Abstract
As a complication of myocardial ischemia, severe elongation of the anterior papillary muscle with resultant mitral valve insufficiency is a rare clinical finding. Using echocardiography, we accurately diagnosed this condition in a 75-year-old man. The patient underwent successful plication of the elongated anterior papillary muscle and the implantation of polytetrafluoroethylene neochordae tendineae.
Key words: Aged; cardiac surgical procedures/methods; echocardiography, Doppler; mitral valve/surgery; mitral valve insufficiency/complications/etiology/pathology/surgery; mitral valve prolapse/etiology/surgery; myocardial ischemia/complications; papillary muscles/pathology/surgery; reconstructive surgical procedures/methods; treatment outcome
An unusual complication of myocardial ischemia—severe elongation of the anterior papillary muscle with resultant mitral regurgitation—has rarely been reported in the medical literature.1–3 Herein, we present the case of a patient who had severe mitral valve insufficiency secondary to ischemic remodeling of the anterior papillary muscle. The condition was accurately diagnosed, and the patient underwent successful surgical correction.
Case Report
In December 2004, a 75-year-old man with a history of gradually increasing exertional dyspnea was admitted to our hospital. The patient had experienced an inferolateral myocardial infarction 13 years previously. Physical examination revealed a grade 4/6 blowing systolic murmur at the apex of the heart, radiating to the left axilla. Standard chest radiography suggested left ventricular enlargement with increased pulmonary vasculature. Electrocardiography showed sinus rhythm, Q waves in the inferior leads, and negative T waves in the lateral leads. Transthoracic echocardiography (TTE) revealed severe mitral regurgitation (100 mL/sec of regurgitant volume, as determined by use of the proximal isovelocity surface area method). The regurgitation was secondary to a loss of leaflet coaptation, which was caused by an isolated prolapse of a thin anterior mitral leaflet (Figs. 1–3). The septal lateral diameter of the mitral annulus was 42 mm. The left ventricle was dilated (end-diastolic volume, 180 mL; end-diastolic diameter, 62 mm); septal hyperkinesia and inferior and lateral akinesia were present (Fig. 4). The left ventricular myocardial contractility was depressed (ejection fraction, 0.35). Severe tricuspid regurgitation and pulmonary hypertension (systolic pulmonary artery pressure, 70 mmHg) were shown. Coronary arteriography revealed complete occlusion of the posterior descending artery and severe stenosis of the obtuse marginal branch of the circumflex coronary artery.

Fig. 1 Preoperative 2-dimensional transthoracic echocardiogram (parasternal long-axis view) shows reduced coaptation of the mitral valve due to marginal prolapse of the medial scallop (A2) of the anterior leaflet. Scarring from an old inferolateral myocardial infarction is visible.

Fig. 2 Preoperative color-flow Doppler transthoracic echocardiogram (parasternal long-axis view: zoom) shows an eccentric jet of mitral regurgitation.

Fig. 3 Preoperative transthoracic echocardiogram (2-dimensional and color-flow Doppler, apical 4-chamber view) shows loss of mitral valve coaptation and severe mitral regurgitation. Note that the anterior papillary muscle appears elongated, with higher echogenicity at its tip.

Fig. 4 Preoperative transthoracic echocardiogram (apical 4-chamber view) shows loss of mitral valve coaptation and marginal prolapse of the anterior leaflet (scallop A3). The anterior papillary muscle appears elongated.
The patient was taken to surgery. Pre-pump transesophageal echocardiography (TEE) confirmed the prolapse of the anterior mitral leaflet. Total hypothermic cardiopulmonary bypass was instituted at 32 °C, the aorta was cross-clamped, and warm-blood cardioplegic solution was administered. Direct inspection of the mitral valve and careful exposure and examination of the papillary muscles revealed that the anterior papillary muscle was fibrotic and severely elongated (Fig. 5). The valve leaflets were morphologically normal, although there was a significant prolapse of the medial portion of the anterior leaflet above the level of the mitral annulus. The chordae tendineae were normally placed and of normal length. The mitral annulus was dilated. The tricuspid valve was dilated but had normal valve leaflets.
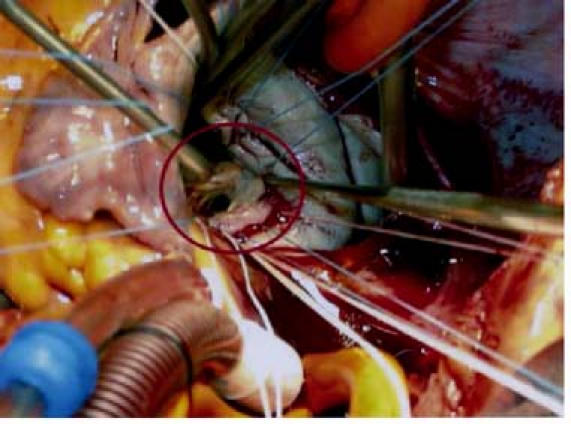
Fig. 5 Intraoperative photo shows elongation of the anterior papillary muscle (circle).
We corrected the mitral insufficiency by plicating and reattaching the fibrotic elongated portion of the papillary muscle to the residual healthy upper extremity of the muscle itself. A pericardium-pledgeted polytetrafluoroethylene (PTFE) suture (GoreTex®, W.L. Gore & Associates, Inc.; Flagstaff, Ariz) was used. To secure the repair, we implanted a pair of artificial PTFE neochordae tendineae between the upper extremity of the residual healthy anterior papillary muscle and the medial portion of the anterior leaflet of the mitral valve. Mitral annuloplasty was performed with a Carpentier-Edwards Physio Annuloplasty Ring (Edwards Lifesciences; Irvine, Calif). Forceful injection of a saline solution into the left ventricle showed good mitral valve competence. Tricuspid valve competence was restored by means of a triple-pledgeted De Vega annuloplasty. Myocardial revascularization was performed with 2 saphenous vein grafts, one anastomosed to the posterior descending artery and the other to a marginal branch of the circumflex coronary artery. Sinus rhythm resumed spontaneously upon the declamping of the aorta, and the patient was weaned from cardiopulmonary bypass without complications.
Post-repair, intraoperative TEE revealed good coaptation of the mitral leaflets and the satisfactory restoration of mitral valve competence, with only trivial residual mitral regurgitation. Postoperative TTE confirmed the good result (Fig. 6) and showed negligible tricuspid regurgitation. The pulmonary artery pressures were normal.

Fig. 6 Postoperative 2-D and color-flow Doppler transthoracic echocardiogram (apical 4-chamber view) shows good mitral valve coaptation and trivial residual mitral regurgitation.
After an uneventful recovery, the patient was discharged from the hospital on the 7th postoperative day in good clinical condition.
At follow-up 13 months after surgery, TTE showed good mitral leaflet coaptation with trivial residual mitral regurgitation. The patient was in New York Heart Association functional class I. Thirty months after surgery, his clinical condition remained good and the echocardiographic findings were unchanged.
Discussion
Severe elongation of the papillary muscle is a rare mechanical complication of myocardial infarction and ischemia.1–3 The mechanism that leads to papillary muscle elongation was described in 2004 by Jouan and colleagues,4 who found a papillary muscle lesion in which an incomplete detachment of a head of a papillary muscle occurred while the muscle remained fixed to the ventricle via muscular bridges (“incomplete” papillary muscle rupture). It remains debatable whether papillary muscle elongation invariably represents a late sequela of an incomplete rupture or whether it is sometimes a primary lesion (the distention of a papillary muscle weakened by ischemic changes).
Late post-ischemic remodeling of the anterior papillary muscle occurs less frequently than does remodeling of the posterior papillary muscle. This is probably due to the anterior muscle's more favorable blood supply, which is provided by diagonal branches that originate from the left anterior descending coronary artery and by marginal branches of the circumflex coronary artery.5 In contrast, the posterior papillary muscle, which is vascularized independently by branches of either the right or the circumflex coronary artery,5 has a more vulnerable blood supply.
Transthoracic echocardiography enables an accurate diagnosis of papillary muscle post-ischemic dysfunction and helps to differentiate it from other causes of mitral regurgitation that occur in the absence of myocardial infarction. Upon TTE, the papillary muscle dysfunction appears as an isolated prolapse of the free border of the morphologically normal mitral valve leaflet. A loss of mitral valve coaptation that is caused by the prolapse of 1 leaflet can also be observed in myxomatous valves; however, in that situation, the mitral valve leaflets appear floppy and redundant. When ischemic injury to the papillary muscle is suspected, a perfusion scintigram may help to clarify the ischemic cause of an isolated free-border prolapse of 1 leaflet by revealing the presence of myocardial scar tissue.
Our patient had severe mitral regurgitation with isolated prolapse of the anterior mitral leaflet. The results of coronary arteriography and TEE were consistent with involvement of the anterior papillary muscle—findings that were confirmed intraoperatively.
We used TTE to accurately diagnose and quantify the severity of the lesion; the echocardiographic results also influenced our selection of the surgical technique. Intraoperatively, TEE was important in the description of the papillary muscle changes and enabled immediate, accurate evaluation of the surgical results.
Shortening of elongated papillary muscles has been reported, with good mid-term results.1–4 In our patient, we reconstructed the subvalvular apparatus by plicating the elongated fibrotic portion of the papillary muscle and, in supplement, by implanting artificial PTFE neochordae. The stability of the repair was confirmed at the patient's 2½-year follow-up examination.
Footnotes
Address for reprints: Ugo F. Tesler, MD, Clinica San Gaudenzio, Via Bottini 3, 28100 Novara, Italy. E-mail: hugin@iol.it
References
- 1.Fasol R, Wild T, Pfannmuller B, Stumpf J, Hacker R. Papillary muscle shortening for mitral valve reconstruction in patients with ischaemic mitral insufficiency. Eur Heart J 1998; 19:1730–4. [DOI] [PubMed]
- 2.Fasol R, Lakew F, Pfannmuller B, Slepian MJ, Joubert-Hubner E. Papillary muscle repair surgery in ischemic mitral valve patients. Ann Thorac Surg 2000;70:771–7. [DOI] [PubMed]
- 3.Fasol R, Lakew F, Wetter S. Mitral repair in patients with a ruptured papillary muscle. Am Heart J 2000;139:549–54. [DOI] [PubMed]
- 4.Jouan J, Tapia M, C Cook R, Lansac E, Acar C. Ischemic mitral valve prolapse: mechanisms and implications for valve repair. Eur J Cardiothorac Surg 2004;26:1112–7. [DOI] [PubMed]
- 5.Estes EH Jr, Dalton FM, Entman ML, Dixon HB 2nd, Hackel DB. The anatomy and blood supply of the papillary muscles of the left ventricle. Am Heart J 1966;71:356–62. [DOI] [PubMed]


