Abstract
Acute fulminant myocarditis commonly manifests itself as severe, rapidly progressive hemodynamic deterioration and circulatory collapse that may be resistant to high doses of inotropic agents and steroids and to mechanical support by intra-aortic balloon pump. Acute myocarditis has a high mortality rate and may necessitate heart transplantation. The best short-term therapy available to support the patient may be a percutaneous left ventricular assist device.
One such unit, the TandemHeart® percutaneous ventricular assist device, can enable patients to recover in a few days. Two of our patients who experienced profound, therapy-resistant heart failure arising from acute myocarditis were successfully supported by the TandemHeart. To the best of our knowledge, these are the 1st reported cases in which the TandemHeart percutaneous ventricular assist device served as a bridge to recovery from acute fulminant myocarditis.
Key words: Assisted circulation/methods; heart-assist devices; myocarditis/complications/surgery/therapy; shock, cardiogenic/therapy; treatment outcome; ventricular dysfunction, left/etiology/therapy
Acute fulminant myocarditis commonly results in severe, rapidly progressive hemodynamic deterioration and circulatory collapse; the condition can be fatal. When acute myocarditis is resistant to medical therapy and to mechanical support from an intra-aortic balloon pump (IABP), a percutaneous left ventricular assist device (pVAD) can support the patient and enable recovery. We report our use of the TandemHeart® pVAD™ (CardiacAssist, Inc.; Pittsburgh, Pa) in the treatment of 2 patients who were experiencing profound heart failure secondary to acute myocarditis.
Case Reports
Patient 1
In March 2006, a previously healthy 38-year-old woman presented at a local hospital with flu-like symptoms, weakness, and hypotension (systolic blood pressure, 75 mmHg). For 4 weeks, she had received outpatient care for upper respiratory symptoms (including cough, fever, and chills) that were unsuccessfully treated with antibiotics and amantadine. She had no history of alcohol, tobacco, or drug use.
She was admitted to that hospital and was started on vasopressin. Transthoracic echocardiography showed poor left ventricular (LV) function: the ejection fraction (LVEF) was <0.20, and the end-diastolic diameter was 4.2 cm. Also evident were mild mitral and tricuspid regurgitation, nondilated ventricles, severely reduced right ventricular (RV) systolic function, and a small pericardial effusion consistent with a diagnosis of myopericarditis. The patient subsequently went into cardiogenic shock, was intubated, underwent placement of an IABP, and was administered high doses of pressors. An angiogram showed normal coronary arteries.
The patient was transferred to our institution for further care. Upon arrival, her systolic blood pressure was 70 mmHg on IABP support and multiple pressors. An electrocardiogram revealed sinus tachycardia with diffuse ST elevation. She was oliguric and displayed evidence of end-organ dysfunction that suggested severe cardiogenic shock resistant to the therapy. The hemodynamic data included a cardiac index of 1.6 L/min and a pulmonary artery wedge pressure of 18 mmHg. Because the patient was critically ill and had been symptomatic for about 4 weeks, the sensitivity of an endomyocardial biopsy would have been decreased; therefore, no biopsy was performed.
To provide the patient with improved mechanical hemodynamic support, we decided to place the TandemHeart® pVAD™, a ventricular assist device that can provide up to 4 L/min of systemic blood flow (Fig. 1).
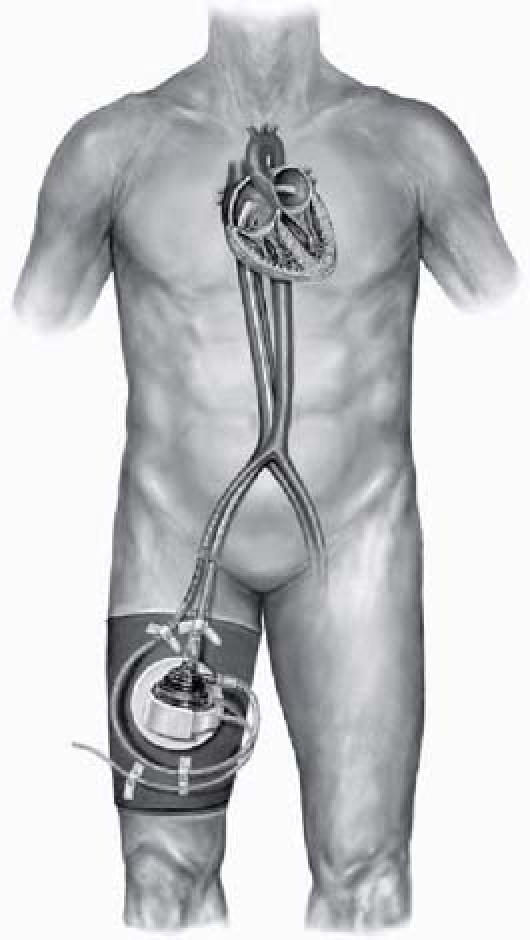
Fig. 1 The TandemHeart® pVAD™ (CardiacAssist, Inc.; Pittsburgh, Pa) and associated blood circuit components. The aortic outflow is positioned in the right femoral artery.
The patient was rushed to the cardiac catheterization laboratory. She was cold and pale, with systolic blood pressure in the range of 60 to 70 mmHg. Her groins were prepared. A transseptal catheter was inserted into the right femoral vein and guided into the right atrium.
The patient then developed ventricular fibrillation. After being defibrillated to sinus rhythm, she experienced ventricular tachycardia that resolved spontaneously. She was promptly intubated and was started on an intravenous drip of amiodarone and lidocaine. Standard transseptal cannulation was then performed, the catheter was placed into the left atrium under fluoroscopic guidance, and 8,000 IU of heparin was administered for anticoagulation. The left femoral artery was progressively dilated. On the basis of an aortogram obtained at the beginning of the procedure in order to determine cannula size, a 15F catheter was placed.
The TandemHeart pump was primed, air was removed from the lines, and the patient was started on pVAD support. When the flow rate reached approximately 2.5 L/min, a chest radiograph showed a heart shadow that suggested RV failure, so in order to decrease the RV preload, the pump speed was maintained at a lower flow rate of approximately 2.4 L/min. The cannulae were secured, the anticoagulant and sedation levels were maintained, and the patient was transferred to the recovery room. She was started on an intravenous drip of vasopressin, epinephrine, dopamine, and milrinone for RV support and was given fluid. The IABP was left in place.
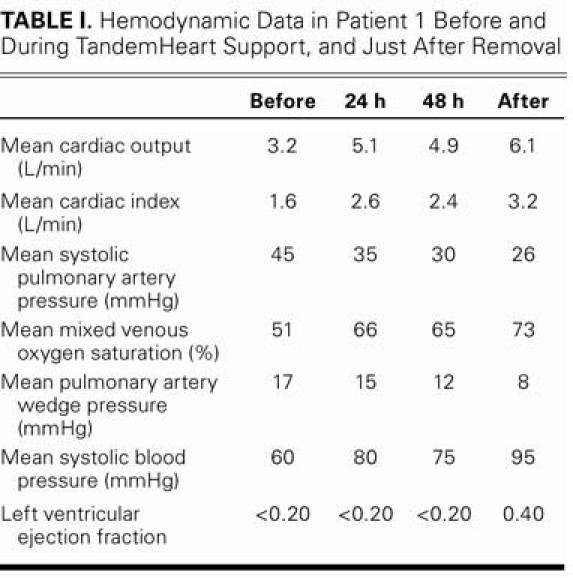
Test results were negative for influenza, herpes simplex virus, human immunodeficiency virus, cytomegalovirus, and hepatitis. The patient was given a bolus of 500 mg of intravenous methylprednisone, followed by 1 mg/kg per day of prednisone (a dosage that was to be tapered to zero over 6 weeks), and 1 intravenous dose of immunoglobulin. After the immunoglobulin infusion, the patient became severely oliguric. Despite treatment with diuretics, her urine output was less than 10 cc/hr, and her levels of urea nitrogen serum and creatinine were elevated. Continuous venovenous hemodialysis was initiated and maintained for 2 days, which improved renal function. Despite hemodynamic stability on the pVAD, the IABP, and the pressors, transthoracic echocardiography showed severe biventricular failure (EF, <0.20), and the patient could not tolerate a lower pVAD pump speed. On day 4 after pVAD placement, ejection had improved, and the patient was getting better. On day 7, after further hemodynamic and clinical progress, we began reducing the pump rate in order to wean her from the pVAD. She was completely dependent on the pVAD to provide the output necessary for organ perfusion, which confirmed that the TandemHeart was chiefly responsible for the clinical and hemodynamic improvements. Table I shows the progressive improvement in the patient's hemodynamic readings.
TABLE I. Hemodynamic Data in Patient 1 Before and During TandemHeart Support, and Just After Removal
We surgically removed the pVAD on day 8. After its removal, cardiac magnetic resonance imaging and transthoracic echocardiography showed markedly improved LV contractility (LVEF, 0.40), normal LV shape and size, and moderate RV systolic dysfunction (EF, 0.35) without echographic evidence of atrial septal defect. Viability studies by cardiac magnetic resonance imaging after 2 weeks of hospitalization revealed patchy epicardial hyperenhancement in the mid-inferoseptum and in the mid-inferior and mid-anterolateral walls of the LV; the remainder of the LV appeared of normal thickness, with viable myocardium (Fig. 2).
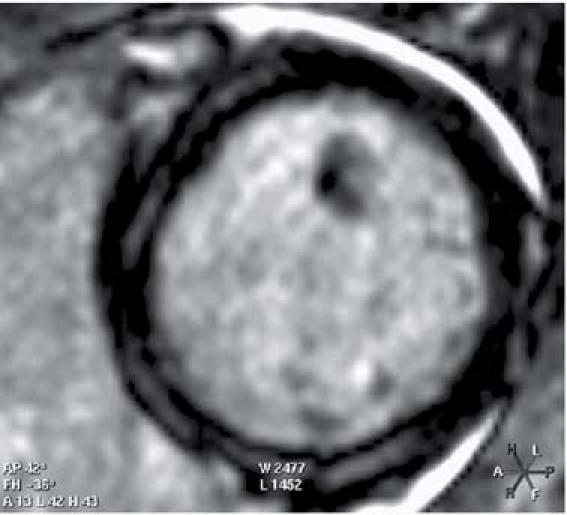
Fig. 2 Patient 1. Cardiac magnetic resonance imaging shows patchy epicardial hyperenhancement after 2 weeks of hospitalization. The remainder of the left ventricle appears of normal thickness, with viable myocardium.
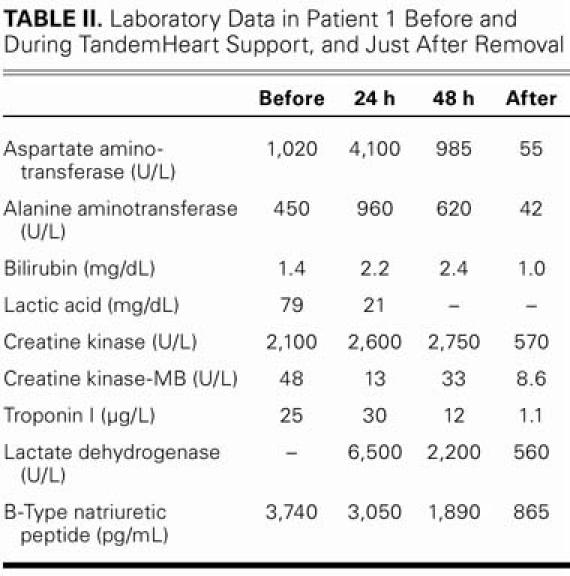
Levels of B-type natriuretic peptide, creatine kinase, creatine kinase-MB, troponin I, lactate dehydrogenase, and uric acid—all of which were very high upon the patient's hospital admission—progressively decreased after pVAD placement. Table II shows the progression of the laboratory data.
TABLE II. Laboratory Data in Patient 1 Before and During TandemHeart Support, and Just After Removal
The patient remained neurologically intact during her 2-week hospitalization and was discharged in good condition. Her outpatient therapy included close follow-up and prescriptions for an angiotensin-converting enzyme inhibitor, a β-adrenergic blocker, an aldosterone antagonist, and steroids (to be tapered to zero over 6 weeks). Six months after hospital discharge, the patient remained stable and asymptomatic, with a low-to-normal LVEF (0.50–0.55) on echocardiography.
In summary, the patient was experiencing multisystemic organ failure upon hospital admission. The pVAD enabled progressively improved organ function via better systemic perfusion.
Patient 2
After a few days of experiencing flu-like symptoms, a 63-year-old woman with a medical history of asthma was admitted to a local hospital in April 2006 for respiratory failure and sudden-onset (“flash”) pulmonary edema. Her condition required prompt intubation. She had mildly elevated cardiac enzymes, leukocytosis, insignificant electrocardiographic changes, and mild ST elevations in the high-lateral leads. A transthoracic echocardiogram showed an LVEF <0.20, with normal LV size. She was hypotensive (systolic blood pressure, 70–75 mmHg) with bradycardia (heart rate, 50–55 beats/min).
The patient was transferred to our institution in cardiogenic shock. She had been given dopamine and heparin. Emergency coronary angiography revealed normal coronary arteries and a LVEF of 0.10 to 0.15. She underwent right heart catheterization that included a RV endomyocardial biopsy, and an IABP was placed. Results of the biopsy were negative for inflammatory changes and for viral, bacterial, and fungal infection. In the intensive care unit, despite the administration of dopamine, dobutamine, and vasopressin in high doses, her hypotension and bradycardia worsened to a systolic blood pressure in the 50- to 60-mmHg range and a heart rate in the range of 30 to 40 beats/min. She experienced ventricular fibrillation and was promptly transferred to the catheteri-zation laboratory for placement of a TandemHeart pVAD. During this procedure, her systolic blood pressure decreased to 30 mmHg and her heart rate slowed to <20 beats/min. Cardiopulmonary resuscitation was initiated, along with transcutaneous pacing. Almost immediately after the pVAD was started, she became more stable hemodynamically. Due to the probability of fulminant myocarditis, she was given intravenous doses of methylprednisone, immunoglobulin, and gamma globulin. A few hours after the placement of the pVAD, her systolic blood pressure fell from 90 to 60 mmHg, and she was given more pressors. Cardiac tamponade was suspected, so she was taken to the operating room. Mediastinal exploration revealed tamponade caused by a RV perforation, which was repaired. The next day, a 2nd exploration was required for tamponade that was caused by a large hematoma. Transesophageal echocardiography revealed a large right-to-left shunt through an artificial atrial septal defect and a patent foramen ovale not much larger than 1 cm in diameter; these 2 conditions were not repaired. After approximately 2 days of hemodynamic stabilization and a return to normal EF, the pVAD and the IABP were removed. Our final diagnosis was severe cardiogenic shock secondary to acute fulminant myocarditis, with a negative endomyocardial biopsy.
The patient remained unresponsive even when not sedated. Computed tomography and magnetic resonance angiography revealed evidence of multiple strokes in the occipital area of the cerebellum. These strokes might have been related to the patent foramen ovale induced by the placement of the pVAD. The patient remained critically ill, then underwent a few days of aggressive physical rehabilitation and began a slow neurologic recovery. She experienced several episodes of paroxysmal atrial fibrillation, for which treatment with amiodarone and aspirin was initiated.
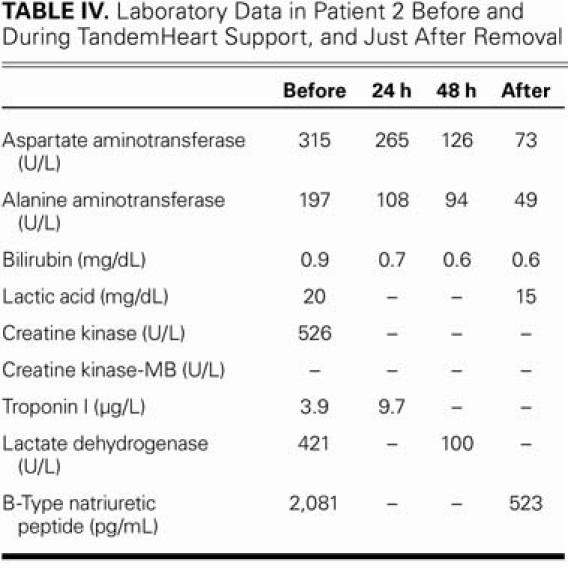
The patient completely recovered hemodynamically after 2 days of mechanical circulatory support from the pVAD. Table III shows the hemodynamic progression and Table IV, the progression of the laboratory data. The patient also experienced tamponade and RV perforation, probably as a consequence of the endomyocardial biopsy.
TABLE III. Hemodynamic Data in Patient 2 Before and During TandemHeart Support, and Just After Removal
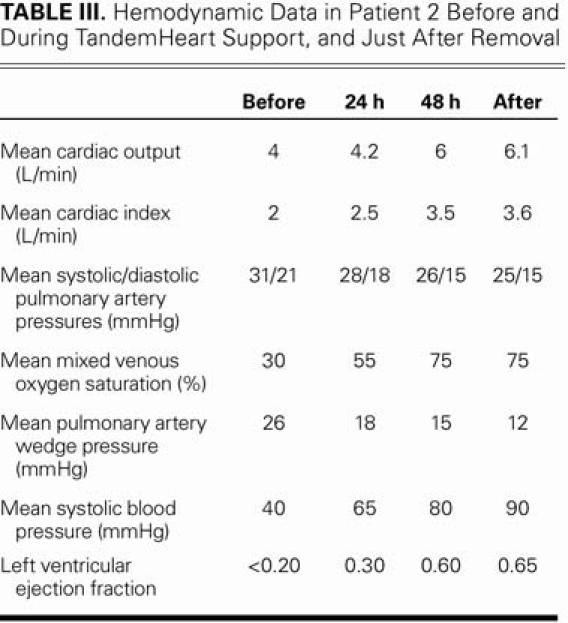
TABLE IV. Laboratory Data in Patient 2 Before and During TandemHeart Support, and Just After Removal
After 3 weeks of hospitalization, the patient had normal cardiac function and no residual neurologic deficit. She was discharged from the hospital in good condition on a regimen of amiodarone, clopidogrel, aspirin, and lisinopril. Four months after hospital discharge, she remained asymptomatic.
Discussion
Myocarditis, defined as inflammation of the myocardium, is characterized by an inflammatory cellular infiltrate with or without evidence of myocyte necrosis. Myocarditis has many causes, but it is most commonly viral. Clinical manifestations of myocarditis range from an asymptomatic state with an abnormal electrocardiogram to a fulminant condition characterized by arrhythmias, heart failure, cardiogenic shock, and death. Myocarditis is a well-established cause of dilated cardiomyopathy (in 9% of cases)1; in prospective postmortem data, myocarditis was implicated in 8% to 12% of sudden cardiac deaths.2,3
Despite the morbidity and death associated with myocarditis, clinical practice guidelines are still lacking. Supportive care is the 1st line of treatment. Fulminant myocarditis can have an excellent prognosis if the patient's hemodynamic collapse is treated aggressively and without delay.4 The necessarily intensive level of care will include IABP support and the aggressive administration of positive inotropic agents, vasopressors, and diuretics. Rarely, in collapses refractory to optimal medical treatment and IABP placement, a ventricular assist device will be required to sustain the patient. Acker5 described a survival rate of 50% to 70% after the implementation of mechanical support. In addition, multiple studies have shown that LVAD support can promote myocardial recovery by producing substantial structural changes in the failing heart, including a reduction in stress wall and LV dimensions, an improvement of myocyte contractility and β-adrenergic-blocker responsiveness, and the promotion of ventricular remodeling.6–10 Treatment after initial hemodynamic stabiliza-tion should follow the American College of Cardiology and American Heart Association guidelines for treatment of heart failure.11
Mechanical devices that have been used in the treatment of acute fulminant myocarditis and other causes of heart failure are generally expensive; their use also requires surgical implantation and removal, with the accompanying risk of morbidity or death. In contrast, the much less expensive TandemHeart pVAD can be rapidly inserted in the catheterization laboratory via a transseptal approach12–14 and can then be removed via a minimally invasive technique.12 The TandemHeart improves hemodynamic conditions by reducing left atrial pressure, increasing mean arterial pressure, and improving cardiac output. In 18 patients who were supported by the TandemHeart, Thiele and colleagues13 reported a relatively low 44% overall mortality rate from cardiogenic shock, and an even lower 31% rate from this cause in patients who had no ventricular septal defect. This device has been used to mitigate LV failure due to cardiogenic shock of various origins, as a bridge for surgical or interventional procedures, and even as a bridge to heart transplantation. The device can usually be explanted when the patient's cardiac index is more than 2 L/min per m2 in the presence of minimal inotropic support or without increasing filling pressures.15
The TandemHeart pVAD, a centrifugal, continuous-flow pump, consists of a single rotor suspended by magnetic force on a thin lubricating-fluid film. Heat and friction are lessened, which reduces risk of thromboembolism. The TandemHeart can be left in place for up to 2 weeks without producing significant thromboembolic or hemorrhagic events.12 This relatively new pVAD seems safer and more promising than its predecessors on the basis of its ease of use, its survival benefit, the fact that it can be rapidly inserted even in urgent circumstances, and its encouraging safety profile.12–14 Although the TandemHeart's contribution to morphologic recovery from acute myocarditis is difficult to isolate from the spontaneous healing process, its mechanical support reduces hemodynamic demand and, by unloading the LV, most likely accelerates the healing mechanisms that follow acute inflammatory damage.
We conclude that short-term circulatory support during acute heart failure can enable ventricular myocytes to undergo substantial, beneficial functional changes as a consequence of hemodynamic unloading and of the reversal of neurohumoral and circulatory derangements caused by the initial cardiac insult. The cases of our 2 patients illustrate the essential role of pVAD support as a bridge to recovery from acute fulminant myocarditis. We believe that this is the 1st report of the TandemHeart pVAD's having conferred this benefit.
Footnotes
Address for reprints: Wissam I. Khalife, MD, 19220 Space Center Blvd. #1527, Houston, TX 77058-3753. E-mail: wkhalife@yahoo.com
References
- 1.Felker GM, Hu W, Hare JM, Hruban RH, Baughman KL, Kasper EK. The spectrum of dilated cardiomyopathy. The Johns Hopkins experience with 1,278 patients. Medicine (Baltimore) 1999;78:270–83. [DOI] [PubMed]
- 2.Fabre A, Sheppard MN. Sudden adult death syndrome and other non-ischaemic causes of sudden cardiac death. Heart 2006;92:316–20. [DOI] [PMC free article] [PubMed]
- 3.Doolan A, Langlois N, Semsarian C. Causes of sudden cardiac death in young Australians. Med J Aust 2004;180:110–2. [DOI] [PubMed]
- 4.McCarthy RE 3rd, Boehmer JP, Hruban RH, Hutchins GM, Kasper EK, Hare JM, Baughman KL. Long-term outcome of fulminant myocarditis as compared with acute (nonfulminant) myocarditis. N Engl J Med 2000;342:690–5. [DOI] [PubMed]
- 5.Acker MA. Mechanical circulatory support for patients with acute-fulminant myocarditis. Ann Thorac Surg 2001;71(3 Suppl):S73–6; discussion S82–5. [DOI] [PubMed]
- 6.Altemose GT, Gritsus V, Jeevanandam V, Goldman B, Margulies KB. Altered myocardial phenotype after mechanical support in human beings with advanced cardiomyopathy. J Heart Lung Transplant 1997;16:765–73. [PubMed]
- 7.Dipla K, Mattiello JA, Jeevanandam V, Houser SR, Margulies KB. Myocyte recovery after mechanical circulatory support in humans with end-stage heart failure. Circulation 1998;97: 2316–22. [DOI] [PubMed]
- 8.Nakatani S, McCarthy PM, Kottke-Marchant K, Harasaki H, James KB, Savage RM, Thomas JD. Left ventricular echocardiographic and histologic changes: impact of chronic unloading by an implantable ventricular assist device. J Am Coll Cardiol 1996;27:894–901. [DOI] [PubMed]
- 9.Levin HR, Oz MC, Chen JM, Packer M, Rose EA, Burkhoff D. Reversal of chronic ventricular dilation in patients with end-stage cardiomyopathy by prolonged mechanical unloading. Circulation 1995;91:2717–20. [DOI] [PubMed]
- 10.Zafeiridis A, Houser SR, Mattiello JA, Jeevanandam V, Margulies KB. LVAD support produces “reverse remodeling” of cardiac myocytes. Circulation 1997;96(Suppl I):I–603.
- 11.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, et al. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation; endorsed by the Heart Rhythm Society. Circulation 2005;112:e154–235. [DOI] [PubMed]
- 12.Vranckx P, Foley DP, de Feijter PJ, Vos J, Smits P, Serruys PW. Clinical introduction of the Tandemheart, a percutaneous left ventricular assist device, for circulatory support during high-risk percutaneous coronary intervention. Int J Cardiovasc Intervent 2003;5:35–9. [DOI] [PubMed]
- 13.Thiele H, Lauer B, Hambrecht R, Boudriot E, Cohen HA, Schuler G. Reversal of cardiogenic shock by percutaneous left atrial-to-femoral arterial bypass assistance. Circulation 2001; 104:2917–22. [DOI] [PubMed]
- 14.Aragon J, Lee MS, Kar S, Makkar RR. Percutaneous left ventricular assist device: “TandemHeart” for high-risk coronary intervention. Catheter Cardiovasc Interv 2005;65:346–52. [DOI] [PubMed]
- 15.Lemos PA, Cummins P, Lee CH, Degertekin M, Gardien M, Ottervanger JP, et al. Usefulness of percutaneous left ventricular assistance to support high-risk percutaneous coronary interventions. Am J Cardiol 2003;91:479–81. [DOI] [PubMed]


