Abstract
A 12-year-old girl with a high fever underwent echocardiography and was found to have a myxoma that arose from the atrial side of the anterior mitral valve leaflet. The tumor was successfully excised. Histologic examination of the tumor showed myxoma cells and an organized thrombus with bacterial colonization. The patient was discharged from the hospital on antibiotic treatment. After remaining asymptomatic for 3 weeks, she was readmitted with acute abdomen. Ultrasonography and magnetic resonance angiography detected intra-abdominal hemorrhaging and a saccular aneurysm of the abdominal aorta. The patient underwent successful emergency surgery.
To our knowledge, no other report has been published concerning an abdominal aortic aneurysm secondary to bacterial infection of a cardiac myxoma. Although complications this severe are rarely observed in patients who have endocarditis, early recognition and treatment can be life-saving.
Key words: Aorta, abdominal; aortic aneurysm/surgery; coronary disease/complications/surgery; child; embolism/diagnosis/pathology; heart atria/pathology/surgery; heart neoplasms/complications/diagnosis/surgery; myxoma/complications/diagnosis/surgery/ultrasonography; staphylococcal infections; treatment outcome
Cardiac tumors in children differ from those in adults. Atrial myxomas occur infrequently in children. Infected cardiac myxomas are even rarer; only 41 cases in all age groups have been reported in the English-language medical literature.1,2 Despite advanced antibiotic therapy, mycotic aneurysms are an important cause of morbidity and death due to endocarditis. In the visceral arteries, aneurysms are uncommon and usually remain clinically silent unless they rupture.3 We report herein the diagnosis and successful surgical treatment of a ruptured saccular abdominal aortic aneurysm in a child. The aneurysm developed from residual endocarditis of a left atrial myxoma that had been excised 3 weeks earlier.
Case Report
In May 2003, a 12-year-old girl was admitted to a pediatric clinic after 2 weeks of high fever, excessive sweating, and fatigue. Because infective endocarditis was suspected, the patient was referred to our echocardiographic unit for further evaluation. On physical examination, her blood pressure was 100/60 mmHg, her heart rate was 109 beats/min and regular, and her temperature was 38.5 °C. A grade 2/6 systolic murmur was best heard at the apex. There were no peripheral signs of infective endocarditis, splenomegaly, hepatomegaly, or edema. An electrocardiogram showed sinus tachycardia and juvenile T waves in leads V1 through V3. Laboratory analysis revealed mild anemia (hemoglobin, 9.3 g/dL) and leukocytosis (white blood count, 16,800/mm3). The erythrocyte sedimentation rate was 56 mm in the 1st hour. Elevated levels of rheumatoid factor and C-reactive protein were detected. The serum complement level and urinalysis results were normal. Methicillin-sensitive Staphylococcus aureus grew in blood cultures. Anticardiolipin antibodies were negative. Protein C, protein S, and antithrombin III levels were normal.
Transthoracic Doppler echocardiography showed left ventricular function within the normal range; the left atrium was dilated and contained a mobile mass. The parasternal short-axis view disclosed a 1.8 × 2.9-cm heterogeneous mass adhering to the atrial side of the anterior mitral valve leaflet (Fig. 1) and prolapsing into the left ventricle during diastole (Fig. 2). Arising from the anterior wall of the mitral valve was an atrial myxoma, with a surface that was friable and not smooth. Doppler echocardiography disclosed mild mitral and tricuspid regurgitation, with a calculated peak systolic pulmonary artery pressure of 45 mmHg.
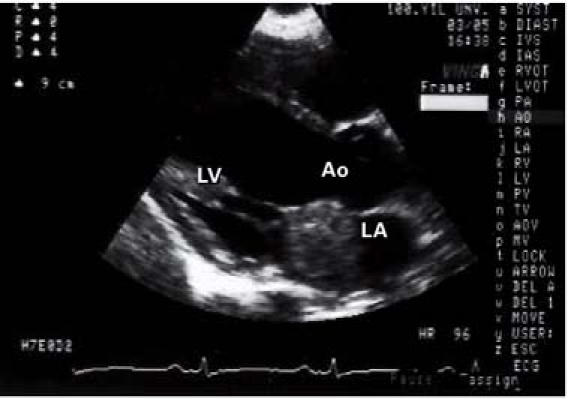
Fig. 1 Transthoracic Doppler echocardiography in the parasternal short-axis view shows a heterogeneous mass in the left atrium.
Ao = aorta; LA = left atrium; LV = left ventricle
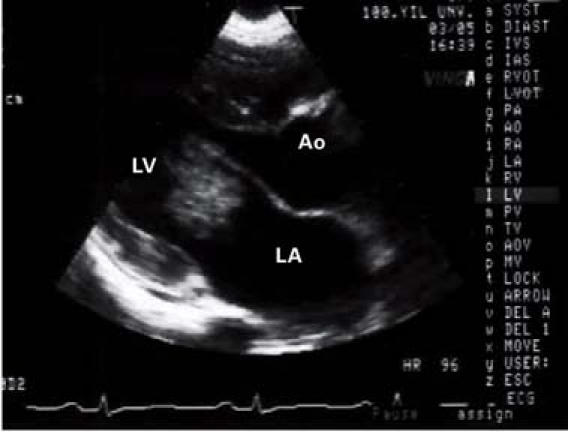
Fig. 2 Transthoracic Doppler echocardiography shows a 1.8 × 2.9-cm mass, which occupied a large part of the mitral valve's opening area. The mass prolapsed into the left ventricular cavity upon the opening of the mitral valve leaflets during diastole.
Ao = aorta; LA = left atrium; LV = left ventricle
Because of the risk of embolism, the patient was taken to emergency surgery, and the mass was resected. Histologic examination of the tumor revealed myxoma cells and an organized thrombus with bacterial colonization. The postoperative course was uneventful. Methicillin and gentamicin sulfate therapies were maintained. Nafcillin (2 g intravenously every 6 hr) and gentamicin sulfate (1 mg/kg) therapies were administered postoperatively. A repeat echocardiogram revealed no mass and no tricuspid or mitral regurgitation. Nine days after the operation, the patient's condition had greatly improved, and she was discharged from the hospital on the same antibiotic treatment.
Three weeks later, the patient was readmitted to our hospital due to the sudden onset of acute abdomen. Ultrasonography and magnetic resonance angiography (Fig. 3) revealed a ruptured saccular abdominal aortic aneurysm; therefore, the patient underwent urgent surgery. A xiphoid-to-pubis midline incision enabled transperitoneal exposure of the abdominal aortic aneurysm. Because of intra-abdominal hemorrhaging, the aortic cross-clamp was placed after the diaphragmatic crux was divided; this placement provided a clear view of the supraceliac aorta. When supraceliac (proximal) aortic control was achieved, the transverse colon and greater omentum were mobilized superiorly. The entire small intestine was moved to the right side of the abdomen. The posterior peritoneum was incised anterior to the aorta and the common iliac arteries. A retroperitoneal hematoma was evacuated. The inferior mesenteric artery was ligated within the sac of the aneurysm. An aortoiliac bypass was performed, with use of a polytetrafluoroethylene Y-shaped graft, because of aneurysmal dilatation of the common iliac arteries. The graft's limbs (8 mm for the iliac artery and 16 mm for the aorta) were joined with 3–0 polypropylene sutures to the distal common iliac arteries at the level of the bifurcation of the common iliac artery. Because the patient was a child, interrupted sutures were used for the anastomoses to allow for growth. During surgery, we saw a lobular bilateral renal infarct that had been caused by thrombosis. Specimens obtained from the aorta for culture and for macroscopic and microscopic analysis showed evidence of septic emboli in the aneurysm. Although no specific microorganism grew in the cultures from the ruptured aneurysm, we administered methicillin and ciprofloxacin to the patient prophylactically.
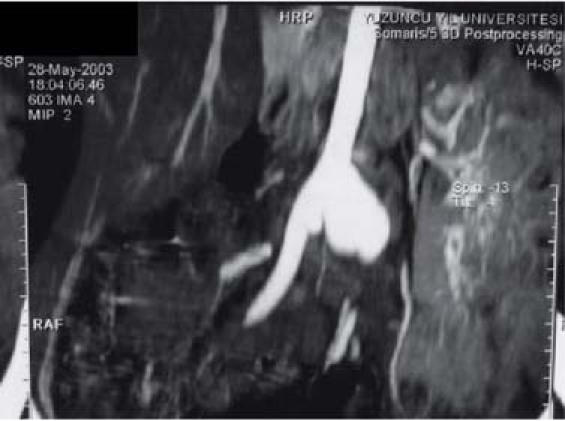
Fig. 3 Magnetic resonance angiography shows a saccular aneurysm of the abdominal aorta, just proximal to the iliac arteries.
The patient's condition improved promptly, and she was discharged from the hospital on the 10th postoperative day. Follow-up examinations have been performed every 6 months; her condition remains good, with no sign of recurrent infection.
Discussion
The clinical diagnosis of cardiac myxoma remains a challenge. Whereas patients with a cardiac myxoma usually present with constitutional, embolic, and obstructive symptoms,3 symptoms can also be nonspecific and extracardiac. Moreover, a variety of clinical and laboratory findings can mimic systemic vasculitis or a connective-tissue disease and thus impede a correct diagnosis.4 Echocardiography, while highly sensitive and specific for intracardiac tumors, does not yield a complete diagnosis. The evaluation of cardiac signs and symptoms is helpful in the diagnosis of cardiac myxoma.
Angiography has on rare occasions revealed multiple cerebral aneurysms that have developed after successful resection of primary left atrial myxomas.5 Arterial embolism of myxoma fragments can cause those aneurysms.6
The incidence of systemic embolization in cases of infected atrial myxoma is extremely high, and the bacterial foci can be the source of persistent bacteremia. In patients who present with embolism, the myxomas usually have a friable surface. Accordingly, we perform open-heart surgery as soon as possible after diagnostic evaluation that suggests an infected cardiac myxoma, in order to prevent both the embolization and the infective progression into other cardiovascular structures.
In 1991, Tsuchiya and colleagues7 described the case of a man who was diagnosed with a left atrial myxoma and an abdominal aortic aneurysm. That aneurysm might have been due to atherosclerosis—the patient was 68 years old, and he required quadruple coronary artery bypass surgery and other procedures as well. In contrast, we infer that the ruptured aneurysm in our much younger patient was caused by a septic embolus from the infected intracardiac tumor.
To our knowledge, ours is the 1st published report of a mycotic abdominal aortic aneurysm secondary to endocarditis of a cardiac myxoma. Our case highlights the importance of careful clinical investigation and of early use of appropriate imaging techniques in order to identify potential locations of septic emboli, which can cause severe clinical problems after the successful resection of an infected cardiac myxoma.
Footnotes
Address for reprints: Niyazi Guler, MD, Yuzuncu Yil Universitesi, Arastirma Hastanesi, Kardiyoloji Anabilim Dali, 65200 Van, Turkey. E-mail: niyaziguler@hotmail.com
References
- 1.Revankar SG, Clark RA. Infected cardiac myxoma: Case report and literature review. Medicine 1998;77:337–44. [DOI] [PubMed]
- 2.Gregory SA, O'Byrne WT 3rd, Fan P. Infected cardiac myxoma. Echocardiography 2004;21:65–7. [DOI] [PubMed]
- 3.Pinede L, Duhaut P, Loire R. Clinical presentation of left atrial cardiac myxoma. A series of 112 consecutive cases. Medicine (Baltimore) 2001;80:159–72. [DOI] [PubMed]
- 4.Boussen K, Moalla M, Blondeau P, Ben Ayed H, Lie JT. Embolization of cardiac myxomas masquerading as polyarteritis nodosa. J Rheumatol 1991;18:283–5. [PubMed]
- 5.Oguz KK, Firat MM, Cila A. Fusiform aneurysms detected 5 years after removal of an atrial myxoma. Neuroradiology 2001;43:990–2. [DOI] [PubMed]
- 6.Furuya K, Sasaki T, Yoshimoto Y, Okada Y, Fujimaki T, Kirino T. Histologically verified cerebral aneurysm formation secondary to embolism from cardiac myxoma. Case report. J Neurosurg 1995;83:170–3. [DOI] [PubMed]
- 7.Tsuchiya K, Takazawa A, Hagino I, Iida Y, Aizawa K, Noda Y. Combined operation for left atrial myxoma, mitral regurgitation, coronary artery stenosis and abdominal aortic aneurysm [in Japanese]. Kyobu Geka 1991;44:569–72. [PubMed]


