Abstract
Inferior vena cava filters are often used as alternatives to anticoagulant therapy for the prevention of pulmonary embolism. Many of the clinical data that support the use of these devices stem from relatively limited retrospective studies.
The dual purpose of this review is to examine the incidence of thrombotic complications associated with inferior vena cava filters and to discuss the role of anticoagulant therapy concurrent with filter placement. Device-associated morbidity and overall efficacy can be considered only in the context of rates of vena cava thrombosis, insertion-site thrombosis, recurrent deep venous thrombosis, and recurrent pulmonary embolism.
Key words: Anticoagulants/contraindications/therapeutic use, combined modality therapy, device removal, equipment design/safety/trends, evaluation studies, patient selection, prosthesis implantation, pulmonary embolism/prevention & control/therapy, recurrence, risk factors, thrombolytic therapy/methods, treatment outcome, vena cava filters/adverse effects/classification/contraindications/history/statistics & numerical data/trends/utilization, venous thrombosis/complications/prevention & control/therapy
Deep venous thrombosis (DVT) predisposes patients to pulmonary vascular occlusion and its secondary effects: an estimated 400,000 to 650,000 patients in the United States develop a pulmonary embolism (PE) each year, and there are 50,000 to 240,000 associated fatalities.1–3 Medical anticoagulation with oral or injectable agents, usually in the outpatient-care setting, remains the treatment of choice for DVT and its sequelae; secondary prevention of PE is achieved in up to 95% of cases.4–6 However, warfarin and heparin may be contraindicated for certain patients, particularly if interactions with concurrent medications or any active bleeding diathesis is suspected; in such cases, the placement of an inferior vena cava (IVC) filter may be appropriate.7,8 Vena cava filter (VCF) placement may be performed via minimally invasive interventional radiologic techniques.1,8 The steel Greenfield filter (Boston Scientific/Meditech; Watertown, Mass), initially used in 1973,9 and its titanium revision (Boston Scientific Corporation; Natick, Mass) are the IVC filters perhaps most familiar to medical practitioners. Alternative filters in common use include the Gianturco-Roehm Bird's Nest® Vena Cava Filter (Cook Medical, Inc.; Bloomington, Ind), the Simon Nitinol Filter® (Bard Peripheral Vascular, Inc.; Tempe, Ariz), the Günther Tulip filter (Cook), and the Vena Tech™–LGR® (B. Braun Medical, Inc.; Bethlehem, Pa) (Figs. 1 and 2). An estimated 49,000 IVC filters are placed annually in the United States.10
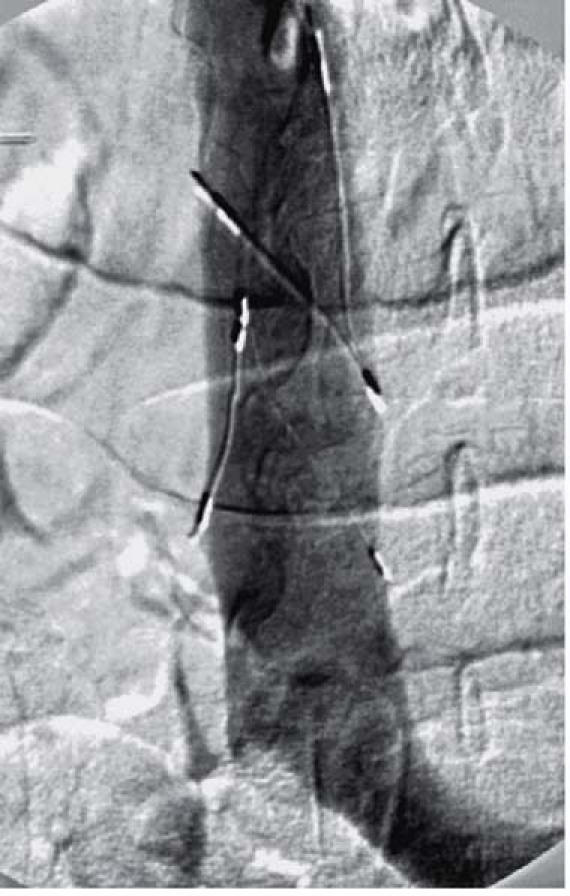
Fig. 1 Manipulation of a Bird's Nest® vena cava filter within the inferior vena cava, under fluoroscopic guidance.
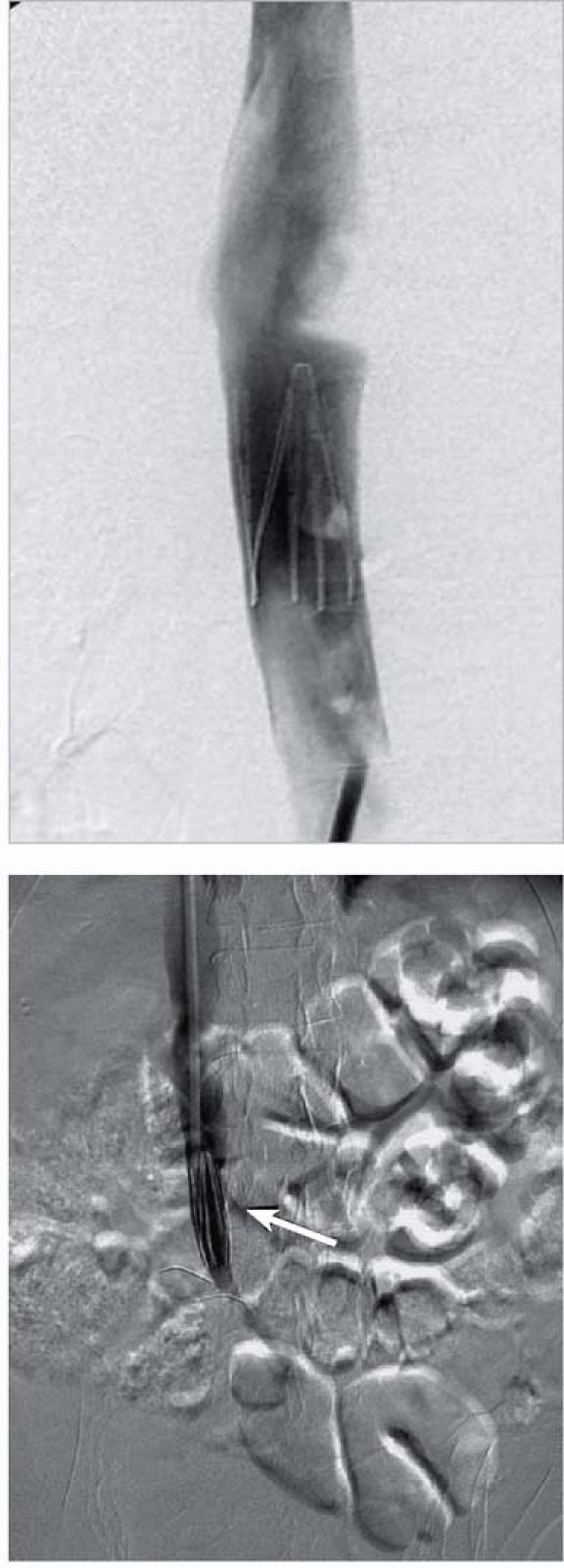
Fig. 2 Vena Tech™–LGM® vena cava filter deployed in a suprarenal position (top); and, in a different patient, in an infrarenal position (bottom, arrow).
Risks of Inferior Vena Cava Filters
Transjugular or transfemoral insertion of IVC filters may result in clinically significant complications, such as vena cava thrombosis, insertion-site thrombosis, intravascular migration, vena cava perforation, and recurrent DVT or PE.8,11 In addition, while the weight of clinical evidence may support the view that IVC filters prevent recurrent PE with a resultant high IVC patency rate, some practitioners have expressed concern that the filters may increase the risk of thrombosis at the insertion site and within the venous system.12,13 Clinical evidence supports the theory that clot accumulation within the device lumen itself is likely the cause of embolic complications.14
A single-center review15 in 2000 found the overall incidence of PE among 1,731 patients with IVC filters to be 5.6%. Death from PE occurred in 3.7% of the patients (median time, 4 days after insertion). Vena cava thrombosis occurred in 2.7% of the patients. Most filters were placed due to a contraindication to, or prior failure of, anticoagulation (94%). Because the procedures were conducted over a 25-year period, some early filter failures may have been attributable to early practitioner inexperience in placement and management, and to new technology.
Inferior Vena Cava Filter Efficacy: Primary Evidence
Before the advent of IVC filters in 1969 in the form of the Mobin-Uddin system,16 interventional approaches to the prevention of PE were largely limited to open surgical femoral vein ligation or IVC clipping, introduced in the 1930s and 1940s, respectively, each with low reported efficacy and high possible operative morbidity. Subsequently, since their introduction, IVC filters have been subject to only a single randomized controlled trial17 in which the efficacy of thrombosis rate reduction in patients was evaluated. A clear understanding of this landmark trial and its construction is important in evaluating the filters' benefits. Four hundred participants were randomly assigned, in a 2 × 2 factorial design, to receive anticoagulation with or without filter placement. Anticoagulation was achieved with either unfractionated heparin (205 patients) or enoxaparin (195 patients) for 8 to 12 days, along with warfarin initiated on day 4 and continued for at least 3 months. If warfarin was contraindicated, unfractionated heparin was substituted, again for at least 3 months. Half of the participants received a filter and half did not.
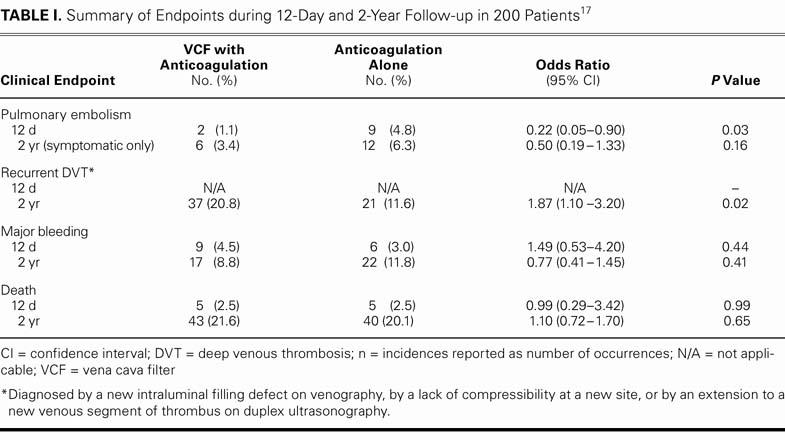
At the study's close,17 a statistically significant reduction of PE at the 12-day mark was observed with filtration, compared with anticoagulation alone; however, this effect was lost upon follow-up at 2 years. Furthermore, the patients who had a filter ran a significantly higher risk of recurrent DVT at 2-year follow-up. Overall, no difference in 2-year mortality rate was observed between the filter and nonfilter groups, and no difference in bleeding was noted between the heparin-treated and enoxaparin-treated groups (Table I). The authors concluded that an IVC filter should be implanted with caution in high-risk patients, because any initial efficacy of the filters was negated in the long term and no favorable impact on mortality rate was noted.
TABLE I. Summary of Endpoints during 12-Day and 2-Year Follow-up in 200 Patients17
Limitations of this trial17 include its not having met original patient enrollment targets (800 subjects were originally envisioned) and the narrow range of the enrolled subjects' clinical diagnoses, which hindered both the study's statistical power and its general application.
Vena Cava Filter Efficacy: Subsequent Trials
Additional evidence has been collected in a systematic manner to determine whether the use of IVC filters is justified. An observational analysis14 with 3,622 IVC-implanted patients and a control population of 64,333 patients with venous thromboembolism determined that an implanted filter was not associated with a significant reduction in later hospitalization for recurrent PE after 1 year. The patients who received filters and those who were medically managed were significantly more likely to be readmitted as inpatients if their initial presentation included PE (relative risk, 6.72; 95% confidence interval, 3.61–12.49) rather than venous thromboembolism (relative risk, 5.30; 95% confidence interval, 4.61–6.10). In addition, the filter was associated with a significantly higher risk of re-hospitalization for venous thromboembolism if the patient's initial presentation included PE (relative hazard, 2.62). The study was limited by a lack of documentation of anticoagulation and by a higher frequency of comorbidities and recurrent PE in the filter group.
A review by Streiff18 reported rates of thrombosis during follow-up of patients who had undergone filter placement; included within the analysis were case series with relatively short follow-up periods of 6 to 18 months. Patients lost to follow-up before the completion of the study were not included in the analysis. The reported rates of complications, such as DVT (5.9%–32%), IVC thrombosis (3.6%–11.2%), and insertion-site thrombosis (23%–36%), were highly variable. However, all filter types were equally effective in preventing PE, which occurred in 2.6% to 3.8% of patients.18 The rates of PE in this study corroborate prior reports of a 4% recurrence with the stainless-steel Greenfield filter, 3.5% with the titanium Greenfield filter, 2.7% with the Bird's Nest filter, and 2.9% with the Simon Nitinol filter.6,19–21
Concurrent Anticoagulation
To prevent thrombosis during filter insertion, anticoagulation is recommended unless otherwise contraindicated.4,22–24 A prospective study of intraprocedural bleeding in 100 patients with concurrent anticoagulation (including 87 patients with prolonged bleeding times) uncovered no cases of arterial puncture or venous bleeding during a venous interventional radiology procedure. The authors concluded that the continuation of anticoagulation therapy is indicated in patients with severe thromboembolic conditions.23
The proper protocol for administration of warfarin or heparin after filter replacement is far less clear. Aside from the randomized trial led by Decousus and colleagues,17 a paucity of complementary studies exists. A long follow-up period distinguishes a study by David and associates,25 which identified 10 patients with recurrent PE; 4 patients (who had thrombi <5 cm in length, measured from the apex of the filter) were treated with anticoagulation, and 6 patients (who had larger thrombi) received a 2nd VCF. Warfarin therapy proved beneficial in the short and long terms. Dissolution of the thrombi was observed in all warfarin-treated patients; at the 5-year mark, recurrent PE occurred in 1 patient after the discontinuation of anticoagulation.
A separate study,26 with shorter follow-up, presented additional (non-thrombus-related) benefits of anticoagulation: 47 patients with previous DVT were treated with anticoagulation after IVC placement. Anticoagulants significantly improved symptoms associated with post-thrombotic syndrome over early (<6-week) and late (>6-week) follow-up periods.
Of note, a small number of well-designed trials have shown no significant association between medical therapy and the reduction of thromboembolism in recipients of VCFs. Ortega and colleagues24 examined the records of 199 patients who received IVC filters, both with and without anticoagulation upon hospital discharge. Warfarin treatment extended from 1 to 8 weeks (mean period, 2 weeks). Data on 89 anticoagulated patients and 81 patients with a VCF alone were available for follow-up. Over a follow-up period of 3 to 60 months (mean, 39 mo), no differences in early recurrent DVT or PE were detected between groups.
In another retrospective study, Poletti and coworkers27 evaluated 114 IVC filter patients who were treated with or without anticoagulation. After a mean period of 27 months, there were no statistically significant differences in recurrence of PE (4.3% vs 3.9%, respectively), IVC filter thrombosis (2.2% vs 5.9%), or insertion-site thrombosis (6.5% vs 2.0%).
In a prospective trial, Greenfield and Proctor28 monitored 465 VCF patients for a mean period of 9 years. New DVT occurred in 12% of 241 patients given anticoagulation versus 15% without anticoagulation (P=0.35); new PE in 2% versus 4%, respectively (P=0.16), and vena cava thrombosis in 0.4% in both groups. The results of this study were weakened by inconsistent documentation of both levels and duration of anticoagulation. Although no statistically supported difference was found, the general trend favored anticoagulant therapy for the prevention of recurrent DVT in VCF patients.
Evolving Filter Systems
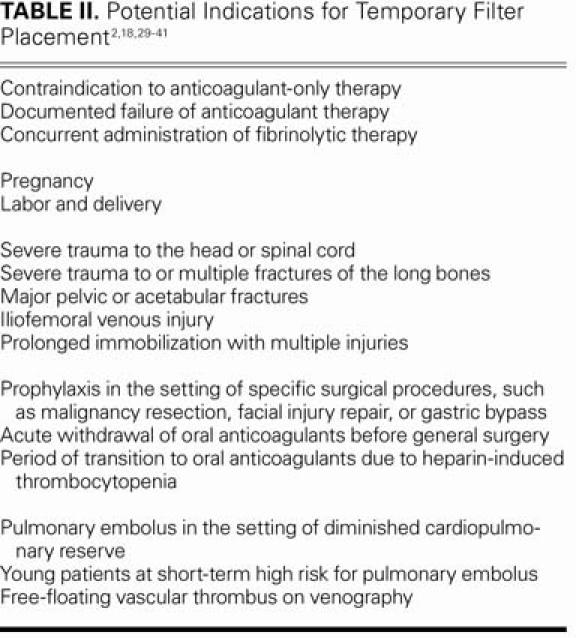
Filters such as the Günther Tulip™ (Cook Medical) and the TrapEase® (Cordis Corp., a Johnson & Johnson company; Miami Lakes, Fla) have been proposed for deployment in thrombosis-prone patients during short, high-risk periods (Fig. 3). The underlying assumption behind such systems is that long-term complications such as recurrent DVT, vena cava thrombosis, and vena cava perforation can be avoided. Indications for nonpermanent filters include pregnancy, trauma, and surgery (Table II). The Günther Tulip and the OptEase® (Cordis) have received approval from the U.S. Food & Drug Administration for use as retrievable devices. Temporary filters usually remain in place for 10 to 14 days; after this window, device endothelialization has been observed.21,40,42 However, Millward and colleagues43 and Pieri and co-authors44 have shown high efficacy rates with such filters in place for as long as 25 days (90 patients) and 63 days (18 patients), respectively. In these nonrandomized, retrospective reports, vena cava thrombosis, insertion-site thrombosis, and recurrent PE occurred rarely, if ever.18,43,44 The results of these studies have been tempered by a retrospective review of 17 patients by Millward,45 in which no reduction in the rate of inferior vena cava and insertion-vein thrombosis was recorded.
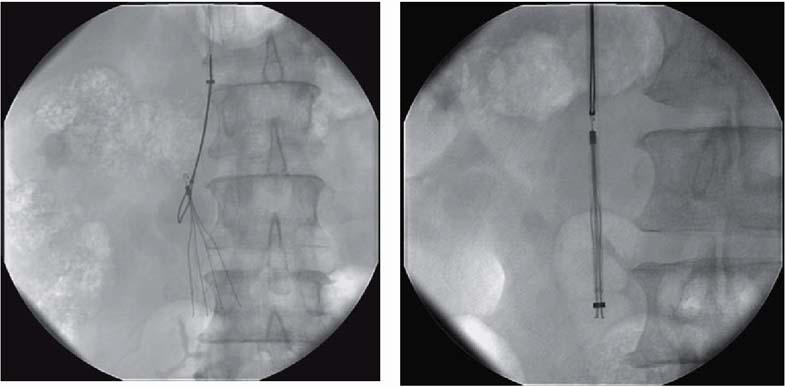
Fig. 3 Snaring of a removable Günther Tulip™ vena cava filter (left) and its capture in a catheter sheath (right).
TABLE II. Potential Indications for Temporary Filter Placement2,18,29–41
Nonpermanent VCF systems are a focus of active basic research; several manufacturers are developing or refining devices. Two such models, the recoverable ALN filter (ALN Implants Chirurgicaux; Ghisonaccia, France),46 and the Recovery nitinol filter (Bard), have been implanted in human beings, with promising initial published data.42 The Recovery was subsequently removed from market availability by the manufacturer. Research into permanent devices is less common, but it continues. For instance, the Cordis Keeper (Cordis) has been tested in a porcine model at the University of Gröningen (The Netherlands) with mixed results: 5 animals showed IVC patency for either 2 or 6 months, but 1 filter caused nonfatal caval wall penetration at 6 months.39
Discussion
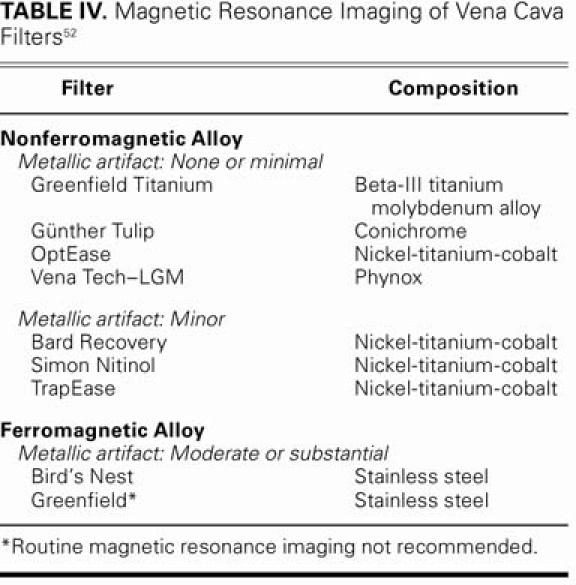
Table III presents a summary of complications that have been reported in the medical literature. The number of patients participating in the trials has varied, and the average follow-up period has been 1 to 1.5 years. In these trials, common forms of routine surveillance for complications consisted of abdominal radiography, ultrasonography, computed tomography, magnetic resonance imaging, and vena-cavography. Should patients require follow-up abdominal magnetic resonance imaging, there are several filters that do not obscure the IVC with a metallic artifact (Table IV).
TABLE III. Summary of Reported Inferior Vena Cava Complications7,13,15,18,27,35,42,43,46–51 (No. = Number of subjects through completion of study. All other values are percentages, with ranges in parentheses.)
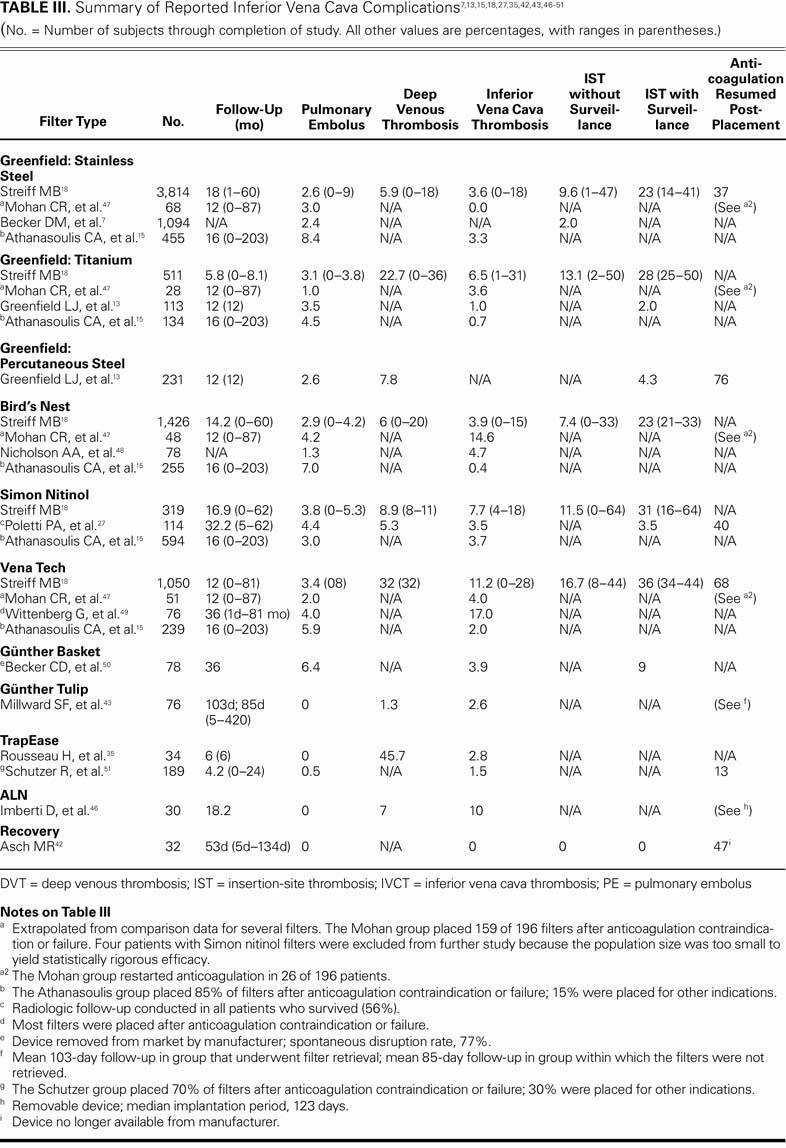
TABLE IV. Magnetic Resonance Imaging of Vena Cava Filters52
Robust evidence indicates that IVC filters effectively reduce the incidence of PE. However, patients in several studies were restarted on anticoagulation after filter placement: as many as 76% of study patients received this concurrent therapy. In addition, the rates of vena cava thrombosis, insertion-site thrombosis, and recurrent DVT are highly variable, and the long-term safety and efficacy of many VCFs remains unknown. Furthermore, the overall complication rates associated with each filter type have yet to be clearly delineated in long-term studies.
In regard to the generally expanding use of IVC filters over the past decade, a prospective study53 (2001–2002) was performed by Buller and colleagues and comprised more than 5,400 U.S. inpatients and outpatients who had ultrasonographically confirmed DVT. That study determined that 14% of those patients had received IVC filters and that 33% of filter recipients had received their filters for primary prevention of PE. The authors of this multicenter study expressed concern that the risks of VCFs ought to temper their use, and suggested that patients without histories of anticoagulant complications or hemorrhage should be strongly considered for medical therapy alone.
Clearly, additional well-designed clinical trials—prospective and sufficiently powered—are warranted in order to define the risk of filter-associated thrombosis. Until further information is available, the weight of evidence dictates VCF use only for patients in whom anticoagulation is contraindicated or ineffective. In support of this viewpoint, the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy has recommended against the use of IVC filters in most patients as a routine addition to an anticoagulant regimen.53
As a means of prophylaxis in high-risk patients who are being successfully treated with anticoagulants, permanent VCF insertion is not an established treatment, and it may increase the risk of thrombus-associated morbidity or death. In the high-embolic-risk patients, however, temporary filters appear to protect against PE and might be particularly useful as a bridge to oral anticoagulation, because the existing evidence most strongly supports VCF efficacy over short periods, such as 1 month.
Further testing is necessary to determine the cost-effectiveness of temporary VCFs. In addition, advances in basic VCF design, such as drug-eluting variants or stent-filter combinations, and in the development of recovery technology, may improve the overall cost-benefit ratio. As yet unclear are the merits of anticoagulation with alternative agents, such as danaparoid, ximelagatran, or lepirudin, in conjunction with VCFs; this subject invites future exploration.
Conclusion
Observational studies and case series have shown that long-term anticoagulation provides no benefit above that of a VCF alone. However, many of these studies were nonrandomized, retrospective, and conducted in selected patient populations; therefore, the validity and applicability of their findings are controversial.
Current best-practice guidelines suggest anticoagulation during and after VCF insertion for patients who have no contraindications. This therapy, adjusted for individual risk factors, should continue for at least 3 months after VCF placement.54
Acknowledgments
The authors thank Henry I. Bussey, PharmD (University of Texas Health Science Center and Anticoagulation Clinics of North America; San Antonio, Texas), for his guidance, and Suresh Vedantam, MD (Mallinckrodt Institute of Radiology, St. Louis), for his assistance with image preparation.
Footnotes
Address for reprints: Salil Patel, MD, Mallinckrodt Institute of Radiology, Washington University in St. Louis, 510 S. Kingshighway Blvd., Box 8131, St. Louis, MO 63110. E-mail: patelsa@mir.wustl.edu
References
- 1.Grassi CJ, Swan TL, Cardella JF, Meranze SG, Oglevie SB, Omary RA, et al. Quality improvement guidelines for percutaneous permanent inferior vena cava filter placement for the prevention of pulmonary embolism. J Vasc Interv Radiol 2003;14(9 Pt 2):S271–5. [PubMed]
- 2.Kinney TB. Update on inferior vena cava filters. J Vasc Interv Radiol 2003;14:425–40. [DOI] [PubMed]
- 3.Olin JW. Pulmonary embolism. Rev Cardiovasc Med 2002;3 Suppl 2:S68–75. [PubMed]
- 4.Hyers TM, Agnelli G, Hull RD, Morris TA, Samama M, Tapson V, Weg JG. Antithrombotic therapy for venous thromboembolic disease. Chest 2001;119(1 Suppl):176S–193S. [DOI] [PubMed]
- 5.Girard P, Tardy B, Decousus H. Inferior vena cava interruption: how and when? Annu Rev Med 2000;51:1–15. [DOI] [PubMed]
- 6.Jacobs DG, Sing RF. The role of vena caval filters in the management of venous thromboembolism. Am Surg 2003;69: 635–42. [PubMed]
- 7.Becker DM, Philbrick JT, Selby JB. Inferior vena cava filters. Indications, safety, effectiveness. Arch Intern Med 1992;152: 1985–94. [PubMed]
- 8.Joels CS, Sing RF, Heniford BT. Complications of inferior vena cava filters. Am Surg 2003;69:654–9. [PubMed]
- 9.Greenfield LJ, McCurdy JR, Brown PP, Elkins RC. A new intracaval filter permitting continued flow and resolution of emboli. Surgery 1973;73:599–606. [PubMed]
- 10.Stein PD, Kayali F, Olson RE. Twenty-one-year trends in the use of inferior vena cava filters. Arch Intern Med 2004;164: 1541–5. [DOI] [PubMed]
- 11.Urena R, Greenwood L. Bird's nest filter migration to the right atrium. AJR Am J Roentgenol 2004;183:1037–9. [DOI] [PubMed]
- 12.Greenfield LJ, Proctor MC. Thromboembolism: prevention and treatment with vena cava filters. Semin Arthroplasty 1992;3:123–7. [PubMed]
- 13.Greenfield LJ, Proctor MC, Cho KJ, Cutler BS, Ferris EJ, McFarland D, et al. Extended evaluation of the titanium Greenfield vena caval filter [published erratum appears in J Vasc Surg 1995;21:162]. J Vasc Surg 1994;20:458–65. [DOI] [PubMed]
- 14.White RH, Zhou H, Kim J, Romano PS. A population-based study of the effectiveness of inferior vena cava filter use among patients with venous thromboembolism. Arch Intern Med 2000;160:2033–41. [DOI] [PubMed]
- 15.Athanasoulis CA, Kaufman JA, Halpern EF, Waltman AC, Geller SC, Fan CM. Inferior vena caval filters: review of a 26-year single-center clinical experience. Radiology 2000;216: 54–66. [DOI] [PubMed]
- 16.Mobin-Uddin K, McLean R, Bolooki H, Jude JR. Caval interruption for prevention of pulmonary embolism. Long-term results of a new method. Arch Surg 1969;99:711–5. [DOI] [PubMed]
- 17.Decousus H, Leizorovicz A, Parent F, Page Y, Tardy B, Girard P, et al. A clinical trial of vena caval filters in the prevention of pulmonary embolism in patients with proximal deep-vein thrombosis. Prevention du Risque d'Embolie Pulmonaire par Interruption Cave Study Group. N Engl J Med 1998;338:409–15. [DOI] [PubMed]
- 18.Streiff MB. Vena caval filters: a comprehensive review. Blood 2000;95:3669–77. [PubMed]
- 19.Cooper JM, Silberzweig J, Mitty HA. Vena cava filters: available devices and current practices. Mt Sinai J Med 1996;63: 273–81. [PubMed]
- 20.Roehm JO Jr, Johnsrude IS, Barth MH, Gianturco C. The bird's nest inferior vena cava filter: progress report. Radiology 1988;168:745–9. [DOI] [PubMed]
- 21.Simon M, Athanasoulis CA, Kim D, Steinberg FL, Porter DH, Byse BH, et al. Simon nitinol inferior vena cava filter: initial clinical experience. Work in progress. Radiology 1989; 172:99–103. [DOI] [PubMed]
- 22.Carman TL, Fernandez BB Jr. Issues and controversies in venous thromboembolism. Cleve Clin J Med 1999;66:113–23. [DOI] [PubMed]
- 23.Gray RR, Sadler DJ, Shulman L, Saliken JC, So CB. Should anticoagulant therapy be stopped or reversed before venous intervention? Can Assoc Radiol J 1999;50:306–9. [PubMed]
- 24.Ortega M, Gahtan V, Roberts A, Matsumoto T, Kerstein M. Efficacy of anticoagulation post-inferior vena caval filter placement. Am Surg 1998;64:419–23. [PubMed]
- 25.David W, Gross WS, Colaiuta E, Gonda R, Osher D, Lanuti S. Pulmonary embolus after vena cava filter placement. Am Surg 1999;65:341–6. [PubMed]
- 26.Vitti M, Gagne PJ. Is anticoagulation justified after inferior vena cava filter placement [abstract]? Proc Intl Soc Cardiovasc Surg 1994. p. 558.
- 27.Poletti PA, Becker CD, Prina L, Ruijs P, Bounameaux H, Didier D, et al. Long-term results of the Simon nitinol inferior vena cava filter. Eur Radiol 1998;8:289–94. [DOI] [PubMed]
- 28.Greenfield LJ, Proctor MC. Recurrent thromboembolism in patients with vena cava filters. J Vasc Surg 2001;33:510–4. [DOI] [PubMed]
- 29.Scholz KH, Just M, Buchwald AB, Werner GS, Stille-Siegener M, Kreuzer H. Experiences with temporary vena cava filters in 114 at-risk patients with thrombosis or thromboembolism [in German]. Dtsch Med Wochenschr 1999;124:307–13. [DOI] [PubMed]
- 30.Backus CL, Heniford BT, Sing RF. Temporary vena cava filter placement for pulmonary embolism. J Am Osteopath Assoc 2002;102:555–6. [PubMed]
- 31.Kutlu R, Alkan A, Sigirci A, Altinok T, Yildirim Z. Temporary and permanent inferior vena cava filter combination in a young patient: to implant or not to implant? Cardiovasc Intervent Radiol 2003;26:492–5. [DOI] [PubMed]
- 32.Langan EM 3rd, Miller RS, Casey WJ 3rd, Carsten CG 3rd, Graham RM, Taylor SM. Prophylactic inferior vena cava filters in trauma patients at high risk: follow-up examination and risk/benefit assessment. J Vasc Surg 1999;30:484–8. [DOI] [PubMed]
- 33.Offner PJ, Hawkes A, Madayag R, Seale F, Maines C. The role of temporary inferior vena cava filters in critically ill surgical patients. Arch Surg 2003;138:591–5. [DOI] [PubMed]
- 34.Rochelson B, Scher L, Warshawsky R, Simon D. Use of a temporary vena cava filter in a woman with septic abortion and inferior vena cava thrombosis. A case report. J Reprod Med 2003;48:557–9. [PubMed]
- 35.Rousseau H, Perreault P, Otal P, Stockx L, Golzarian J, Oliva V, et al. The 6-F nitinol TrapEase inferior vena cava filter: results of a prospective multicenter trial. J Vasc Interv Radiol 2001;12:299–304. [DOI] [PubMed]
- 36.Ferrell A, Byrne TK, Robison JG. Placement of inferior vena cava filters in bariatric surgical patients–possible indications and technical considerations. Obes Surg 2004;14:738–43. [DOI] [PubMed]
- 37.Sue LP, Davis JW, Parks SN. Iliofemoral venous injuries: an indication for prophylactic caval filter placement. J Trauma 1995;39:693–5. [DOI] [PubMed]
- 38.Yamagami T, Kato T, Iida S, Tanaka O, Nishimura T. Retrievable vena cava filter placement during treatment for deep venous thrombosis. Br J Radiol 2003;76:712–8. [DOI] [PubMed]
- 39.Hoekstra A, Elstrodt JM, Nikkels PG, Tiebosch AT. Vessel wall reaction after vena cava filter placement. Cardiovasc Intervent Radiol 2002;25:53–6. [DOI] [PubMed]
- 40.Ricco JB, Bouin-Pineau MH, Camiade C, Blecha LM, Reynaud P, Faroy F, Marchand C. Emergency interruption of the inferior vena cava: a debatable issue. Cardiovasc Surg 2000; 8:411–21. [DOI] [PubMed]
- 41.Ponchon M, Goffette P, Hainaut P. Temporary vena caval filtration. Preliminary clinical experience with removable vena caval filters. Acta Clin Belg 1999;54:223–8. [PubMed]
- 42.Asch MR. Initial experience in humans with a new retrievable inferior vena cava filter. Radiology 2002;225:835–44. [DOI] [PubMed]
- 43.Millward SF, Oliva VL, Bell SD, Valenti DA, Rasuli P, Asch M, et al. Gunther Tulip retrievable vena cava filter: results from the Registry of the Canadian Interventional Radiology Association. J Vasc Interv Radiol 2001;12:1053–8. [DOI] [PubMed]
- 44.Pieri S, Agresti P, Morucci M, de' Medici L. Optional vena cava filters: preliminary experience with a new vena cava filter [in English, Italian]. Radiol Med (Torino) 2003;105:56–62. [PubMed]
- 45.Millward SF, Bormanis J, Burbridge BE, Markman SJ, Peterson RA. Preliminary clinical experience with the Gunther temporary inferior vena cava filter. J Vasc Interv Radiol 1994; 5:863–8. [DOI] [PubMed]
- 46.Imberti D, Bianchi M, Farina A, Siragusa S, Silingardi M, Ageno W. Clinical experience with retrievable vena cava filters: results of a prospective observational multicenter study. J Thromb Haemost 2005;3:1370–5. [DOI] [PubMed]
- 47.Mohan CR, Hoballah JJ, Sharp WJ, Kresowik TF, Lu CT, Corson JD. Comparative efficacy and complications of vena caval filters. J Vasc Surg 1995;21:235–46. [DOI] [PubMed]
- 48.Nicholson AA, Ettles DF, Paddon AJ, Dyet JF. Long-term follow-up of the Bird's Nest IVC filter. Clin Radiol 1999; 54:759–64. [DOI] [PubMed]
- 49.Wittenberg G, Kueppers V, Tschammler A, Scheppach W, Kenn W, Hahn D. Long-term results of vena cava filters: experiences with the LGM and the Titanium Greenfield devices. Cardiovasc Intervent Radiol 1998;21:225–9. [DOI] [PubMed]
- 50.Becker CD, Hoogewoud HM, Felder P, Gal I, Ruijs PA, Triller J. Long-term follow-up of the Gunther basket inferior vena cava filter: does mechanical instability cause complications? Cardiovasc Intervent Radiol 1994;17:247–51. [DOI] [PubMed]
- 51.Schutzer R, Ascher E, Hingorani A, Jacob T, Kallakuri S. Preliminary results of the new 6F TrapEase inferior vena cava filter. Ann Vasc Surg 2003;17:103–6. [DOI] [PubMed]
- 52.Teitelbaum GP, Bradley WG Jr, Klein BD. MR imaging artifacts, ferromagnetism, and magnetic torque of intravascular filters, stents, and coils. Radiology 1988;166:657–64. [DOI] [PubMed]
- 53.Buller HR, Agnelli G, Hull RD, Hyers TM, Prins MH, Raskob GE. Antithrombotic therapy for venous thromboembolic disease: the Seventh ACCP Conference on Antithrombotic Therapy [published erratum appears in Chest 2005;127:416]. Chest 2004;126(3 Suppl):401S–28S. [DOI] [PubMed]
- 54.Ballew KA, Philbrick JT, Becker DM. Vena cava filter devices. Clin Chest Med 1995;16:295–305. [PubMed]


