Abstract
Cardiac myxomas are the most common primary cardiac tumors. Surgical resection usually provides definitive treatment; however, postoperative tumor recurrence has been reported, especially when myxomas occur as part of a familial pattern. Only a few cases of 2nd recurrence of nonfamilial cardiac myxoma have been reported. We report 2 cases of nonfamilial cardiac myxoma, with multiple recurrences after surgical resection. The possibility of repeated recurrence of cardiac myxomas demonstrates the importance of regular echocardiography after surgical resection in order to detect such recurrence. Future studies, including genetic analysis of patients with recurrent cardiac myxomas, are warranted to investigate the nature of these tumors.
Key words: Echocardiography; heart neoplasms/ ultrasonography; myxoma/ pathology/surgery; neoplasm recurrence, local/epidemiology; neoplasms, multiple primary/genetics/surgery
Cardiac myxomas are the most common primary cardiac tumors, with an estimated incidence of 0.5 per million individuals annually.1,2 Patients are often asymptomatic but may present with congestive heart failure, thromboembolic disease, chest discomfort, heart murmurs, or constitutional symptoms.1,3 Echocardiography is the preferred method of diagnosis. Surgical resection is the definitive treatment for cardiac myxomas, with postoperative tumor recurrence observed in up to 3% of cases in several large series, except when myxomas occur as part of an underlying genetic syndrome.4,5 Only a few cases of 2nd recurrence of nonfamilial cardiacmyxomas have been reported3,4,6–12 (Table I). We report 2 cases of nonfamilial cardiac myxoma in which there were multiple recurrences after surgical resection.
TABLE I. Nonfamilial 2nd Recurrences of Benign Cardiac Myxomas
Case Reports
Patient 1
A 42-year-old woman who had undergone surgical removal of a left atrial myxoma in August 1992 presented at a hospital in Lima, Peru, in April 1993 with an asymptomatic recurrence in the left atrium. The recurrence was detected on routine echocardiography, which also showed that she had developed moderate mitral valve regurgitation. The patient underwent reoperation through a biatrial approach, during the course of which a 2.7 × 2.6 × 2.3-cm pedunculated tumor was resected. The tumor was attached to the inferior border of a Dacron patch that had been placed in the interatrial septum during the 1st operation. The root of the pedicle, the Dacron patch, and the full thickness of the adjacent interatrial septum were excised, and the resulting atrial septal defect was closed with a pericardial patch. Before the patch was closed, all cardiac chambers were inspected and no additional tumors were seen. Moreover, the mitral valve was replaced with a bileaflet mechanical valve. The histopathologic findings from examination of the excised tumor were consistent with benign cardiac myxoma, and there was no evidence of residual tumor in the surgical margins.
Fourteen months later, follow-up echocardiography revealed a 2.0 × 1.8-cm mass attached to the posterior wall of the left atrium. The patient refused reoperation for an additional 6 months, by which point she had developed malaise, arthralgia, and the sudden onset of transient visual loss in her left eye. At the 2nd reoperation, a 3.6 × 3.3 × 3.3-cm pedunculated tumor was excised, following the same surgical technique that had been used earlier. This tumor, upon histopathologic study, was again consistent with benign cardiac myxoma and was indistinguishable from the previous lesions.
At the time of writing, no other myxoma has been diagnosed in this patient. In addition, further questioning has revealed no history of myxoma in any of her close relatives.
Patient 2
A man with a history of hypertension and coronary artery disease had undergone surgical removal of a left atrial myxoma in 1969 and again in 1972. In November 2005, routine echocardiography revealed a new mass in the right atrium, although the patient—by then 77 years of age—did not complain of any associated symptoms. The mass was estimated by echocardiography to measure 3.2 × 5 cm (Figs. 1–3). Surgical removal of the mass was planned, and this was preceded by preoperative coronary angiography that revealed a 70% stenosis of the left anterior descending coronary artery. Coronary angiography also showed prominent neovascularization in the area of the right atrium where the myxoma was seen on echocardiography. Surgical resection of the myxoma included removal of a 5-mm margin of native cardiac tissue, together with the interatrial patch placed at an earlier surgery. Additionally, a single-vessel bypass was performed from the left internal mammary artery to the left anterior descending artery. Histopathologic findings in regard to the excised mass were consistent with benign cardiac myxoma.
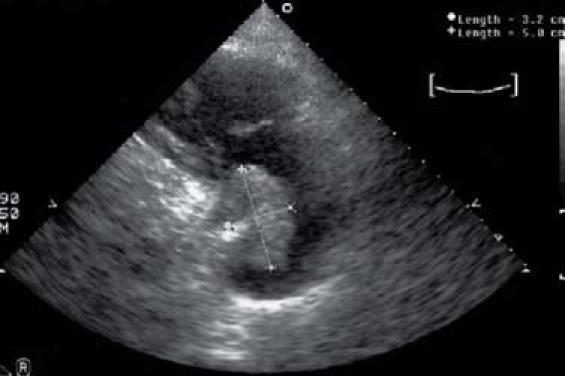
Fig. 1 Patient 2. Two-dimensional transthoracic echocardiogram of right ventricular inflow tract shows recurrent cardiac myxoma and its estimated size.
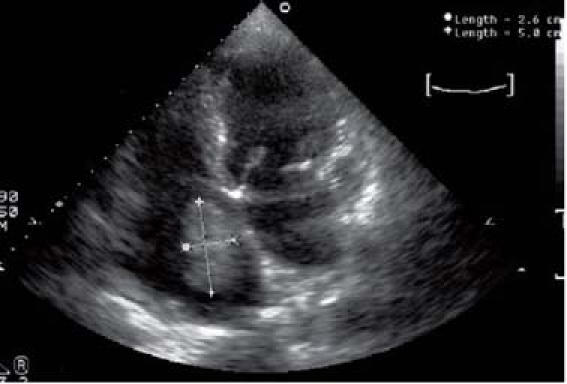
Fig. 2 Patient 2. Four-chamber transthoracic echocardiographic view shows recurrence of a cardiac myxoma.
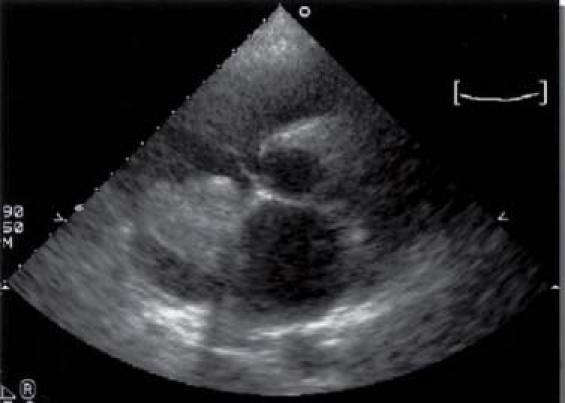
Fig. 3 Patient 2. Short-axis transthoracic echocardiographic view shows recurrence of a cardiac myxoma.
As in the previous case, the patient was unaware of any history of cardiac myxoma among his close relatives. At the time of this writing, he has not had any additional recurrences.
Discussion
Several large case series of patients with nonfamilial cardiac myxomas have demonstrated recurrence rates of up to 3% after surgical resection.3,5,13 However, none of these series has reported multiple recurrence except within the context of a familial pattern.5 In fact, only 10 cases of re-recurrent nonfamilial cardiac myxoma have been reported in the literature in the past 30 years, including our 2 cases described above (Table I). Of these patients, 6 were men and 4 were women. The mean age at which the primary tumor was discovered was 39 years.
The primary tumor was found in the left atrium in 8 patients, in the right atrium in 1 patient, and in both the right atrium and the right ventricle in the remaining patient. Information was cited on intervals of recurrence and 2nd recurrence for all but 1 case. The average intervals for recurrence and 2nd recurrence were 2.9 years and 9.4 years, respectively. The recurrent tumor appeared in the same chamber as the primary tumor in 5 patients, in a different chamber in 2 patients, in multiple chambers in 1 patient, on the tricuspid valve in 1 patient, and in the right ventricular outflow tract in 1 patient.
At 2nd recurrence, 4 patients showed multiple synchronic tumors, and 3 patients had multiple-chamber involvement. The remaining patients were affected by single tumors, 4 of which recurred in the left atrium and 2 in the right atrium.
Histopathologic analysis of the original and all recurrent tumors showed the features typical of a benign cardiac myxoma in all but 1 case. In that patient, the original myxoma was typically benign, but the 1st and 2nd recurrent tumors were reported to have a more aggressive histologic appearance.6
Two patients with a 2nd recurrence died as a result of the tumor—sudden death in 1 case and death from postoperative complications in the other. The available data revealed that the remaining patients were doing well and were free of tumor at the latest follow-up.
Possible causes of multiple recurrence include incomplete excision of the original tumor, intracardiac implantation from the original tumor, malignant transformation, and growth from secondary “pre-tumorous” foci.
In both of our patients, the initial operation and the reoperation were performed by use of a traditional surgical approach, with clean surgical margins demonstrated after reoperation. Successful resection of large cardiac tumors has also been accomplished by means of cardiac autotransplantation. This emerging surgical technique enables improved access to left-sided tumors and reliable cardiac reconstruction.14 Autotransplantation, which was first applied to the resection of a cardiac myxoma in 1987,15 is highlighted in a more recent case series that includes both benign myxomas and malignant cardiac tumors.16 The surgical approach chosen for resecting cardiac tumors should allow clean surgical margins, in order to avoid recurrence.
In cases of recurrent cardiac myxoma in which macroscopic and microscopic specimens from the previous surgery have shown clean surgical margins, other potential causes of recurrence, including malignant transformation, should be considered. Although these tumors are usually considered benign, a subpopulation of cardiac myxomas may have intrinsic malignant potential, as demonstrated by more aggressive patterns of recurrence, a progressive change in the histologic nature of a tumor from myxoma to sarcoma, an abnormal DNA ploidy pattern (in up to 40% of patients who experience recurrence), and distant metastases. Multifocality has been reported to be the reason for recurrence in most cases; in the present series, several recurrent and re-recurrent myxomas arose concurrently at different sites, which suggests multifocal disease.
Although neither of the patients in our report was aware of other occurrences of cardiac myxomas in family members, these tumors are frequently asymptomatic and might go undiagnosed. Genetic analysis was not available for cases reported elsewhere in the literature, nor has such analysis been performed on our 2 patients. Previous studies have identified genetic differences between patients with familial and nonfamilial cardiac myxomas.17 Genetic analysis combined with echocardiographic surveillance of family members would enable more decisive exclusion of familial syndromes than was obtained in our study. In the future, genetic screening of patients with recurrent cardiac myxomas might help to identify patients at risk for additional recurrence.
The previously reported cases of repeatedly recurrent benign cardiac myxomas, along with our 2 new cases, demonstrate the importance of regular echocardiography after surgical resection of benign cardiac myxomas, in order to detect recurrence and avoid potential complications. Whether there is a subpopulation of cardiac myxomas with a tendency to recur or with a malignant potential is open to question but is worthy of consideration. Future studies, including genetic analysis of patients with recurrent cardiac myxomas, are warranted in order to investigate the nature of these tumors.
Footnotes
Address for reprints: Erik Bernstein, MD, 540 Brickell Key Drive #1805, Miami, FL 33131. E-mail: ebernstein@med.miami.edu
References
- 1.Odim J, Reehal V, Laks H, Mehta U, Fishbein MC. Surgical pathology of cardiac tumors. Two decades at an urban institution. Cardiovasc Pathol 2003;12:267–70. [DOI] [PubMed]
- 2.Bjessmo S, Ivert T. Cardiac myxoma: 40 years' experience in 63 patients. Ann Thorac Surg 1997;63:697–700. [DOI] [PubMed]
- 3.Centofanti P, Di Rosa E, Deorsola L, Dato GM, Patane F, La Torre M, et al. Primary cardiac tumors: early and late results of surgical treatment in 91 patients. Ann Thorac Surg 1999; 68:1236–41. [DOI] [PubMed]
- 4.Hermans K, Jaarsma W, Plokker HW, Cramer MJ, Morshuis WJ. Four cardiac myxomas diagnosed three times in one patient. Eur J Echocardiogr 2003;4:336–8. [DOI] [PubMed]
- 5.Pinede L, Duhaut P, Loire R. Clinical presentation of left atrial cardiac myxoma. A series of 112 consecutive cases. Medicine (Baltimore) 2001;80:159–72. [DOI] [PubMed]
- 6.Cleveland DC, Westaby S, Karp RB. Treatment of intra-atrial cardiac tumors. JAMA 1983;249:2799–802. [PubMed]
- 7.Jugdutt BI, Rossall RE, Sterns LP. An unusual case of recurrent left atrial myxoma. Can Med Assoc J 1975;112:1099–100. [PMC free article] [PubMed]
- 8.O'Neil MB Jr, Grehl TM, Hurley EJ. Cardial myxomas: a clinical diagnostic challenge. Am J Surg 1979;138:68–76. [DOI] [PubMed]
- 9.Gray IR, Williams WG. Recurring cardiac myxoma. Br Heart J 1985;53:645–9. [DOI] [PMC free article] [PubMed]
- 10.McCarthy PM, Piehler JM, Schaff HV, Pluth JR, Orszulak TA, Vidaillet HJ Jr, Carney JA. The significance of multiple, recurrent, and “complex” cardiac myxomas. J Thorac Cardiovasc Surg 1986;91:389–96. [PubMed]
- 11.Aroca A, Mesa JM, Dominguez F, Oliver JM, Ramirez U, Centeno JE. Multiple recurrence of a “sporadic” (non-familial) cardiac myxoma. Eur J Cardiothorac Surg 1996;10:919–21. [DOI] [PubMed]
- 12.Moyssakis I, Anastasiadis G, Papadopoulos D, Margos P, Votteas V. Second recurrence of cardiac myxoma in a young patient. A case report. Int J Cardiol 2005;101:501–2. [DOI] [PubMed]
- 13.Reynen K. Cardiac myxomas. N Engl J Med 1995;333:1610–7. [DOI] [PubMed]
- 14.Reardon MJ, DeFelice CA, Sheinbaum R, Baldwin JC. Car-diac autotransplant for surgical treatment of a malignant neoplasm. Ann Thorac Surg 1999;67:1793–5. [DOI] [PubMed]
- 15.Scheld HH, Nestle HW, Kling D, Stertmann WA, Langebartels H, Hehrlein FW. Resection of a heart tumor using autotransplantation. Thorac Cardiovasc Surg 1988;36:40–3. [DOI] [PubMed]
- 16.Reardon MJ, Malaisrie SC, Walkes JC, Vaporciyan AA, Rice DC, Smythe WR, et al. Cardiac autotransplantation for primary cardiac tumors. Ann Thorac Surg 2006;82:645–50. [DOI] [PubMed]
- 17.Mabuchi T, Shimizu M, Ino H, Yamguchi M, Terai H, Fujino N, et al. PRKAR1A gene mutation in patients with cardiac myxoma. Int J Cardiol 2005;102:273–7. [DOI] [PubMed]


