/drug effects; history of medicine, 19th cent.; history of medicine, 20th cent.; intracranial embolism and thrombosis/prevention & control; Lawrence Craven; myocardial infarction/prevention & control; platelet aggregation inhibitors; salicylic acids; stroke; thrombosis/prevention & control
Willow bark and other salicylate-containing plants have been used for pain relief since ancient times. For example, the Greek physicians Galen and Hippocrates described the analgesic effects of willow bark; Galen was the first to record its antipyretic and anti-inflammatory effects. The Assyrians of the Sumerian period and the ancient Egyptians recorded that willow could be used to alleviate pain.1 Of course, these observations were made long before the advent of modern evidence-based medicine, and therefore the use of willow in ancient medicine had its foundation in observational or anecdotal evidence. Nevertheless, the claims of willow bark's analgesic properties stood the test of time and were scientifically validated in the modern era. Although ancient physicians had no way of understanding the mechanism by which willow bark might relieve pain, lack of understanding did not stop them from prescribing this relatively safe and helpful herbal remedy.
In 1763, the Reverend Edward Stone wrote a letter to the Earl of Macclesfield wherein he described the use of powder derived from willow bark for treating ague (malarial fever) in 50 patients. Stone's work is generally regarded as the 1st modern scientific description of the medicinal use of willow bark.2 The development of chemical techniques in the 18th and 19th centuries enabled scientists to characterize the compounds that were extracted from willow bark. In 1826, Henri Leroux isolated what was later to be called “salicin” from willow bark.3 Two years later, Johann Buchner also purified the same compound and named it salicin, which means “willow” in Latin. Other groups simultaneously purified salicin and optimized the protocol for its isolation from willow bark. In 1838, Raffaele Piria at the Sorbonne generated salicylic acid from salicin. In 1853, Charles Frederic Gerhardt created acetylsalicylic acid for the 1st time, but he did not use or market this modified version of salicylic acid.4 At about the same time, physicians began prescribing the purified compounds to relieve pain. A Dundee physician, Thomas Maclagan, used salicin to treat patients who had rheumatism, and he reported its beneficial effects in The Lancet in 1876.5
In 1897, Felix Hoffman, a German chemist working for the Bayer company, was able to modify salicylic acid to create acetylsalicylic acid, which was named aspirin (Fig. 1). The following year, Heinrich Dreser at Bayer dismissed the market potential of aspirin on the ground that it had an “enfeebling” action on the heart (“The product has no value”). His real reason for ignoring aspirin was his preoccupation with the sales potential of another new drug—heroin (first synthesized in the Bayer laboratory in 1897)—which Bayer was about to launch as a cough remedy.6 Arthur Eichengruen (whose job it was to originate new products at Bayer) refused to accept Dreser's rejection of acetylsalicylic acid and continued to press for its development.6 Eventually, Dreser reneged and tested aspirin on himself and on his rabbits, confirming its therapeutic properties. Subsequently, aspirin was found to be more tolerable to the stomach than salicylic acid.7 Felix Hoffmann's innovation led to the widespread modern use of aspirin for pain relief. His acetylation of salicylic acid also proved fortunate in another way, because the modification is important to aspirin's ability to prevent cardiovascular events.8
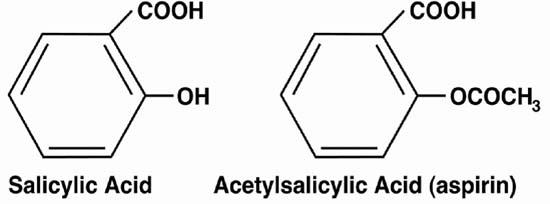
Fig. 1 Acetylsalicylic acid (aspirin) retains the carboxyl group (COOH) of salicylic acid and makes a substitution in the hydroxyl group (OH). The drug was developed at Bayer by Felix Hoffmann. Acetylation made aspirin more tolerable to the gastrointestinal tract, which led to widespread use.
In this manner, an ancient herbal remedy became “aspirin,” a wonder drug, albeit with some persistent side effects, including gastrointestinal irritation. Angina pectoris, which was at one time written off as “stomach pain” or other ailments, was already linked to myocardial infarction by the 18th century. Large atherosclerotic plaques were found in patients who had suffered heart attacks. However, even as late as the 1940s, many questions lingered about why heart attacks sometimes occur precipitously—especially in light of the knowledge that atherosclerotic plaques form gradually and grow slowly over time.9 In the early 20th century, physicians began using the anticoagulant dicumarol to treat myocardial infarction, but there were still questions about the role of thrombosis in the often precipitous nature of cardiovascular events. Indeed, there was considerable controversy about the use of any anticoagulant in the prevention of myocardial infarction.10 It was at this time, when myocardial infarction and the effects of aspirin were still poorly understood, that a suburban general practitioner named Lawrence Craven began to test whether aspirin might prevent myocardial infarction.
Craven's Life
Little is known about Dr. Lawrence L. Craven's life. The only readily accessible documents that give insight into Craven's personality and life experiences are 4 papers published under his name, a Los Angeles Times article that concerned 1 of those papers, and an obituary. Craven was born in Truro, Iowa, in 1883.11 He graduated from the University of Minnesota in 1913 with a bachelor of science degree. One year later, he received his MD degree from the University of Minnesota College of Medicine and Surgery (Fig. 2). During World War I, Craven served as a captain in the military. Later, he and his wife, Mabel, moved to Glendale, California, where he worked as a general practitioner at Glendale Memorial Hospital.11 His younger brother, Earl, was an assistant editor of the Los Angeles Times,12 which probably facilitated the publication of that newspaper article about one of Dr. Craven's papers.13 Little else is known about Craven's life, although he posthumously became famous for his prophetic papers describing aspirin's ability to prevent myocardial infarction and stroke. In regard to his life outside of medicine, Craven's obituary states that he was president of the Glendale Optimist Club and was actively involved in numerous civic activities.11

Fig. 2 Photograph of Dr. Lawrence L. Craven in 1914, at the age of 31, when he graduated from the University of Minnesota College of Medicine and Surgery. Photo courtesy of the University of Minnesota Archives.11
Ironically, on 18 August 1957, Lawrence Craven died after experiencing a myocardial infarction. At the time of his death, he was 74 years old and still actively practicing medicine.11 Of possible interest is Craven's recommendation of aspirin as prophylactic therapy only for patients 45 to 65 years of age—so at the time of his death he would have fallen outside the age range embraced by his own recommendation. It is noteworthy, however, that Craven's recommendations are not far from today's widely accepted standards.14
Craven's Discoveries
The cause of myocardial infarction was a topic of particular interest to Dr. Craven. He was not a formally trained scientist, and he knew his limitations. Indeed, some of the most remarkable aspects of his papers are his humility and his repeated caveats that more rigorous scientific studies had to be conducted to prove his hypotheses. Despite the absence of control groups, Craven's studies had their basis in sound reasoning and in the observation of large numbers of patients. For example, in 1950 he published his 1st letter,15 in the Annals of Western Medicine and Surgery, in which he introduced his hypothesis that aspirin was preventive of coronary thrombosis. He cited evidence that aspirin prolonged prothrombin time16 and mentioned reports of more frequent hemorrhaging among patients who chewed aspirin gum after a tonsillectomy17 or a tooth extraction.18 He prescribed daily aspirin to 400 patients in 1948, and he reported in 1950 that none had suffered a myocardial infarction during that 2-year period.15 Later in 1950, Craven published a 2nd letter,19 in the Journal of Insurance Medicine, that reiterated many of these ideas. He remarked, “For 36 years my surgical work has been primarily removal of tonsils and adenoids. Of the hundreds of cases handled, only 5 were performed in hospitals. Surgery was performed during morning office hours and practically all patients were released to their homes by early afternoon without question of possible hemorrhage—practically none occurring until about 6 years ago, at which time an alarming number of hemorrhages were evidenced in disturbing frequency.”19 Craven was convinced that this increased bleeding resulted from the chewing of aspirin gum to relieve pain.
Craven's hypothesis also arose from his perception that men suffer from heart attacks more often than women. He suggested that men of his time were much less likely than women to take aspirin for everyday aches and pains, which might explain sex differences in the incidence of myocardial infarction. Initially, Craven recommended aspirin to all of his male patients and friends between the ages of 30 and 90, saying that his patients “… ‘might’ prevent dying a horribly painful death if they would take a couple of aspirins daily for the rest of their lives. They evidenced the usual male reaction of skepticism (‘taking aspirin is effeminate!’). They did, however, prove the point that practically every man in the country today has a secret dread of death from ‘heart disease’ to the extent that he will try anything which offers even a remote hope of prevention.”19 Craven reported that many physicians from across the country had written to him in response to his 1st paper, and he mentioned that these corresponding physicians had “enjoyed similar successful results in the administration of aspirin for prevention of coronary thrombosis in their own practices.”19
In 1953, Dr. Craven published his 3rd paper,20 in the Mississippi Valley Medical Journal. By this time, he had changed his age recommendations for daily aspirin prescription. He now prescribed daily aspirin to men between the ages of 45 and 65 who were overweight and led sedentary lifestyles, factors that predispose a patient to myocardial infarction. He found that not one of these patients experienced a myocardial infarction over a several-year period.20 Dr. Craven eloquently justified his method of determining the effects of aspirin:
The value of Aspirin (acetylsalicylic acid) in the general prophylaxis of coronary occlusion is suggested by observations accumulated during the past seven years. Concededly, the effectiveness of any type of prophylactic treatment is difficult to prove, and this applies especially to a procedure aiming merely at nonspecific prevention. Observations on healthy subjects can never be made under strictly scientific conditions, and resulting figures are only within limits suitable for statistical evaluation. Such findings may therefore merely have the value of preliminary impressions, and will be substantiated or refuted by subsequent clinical research. But as long as the field of general prophylaxis of coronary thrombosis is still outside the limits of present-day research procedures, preliminary observations may still be of practical importance provided: 1. the measure is safe in all subjects and throughout the entire extended period of medication; 2. the observations are not in opposition to trend and results of clinical and experimental research; and 3. it is well understood that the findings were not arrived at under strictly scientific conditions.20
According to this same 1953 paper,20 approximately 1,500 at-risk patients faithfully took a small daily dose of aspirin, and not one suffered a myocardial infarction. Craven's discussion centered on 4 points:
Myocardial infarction is likely due to rapid thrombosis at sites of atherosclerotic narrowing.
Aspirin is known to cause hemorrhagic complications after tonsillectomy, and there are significant data to suggest that aspirin has anticoagulant properties.
Craven personally observed that patients who are prescribed aspirin are much less likely to experience myocardial infarction and that small doses of aspirin are generally sufficient to prevent myocardial infarction.
Craven called upon the medical community to study aspirin's effects on coagulation and on the prevention of coronary thrombosis.
Craven continually and urgently expanded his observational studies, for he wanted to convince people that his observations were true. To verify aspirin's ability to impair clotting, he performed a “personal experiment” in 1950. He wrote, “Ingestion of 12 aspirin tablets daily resulted after five days in spontaneous profuse nosebleed. In order to check on the reliability of this observation the test was repeated twice over, with precisely the same results. The proof seemed to be all the more convincing as the author had not experienced nosebleed for more than fifty years.”20
Most of Craven's writing is speculative and descriptive, lacking any statistics or formal presentation of data. So naturally, the question arises: what supports Craven's hypothesis other than anecdotal evidence and speculation thereupon? Craven's idea was eventually proved true, yet his work was not powerful from a scientific perspective. Nevertheless, his cohort sizes were large (a total of 8,000 patients), and his results were striking (no myocardial infarctions in at-risk patients who faithfully stuck to their dosage).21 Perhaps the finest aspect of Craven's work was his reasoning and his review of the literature, which supported his novel therapeutic approach. Craven's papers reflect a deep personal commitment to learning and to the advancement of medicine.
However, Craven's papers went largely unnoticed. It is interesting to speculate about whether more prestigious medical journals might have rejected his papers and, if so, on what basis. We do not have an answer to this question, but it is tempting to conclude that Craven's writings might have been rejected for lack of rigor. However, methods for more stringent clinical trials were still being developed in the 1950s, and Craven likely did everything within his power to prove his observations, considering the limited resources and statistical methods that were available to him.
In Craven's 4th and final paper,21 published less than a year before his death, he updated his trial of aspirin as a prophylactic against coronary thrombosis. His final count was 8,000 patients who had taken aspirin daily, 9 of whom had died of what appeared to be “heart attacks.” Autopsies were performed on all 9 patients who died, and the cause of death proved to be ruptured aortic aneurysm rather than coronary thrombosis. Once again, these observations were presented with the caveat that they were not obtained under controlled conditions. This 1956 paper conveyed another significant observation: aspirin might also prevent “little strokes” (or transient ischemic attacks): no patient had experienced stroke. Finally, Craven answered skeptics who claimed that low doses of aspirin were insufficient to prolong prothrombin time and could not, therefore, have had any antithrombotic effect. He responded:
I might answer that the mechanism whereby electroshock helps the confused is as yet unknown, yet few psychiatrists would discard electroshock treatment because they have seen and welcomed the improvement in their patients. Again, quinine is known as a specific for malaria—but can its worth be demonstrated by laboratory technics? To any physician who has witnessed the results of long-term aspirin administration—who has seen his patients freed of their fear of possible “heart attacks” at a time when their contemporaries are stricken down with coronary and cerebral thrombosis, the evidence speaks for itself.21
Aspirin's Antiplatelet Effect
Long before clinicians conducted clinical trials that tested Dr. Craven's observations, clinician-investigators and basic scientists were asking questions about the basic mechanisms of platelet activation and about bleeding disorders associated with platelet defects. By 1950, it was established that high doses of aspirin prolonged prothrombin time22–24—a fact also noted by Craven.20 However, even doses too low to affect the prothrombin time appeared sufficient to prevent coronary thrombosis, and this bewildered Craven. Because aspirin was associated with bleeding and unusually high doses prolonged prothrombin time, many physicians in the 1940s were prescribing aspirin together with vitamin K (even in a combined pill form), under the false assumption that additional vitamin K might somehow compensate for the increased bleeding. Even Craven made this incorrect assumption.19 Later studies by basic scientists and clinician-investigators would address themselves to these and other fundamental issues.
Atherosclerosis and myocardial infarction are the result of both inflammatory and thrombotic processes. In the latter half of the 20th century, humankind greatly advanced its understanding of these processes. Hemostasis and inflammation were once thought to be distinct, but we now know that these 2 processes are often linked. During inflammation or injury, leukocytes roll along and adhere to activated endothelial cells that line the blood vessel wall. Leukocyte rolling is mediated primarily by selectins and their ligands,25 while firm adhesion is mediated by integrins and their ligands.26 Firmly adherent leukocytes subsequently transmigrate across the endothelium. In contrast with leukocytes, adherent platelets remain in the blood vessel lumen and continue to accumulate toward the center of the lumen. When the blood vessel wall is injured severely enough to denude the endothelium, platelets attach to subendothelial von Willebrand factor and collagen, and flowing platelets tether to the already adherent, activated platelets.27
A variety of stimuli, including thrombin, adenosine diphosphate (ADP), and collagen, are known to cause platelet activation, spreading, and aggregation. Adenosine diphosphate is stored within the dense granules of platelets and is released upon cell activation, but it causes only modest reversible aggregation. Nonetheless, ADP plays an important role in initiating signals that lead to platelet shape changes and to the synthesis of thromboxane A2, which is a potent activator of platelets. When a platelet is activated and its dense granules release their contents, ADP binds to its receptors on the same and neighboring platelets, as does thromboxane A2 to its receptor. Adenosine diphosphate amplifies the platelet's response to other agonists. This adhesion cascade leads to the formation of large platelet aggregates that are both procoagulant and proinflammatory. The resulting thrombus can easily occlude the lumen of an already narrowed atherosclerotic coronary artery, thereby causing a myocardial infarction.28,29
Many scientists have contributed to our current understanding of aspirin and platelet function, and it is not practical to tell each of their stories. Instead, this paper focuses on a few of the investigators whose discoveries helped reinvigorate clinical interest in the use of aspirin for preventing cardiovascular events. In the late 1960s, Dr. Harvey J. Weiss30,31 asked the next important question: “Does aspirin affect platelets?” Weiss had been studying patients who had platelet factor 3 deficiency, a disorder in which platelets exhibit defective release of ADP.8 Simultaneously, Dr. Armand Quick29 was examining the effect of aspirin on bleeding time, finding that very low doses of aspirin prolonged the bleeding time, even though low doses had no effect on prothrombin time. Quick also found that aspirin had a disproportionately large effect on the bleeding times of patients with von Willebrand disease, and he hypothesized that low doses of aspirin might prolong the bleeding time in normal patients by creating a defect similar to that found in von Willebrand disease.29 Weiss theorized that aspirin-associated prolongation of the bleeding time might result from defective platelet aggregation due to impaired ADP release. His findings, published in several papers,30–32 showed that aspirin impairs both ADP release and secondary ADP-dependent platelet aggregation (Fig. 3). Remarkably, Weiss also found that sodium salicylate had no such effect on ADP release or platelet aggregation, which suggested that aspirin's antiplatelet activity was dependent on the acetyl modification that differentiates “aspirin” from salicylic acid. Other groups confirmed this difference between salicylic acid and aspirin.29,33 Weiss reported that aspirin's effect on platelets was rapid and irreversible, inhibiting platelet aggregation for the duration of a platelet's life. Together with the findings from several other groups, Dr. Weiss's discovery was a key step in understanding the mechanism by which low doses of aspirin could prevent coronary and cerebral thrombosis. For example, James Mustard, Marian Packham, and Geoffrey Evans and their colleagues34–36 also demonstrated that aspirin could inhibit the aggregation of platelets. Although this work showed that aspirin had antiplatelet effects, it did not reveal the specific molecular mechanism by which aspirin exerted those effects. Differences in the effects of salicylic acid and aspirin (acetylsalicylic acid) suggested that the acetyl group was somehow involved. One laboratory reported that a radiolabeled acetyl group from acetylsalicylic acid was selectively found in platelets after aspirin treatment, while a radiolabeled carboxyl group was not incorporated into platelets.37
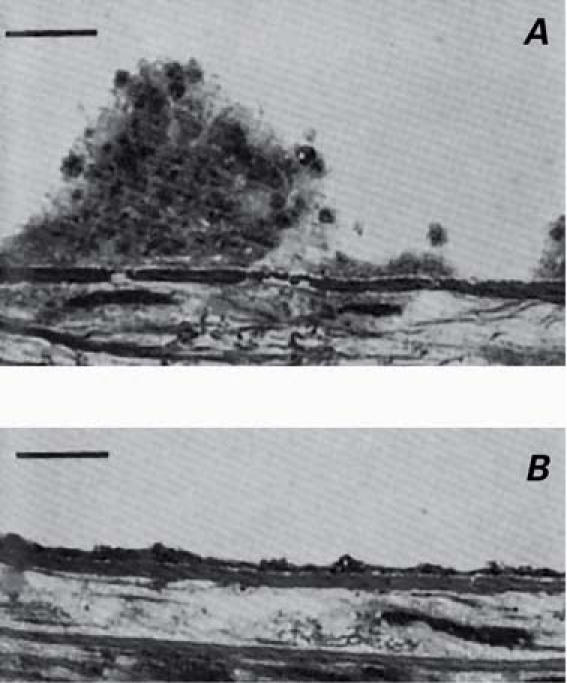
Fig. 3 Inhibition of platelet thrombus formation by aspirin. Citrated blood was perfused over de-endothelialized rabbit aorta at arterial shear rates A) before ingesting aspirin, and B) 2.5 hr after ingesting 0.9 g of aspirin.32 The images were acquired by light microscopy. The black bar represents 10 μm.
(Reprinted, with permission, from Weiss HJ, Tschopp TB, Baumgartner HR. Impaired interaction [adhesion-aggregation] of platelets with the subendothelium in storage-pool disease and after aspirin ingestion. A comparison with von Willebrand's disease. N Engl J Med 1975;293:619–23. Copyright © 1975, Massachusetts Medical Society. All rights reserved.)
While Harvey Weiss was examining the effect of aspirin on platelet aggregation, others were conducting experiments to study in vivo the functions of prostaglandins and the effects of aspirin on their release. Weiss had already solved a big piece of the puzzle, having shown that aspirin inhibited platelet aggregation. At almost the same time, the prostaglandin researchers Priscilla Piper and Sir John Vane were asking whether aspirin might affect the biosynthesis of prostaglandins. In the early 1960s, Vane developed a method for assaying the production of various substances by means of isolated organ perfusion, which he called cascade superfusion bioassay.38 In this assay, blood or artificial salt solution was perfused over isolated assay tissue, and various test substances were also introduced. Vane made several innovations to previous bioassay techniques, such as superfusing an organ with blood from an animal's vein or artery and then returning the blood to a large vein. In 1982, Vane won the Nobel Prize in Physiology or Medicine for his contributions to this field. These innovations would prove vital for understanding the effects of aspirin at the molecular level. Vane commented that the “element of instantaneity is an important aspect of cascade superfusion bioassay in that it detects the biological activity of chemically unstable compounds whose activity would otherwise be lost in the extraction process.”39 Using this approach, Vane and Piper studied substances released during anaphylaxis.40 They discovered the release of prostaglandins and a molecule called “rabbit aorta contracting substance” (RCS, later renamed thromboxane A2). The RCS was found to be highly unstable, and that fact was crucial to their later discoveries: a delay of even a few minutes was sufficient to prevent the effect of RCS on the assay tissue. In assays using guinea pig lung, they found that aspirin blocked the release of RCS and also prostaglandins. Vane described his experiences:
While I was writing a review paper over the weekend, including the results of some of these experiments, a thought occurred to me that perhaps should have been obvious earlier on. In all these experiments (and in those of many other workers), the “release” of prostaglandins must in fact amount to fresh synthesis of prostaglandins. That is, prostaglandin output in these experiments, though very low, was still far higher than the tissues' initial content of the hormones. Evidently, then, the various stimuli, mechanical and chemical, which released prostaglandins, were in fact “turning on” the synthesis of these compounds. A logical corollary was that aspirin might well be blocking the synthesis of prostaglandins.39
Vane tested his hypothesis by introducing aspirin in an experiment that used the supernatant of a cell homogenate known to generate prostaglandins. His hypothesis proved to be correct, for aspirin inhibited the generation of RCS and prostaglandins in a dose-dependent manner.41,42 Smith and Willis43 tested the same hypothesis in patients who had taken 600 mg of aspirin, after which platelets were isolated and stimulated with thrombin. They found that prostaglandin synthesis was specifically inhibited by aspirin,43 which was consistent with Vane's report. The enzyme that aspirin inhibits—later revealed to be cyclooxygenase-1 (COX-1) (Fig. 4) —plays a key role in the synthesis of prostaglandins and thromboxane A2. Collectively, the work of these scientists revealed that aspirin's effects on platelet aggregation result from inhibition of COX-1 (thereby reducing thromboxane-A2 synthesis) and inhibition of the response to thromboxane (which is dependent upon ADP for amplification). Roth and associates44 showed that aspirin irreversibly inhibits COX-1 by acetylating a serine residue, thereby preventing the binding of arachidonic acid. In platelets, irreversible inhibition of COX-1 is of particular consequence, since the synthesis of any new enzyme is minimal in these anuclear cells. This characteristic of platelets leads to a more profound and prolonged inhibition of platelet function, in comparison with aspirin's effects on cells that contain nuclei. The contributions of these and other scientists likely reinvigorated interest in the use of aspirin to prevent cardiovascular events, which led to the 1st clinical trials that directly tested Craven's claims.
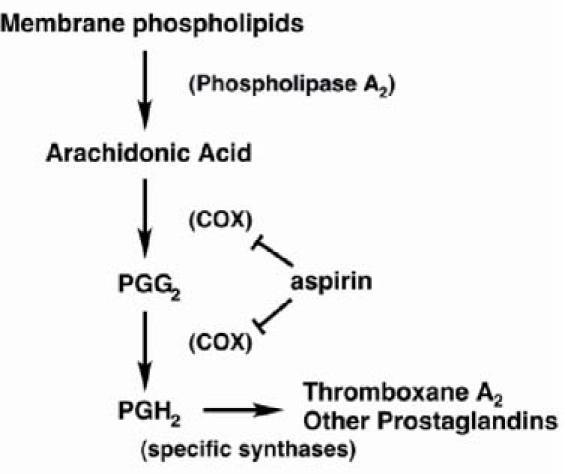
Fig. 4 Synthetic pathway for prostaglandins and thromboxane A2. Aspirin inhibits cyclooxygenase-1, which is necessary for the synthesis of thromboxane A2 and prostaglandins.
COX = cyclooxygenase; PGG2 = prostaglandin G2; PGH2 = prostaglandin H2
Conclusions
Since the publication of Craven's clinical observations, hundreds of clinical trials have tested aspirin's ability to prevent cardiovascular events, and it is now accepted that aspirin can prevent both myocardial infarction and stroke.45 In 1989, this work culminated in the Physicians' Health Study,46 a pivotal clinical trial that confirmed Craven's hypothesis. The remaining questions relate to proper dosage, although the answer to this question is also becoming clear. Clinical trials and aspirin dosage for the prevention of myocardial infarction and stroke were recently reviewed by James E. Dalen.47 Dalen's review concludes that the optimal aspirin dosage should be able to prevent both myocardial infarction and stroke; current data indicate that a dosage of 160 mg/day may be most appropriate for these indications. This dosage is lower than the 325 mg/day prescribed by Craven, but it is remarkable that Craven also detected the efficacy of small doses.
Dr. Craven's observations suggested that aspirin prophylaxis completely prevented myocardial infarction. However, clinical trials have shown that, while aspirin significantly reduces the risk of myocardial infarction and stroke, it clearly is not universally protective.47 Although Craven was not correct on every point, his observations would have saved many lives had they been tested and implemented decades earlier. Craven was intellectually curious, ahead of his time, and maybe a little lucky. His story illustrates the value of a single physician's efforts, when he or she continually observes patients and then strives to improve medical care. Fortunately, basic scientists and clinician-investigators asked fundamental biological questions, and by doing so they were able to confirm and eventually to revive an insight that had temporarily fallen by the wayside.
Footnotes
Address for reprints:Jonathan Miner, Oklahoma Medical Research Foundation, 825 NE 13th Street, MS 45, Oklahoma City, OK 73104. E-mail: jonathan-miner@ouhsc.edu
References
- 1.Jack DB. One hundred years of aspirin. Lancet 1997;350:437–9. [DOI] [PubMed]
- 2.Stone E. An account of the success of the bark of the willow tree in the cure of agues. Philos Trans R Soc Lond 1763;53: 195–200.
- 3.Leroux H. Discovery of salicine. J Chim Med 1830;6:340–432.
- 4.Hawkey CJ. COX-2 chronology. Gut 2005;54:1509–14. [DOI] [PMC free article] [PubMed]
- 5.MacLagan T. The treatment of acute rheumatism by salicin and salicylic acid. Lancet 1876;1:342–4. [DOI] [PMC free article] [PubMed]
- 6.Askwith R. How aspirin turned hero. Sunday Times [London]. 1998 Sep 13.
- 7.Dreser H. Pharmakologisches uber aspirin (acetylsalicylsaure). Pflugers Arch 1899;76:306–18.
- 8.Weiss HJ. The discovery of the antiplatelet effect of aspirin: a personal reminiscence [published erratum appears in J Thromb Haemost 2003;1:2266]. J Thromb Haemost 2003; 1:1869–75. [DOI] [PubMed]
- 9.Master AM, Jaffe HL. Factors in the onset of coronary occlusion and coronary insufficiency; effort, occupation, trauma and emotion. J Am Med Assoc 1952;148:794–8. [DOI] [PubMed]
- 10.Plotz M. The preventive aspects of coronary disease and myocardial infarction. N Y State J Med 1944;4:1227–9.
- 11.Dr. Lawrence Craven, Glendale physician, dies. Los Angeles Times. 1957 Aug 19;5.
- 12.Times asst. editor Earl Craven dies. Los Angeles Times. 1956 Jan 9;A1.
- 13.Barton WS. Glendale physician wins award for heart paper. Los Angeles Times. 1952 Oct 3;A3.
- 14.Dalen JE. An apple a day or an aspirin a day? Arch Intern Med 1991;151:1066–9. [PubMed]
- 15.Craven LL. Acetylsalicylic acid: possible preventive of coronary thrombosis. Ann West Med Surg 1950;4:95–9. [PubMed]
- 16.Govan CD. The effect of salicylate administration on prothrombin time. J Pediatr 1946;29:629–36. [DOI] [PubMed]
- 17.Singer R. Acetylsalicylic acid, a probable cause for secondary post-tonsillectomy hemorrhage. Arch Otolaryngol 1945;42: 19–20.
- 18.Rapp G. Cause of delayed hemorrhage after tooth extraction. J Am Dent Assoc 1947;34:484. [DOI] [PubMed]
- 19.Craven LL. Coronary thrombosis can be prevented. J Insur Med 1950;5:47–8. [PubMed]
- 20.Craven LL. Experiences with aspirin (acetylsalicylic acid) in the nonspecific prophylaxis of coronary thrombosis. Miss Valley Med J 1953;75:38–44. [PubMed]
- 21.Craven LL. Prevention of coronary and cerebral thrombosis. Miss Valley Med J 1956;78:213–5. [PubMed]
- 22.Solley RF, Griffith GC, Huntington RW, Montgomery H. Studies in rheumatic fever. I. The physiologic effect of sodium salicylate on the human being, with particular reference to the prothrombin level of the blood and the effect on hepatic parenchyma. J Amer Med Assoc 1945;128:1195–200.
- 23.Coombs FS, Higley CS, Warren HA. The magnitude of salicylate hypoprothrombinemia. Proc Am Fed Clin Res 1945;2: 57–8. [PubMed]
- 24.Clausen FW, Jager BV. The relation of the plasma salicylate level to the degree of hypoprothrombinemia. J Lab Clin Med 1946;31:428–36. [PubMed]
- 25.McEver RP, Cummings RD. Perspectives series: cell adhesion in vascular biology. Role of PSGL-1 binding to selectins in leukocyte recruitment. J Clin Invest 1997;100:485–91. [DOI] [PMC free article] [PubMed]
- 26.Ruggeri ZM. von Willebrand factor [published erratum appears in J Clin Invest 1997;100:237]. J Clin Invest 1997;99: 559–64. [DOI] [PMC free article] [PubMed]
- 27.Ruggeri ZM. Mechanisms initiating platelet thrombus formation [published erratum appears in Thromb Haemost 1997;78:1304]. Thromb Haemost 1997;78:611–6. [PubMed]
- 28.Ruggeri ZM. Platelets in atherothrombosis. Nat Med 2002; 8:1227–34. [DOI] [PubMed]
- 29.Quick AJ. Salicylates and bleeding: the aspirin tolerance test. Am J Med Sci 1966;252:265–9. [DOI] [PubMed]
- 30.Weiss HJ, Aledort LM. Impaired platelet-connective-tissue reaction in man after aspirin ingestion. Lancet 1967;2:495–7. [DOI] [PubMed]
- 31.Weiss HJ, Aledort LM, Kochwa S. The effect of salicylates on the hemostatic properties of platelets in man. J Clin Invest 1968;47:2169–80. [DOI] [PMC free article] [PubMed]
- 32.Weiss HJ, Tschopp TB, Baumgartner HR. Impaired interaction (adhesion-aggregation) of platelets with the subendothelium in storage-pool disease and after aspirin ingestion. A comparison with von Willebrand's disease. N Engl J Med 1975;293:619–23. [DOI] [PubMed]
- 33.O'Brien JR. Effect of salicylates on human platelets. Lancet 1968;1:1431. [DOI] [PubMed]
- 34.Mustard JF, Glynn MF, Nishizawa EE, Packham MA. Platelet-surface interactions: relationship to thrombosis and hemostasis. Fed Proc 1967;26:106–14. [PubMed]
- 35.Packham MA, Warrior ES, Glynn MF, Senyi AS, Mustard JF. Alteration of the response of platelets to surface stimuli by pyrazole compounds. J Exp Med 1967;126:171–88. [DOI] [PMC free article] [PubMed]
- 36.Evans G, Packham MA, Nishizawa EE, Mustard JF, Murphy EA. The effect of acetylsalicyclic acid on platelet function. J Exp Med 1968;128:877–94. [DOI] [PMC free article] [PubMed]
- 37.al-Mondhiry H, Marcus AJ, Spaet TH. On the mechanism of platelet function inhibition by acetylsalicylic acid. Proc Soc Exp Biol Med 1970;133:632–6. [DOI] [PubMed]
- 38.Vane JR. The use of isolated organs for detecting active substances in the circulating blood. Br J Pharmacol Chemother 1964;23:360–73. [DOI] [PMC free article] [PubMed]
- 39.Vane JR. Adventures and excursions in bioassay: the stepping stones to prostacylin. Biosci Rep 2004;24:254–79. [DOI] [PubMed]
- 40.Piper PJ, Vane JR. The release of prostaglandins during anaphylaxis in guinea-pig isolated lungs. In: Mantegazza P, Horton EW, editors. Prostaglandins, peptides, and amines. London: Academic Press; 1969. p. 15–9.
- 41.Vane JR. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol 1971;231: 232–5. [DOI] [PubMed]
- 42.Hamberg M, Svensson J, Samuelsson B. Thromboxanes: a new group of biologically active compounds derived from prostaglandin endoperoxides. Proc Natl Acad Sci U S A 1975; 72:2994–8. [DOI] [PMC free article] [PubMed]
- 43.Smith JB, Willis AL. Aspirin selectively inhibits prostaglandin production in human platelets. Nat New Biol 1971;231: 235–7. [DOI] [PubMed]
- 44.Roth GJ, Stanford N, Majerus PW. Acetylation of prostaglandin synthase by aspirin. Proc Natl Acad Sci U S A 1975; 72:3073–6. [DOI] [PMC free article] [PubMed]
- 45.Antithrombotic Trialists' Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients [published erratum appears in BMJ 2002;324:141]. BMJ 2002;324:71–86. [DOI] [PMC free article] [PubMed]
- 46.Final report on the aspirin component of the ongoing Physicians' Health Study. Steering Committee of the Physicians' Health Study Research Group. N Engl J Med 1989;321:129–35. [DOI] [PubMed]
- 47.Dalen JE. Aspirin to prevent heart attack and stroke: what's the right dose? Am J Med 2006;119:198–202. [DOI] [PubMed]


